Onset Mechanisms and Prognosis of Neurally Mediated Syncope
Abstract
1. Introduction
2. Mechanism Underlying the Molecular Interactions between α2B-AR Gene Polymorphisms and Gi Protein in NMS
3. Relationship between AC Activity and the Onset of NMS
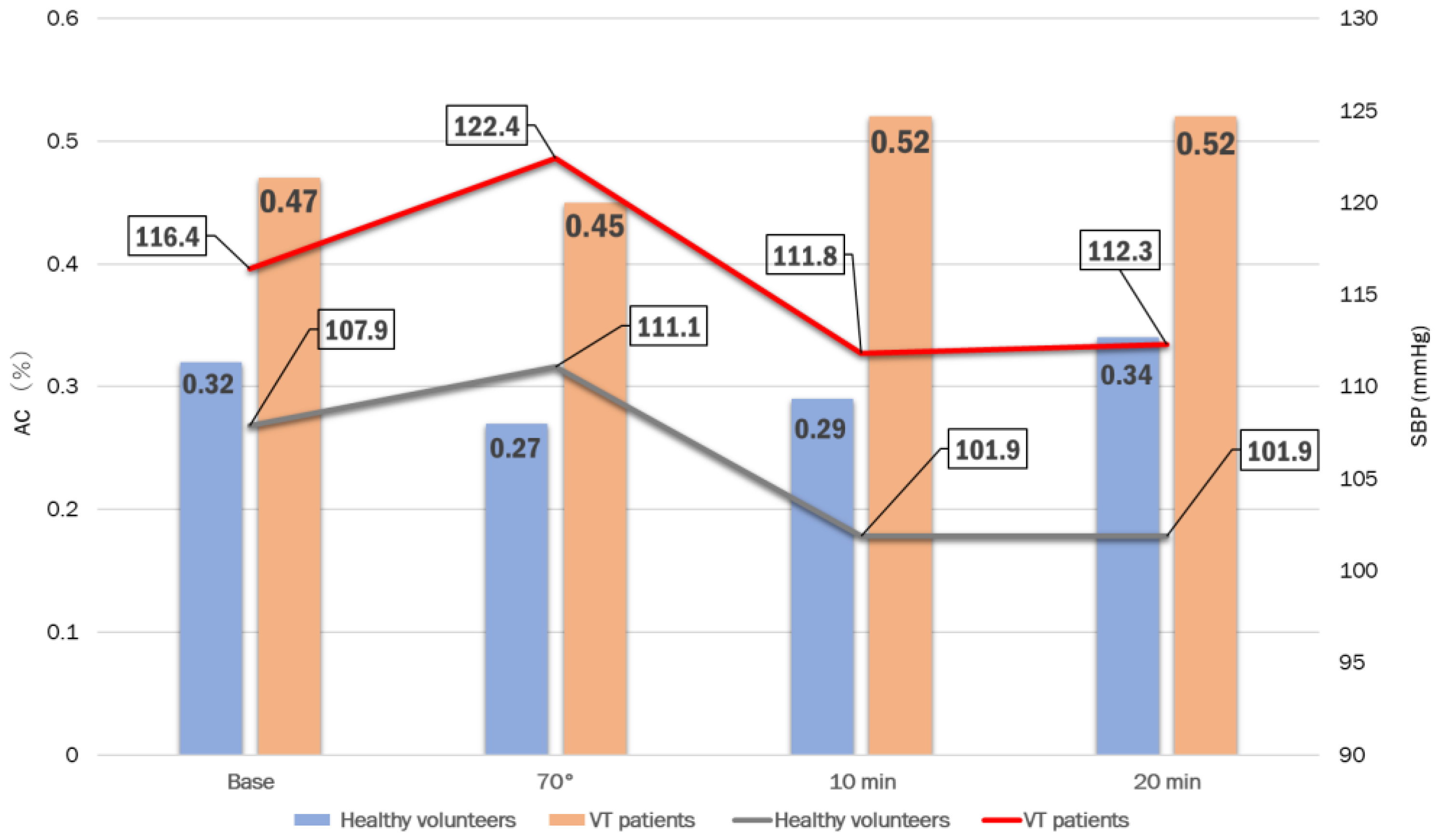
4. Systolic Blood Pressure (SBP) and AC Activity in Japanese Patients with VT-NMS
5. Clinical Significance of the HUT Test for Improving the Prognosis of Patients with Possible NMS
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, K.; Yukiiri, K.; Mizushige, K.; Takagi, Y.; Masugata, H.; Shinomiya, K.; Hosomi, N.; Takahashi, T.; Ohmori, K.; Kohno, M. Usefulness of brain natriuretic peptide as a marker for separating cardiac and noncardiac causes of syncope. Am. J. Cardiol. 2004, 93, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, R. Pathophysiology of syncope. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2004, 14, i18–i24. [Google Scholar] [CrossRef] [PubMed]
- Saklani, P.; Krahn, A.; Klein, G.J.C. Syncope. Circulation 2013, 127, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Alboni, P. The different clinical presentations of vasovagal syncope. Heart 2015, 101, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Ungar, A.; Sgobino, P.; Russo, V.; Vitale, E.; Sutton, R.; Melissano, D.; Beiras, X.; Bottoni, N.; Ebert, H.H.; Gulizia, M. Diagnosis of neurally mediated syncope at initial evaluation and with tilt table testing compared with that revealed by prolonged ECG monitoring. An analysis from the Third International Study on Syncope of Uncertain Etiology (ISSUE-3). Heart 2013, 99, 1825–1831. [Google Scholar] [CrossRef]
- Mosqueda-Garcia, R.; Furlan, R.; MD, J.T.; Fernandez-Violante, R. The elusive pathophysiology of neurally mediated syncope. Circulation 2000, 102, 2898–2906. [Google Scholar] [CrossRef]
- Martin, K.; Bates, G.; Whitehouse, W. Transient loss of consciousness and syncope in children and young people: What you need to know. Arch. Dis. Child.-Educ. Pract. 2010, 95, 66–72. [Google Scholar] [CrossRef]
- Mercader, M.; Varghese, P.; Potolicchio, S.; Venkatraman, G.; Lee, S. New insights into the mechanism of neurally mediated syncope. Heart 2002, 88, 217–221. [Google Scholar] [CrossRef]
- Silvani, S.; Padoan, G.; Guidi, A.R.; Bianchedi, G.; Maresta, A. Cerebral vasoconstriction in neurally mediated syncope: Relationship with type of head-up tilt test response. Ital. Heart J. 2003, 4, 768–775. [Google Scholar]
- Schondorf, R.; Stein, R.; Roberts, R.; Benoit, J.; Cupples, W. Dynamic cerebral autoregulation is preserved in neurally mediated syncope. J. Appl. Physiol. 2001, 91, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Jhanjee, R.; Van Dijk, J.G.; Sakaguchi, S.; Benditt, D.G. Syncope in adults: Terminology, classification, and diagnostic strategy. Pacing Clin. Electrophysiol. 2006, 29, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Zaqqa, M.; Massumi, A. Neurally mediated syncope. Tex. Heart Inst. J. 2000, 27, 268. [Google Scholar] [PubMed]
- Kapoor, W.N. Evaluation and outcome of patients with syncope. Medicine 1990, 69, 160–175. [Google Scholar] [CrossRef]
- Linzer, M.; Yang, E.H.; Estes, N.M.; Wang, P.; Vorperian, V.R.; Kapoor, W.N. Clinical guideline: Diagnosing syncope: Part 1: Value of history, physical examination, and electrocardiography. Ann. Intern. Med. 1997, 126, 989–996. [Google Scholar] [CrossRef]
- Liao, D.; Xu, Y.; Zou, R.; Wu, L.; Luo, X.; Li, F.; Lin, P.; Wang, X.; Xie, Z.; Wang, C. The circadian rhythm of syncopal episodes in patients with neurally mediated syncope. Int. J. Cardiol. 2016, 215, 186–192. [Google Scholar] [CrossRef]
- Chu, W.; Wang, C.; Lin, P.; Li, F.; Wu, L.; Xie, Z. Transient aphasia: A rare complication of head-up tilt test. Neurol. Sci. 2014, 35, 1127–1132. [Google Scholar] [CrossRef]
- Komiyama, T.; Hirokawa, T.; Sato, K.; Oka, A.; Kamiguchi, H.; Nagata, E.; Sakura, H.; Otsuka, K.; Kobayashi, H. Relationship between human evolution and neurally mediated syncope disclosed by the polymorphic sites of the adrenergic receptor gene α2B-AR. PLoS ONE 2015, 10, e0120788. [Google Scholar] [CrossRef]
- Komiyama, T.; Nagata, E.; Hashida, T.; Sakama, S.; Ayabe, K.; Kamiguchi, H.; Sasaki, A.; Yoshioka, K.; Kobayashi, H. Neurally mediated syncope diagnosis based on adenylate cyclase activity in Japanese patients. PLoS ONE 2019, 14, e0214733. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Cornwell, T.L.; Taylor, A.E. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am. J. Physiol. 1990, 258, C399–C407. [Google Scholar] [CrossRef]
- Ishikawa, T.; Hume, J.R.; Keef, K.D. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ. Res. 1993, 73, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Keef, K.D.; Hume, J.R.; Zhong, J. Regulation of cardiac and smooth muscle Ca2+ channels (CaV1. 2a, b) by protein kinases. Am. J. Physiol.-Cell Physiol. 2001, 281, C1743–C1756. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Ikebe, M.; Kargacin, G.J.; Hartshorne, D.J.; Kemp, B.E.; Fay, F.S. Effects of modulators of myosin light-chain kinase activity in single smooth muscle cells. Nature 1989, 338, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, K.; Komiyama, T.; Hasegawa, M.; Sakai, T.; Morise, M.; Sakama, S.; Yagishita, A.; Amino, M.; Ikari, Y.; Yoshioka, K. Clinical significance of the head-up tilt test in improving prognosis in patients with possible neurally mediated syncope. Biology 2021, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Komiyama, T.; Ayabe, K.; Sakama, S.; Sakai, T.; Lee, K.H.; Morise, M.; Yagishita, A.; Amino, M.; Sasaki, A. Diagnosis and prevention of the vasodepressor type of neurally mediated syncope in Japanese patients. PLoS ONE 2021, 16, e0251450. [Google Scholar] [CrossRef] [PubMed]
- Small, K.M.; Brown, K.M.; Forbes, S.L.; Liggett, S.B. Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J. Biol. Chem. 2001, 276, 4917–4922. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, J.R.; Gazis, A.G.; Cross, D.J.; Wheatley, A.; Dewar, J.; Hall, I.P.; Noon, J.P. β2-adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension 2000, 36, 371–375. [Google Scholar] [CrossRef]
- Dishy, V.; Sofowora, G.G.; Xie, H.-G.; Kim, R.B.; Byrne, D.W.; Stein, C.M.; Wood, A.J. The effect of common polymorphisms of the β2-adrenergic receptor on agonist-mediated vascular desensitization. N. Engl. J. Med. 2001, 345, 1030–1035. [Google Scholar] [CrossRef]
- Galandrin, S.; Bouvier, M. Distinct signaling profiles of β1 and β2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol. Pharmacol. 2006, 70, 1575–1584. [Google Scholar] [CrossRef]
- Snapir, A.; Heinonen, P.; Tuomainen, T.-P.; Alhopuro, P.; Karvonen, M.K.; Lakka, T.A.; Nyyssönen, K.; Salonen, R.; Kauhanen, J.; Valkonen, V.-P. An insertion/deletion polymorphism in the α2B-adrenergic receptor gene is a novel genetic risk factor for acute coronary events. J. Am. Coll. Cardiol. 2001, 37, 1516–1522. [Google Scholar] [CrossRef]
- Billington, C.K.; Penn, R.B. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.C.; Scott, J.D.; Catterall, W.A. Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr. Opin. Neurobiol. 1998, 8, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Meinkoth, J.L.; Alberts, A.S.; Went, W.; Fantozzi, D.; Taylor, S.S.; Hagiwara, M.; Montminy, M.; Feramisco, J.R. Signal transduction through the cAMP-dependent protein kinase. Mol. Cell. Biochem. 1993, 127/128, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.; Cooper, D.M. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 2007, 87, 965–1010. [Google Scholar] [CrossRef] [PubMed]
- Kikushima, S.; Kobayashi, Y.; Nakagawa, H.; Katagiri, T. Triggering mechanism for neurally mediated syncope induced by head-up tilt test: Role of catecholamines and response to propranolol. J. Am. Coll. Cardiol. 1999, 33, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Sra, J.S.; Murthy, V.; Natale, A.; Jazayeri, M.R.; Dhala, A.; Deshpande, S.; Sheth, M.; Akhtar, M. Circulatory and catecholamine changes during head-up tilt testing in neurocardiogenic (vasovagal) syncope. Am. J. Cardiol. 1994, 73, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Fitzpartick, A. Echocardiographic and endocrine changes during vasovagal syncope induced by prolonged head-up tilt. Eur. JCPE 1992, 2, 121–128. [Google Scholar]
- Goldstein, D.S.; Spanarkel, M.; Pitterman, A.; Toltzis, R.; Gratz, E.; Epstein, S.; Keiser, H.R. Circulatory control mechanisms in vasodepressor syncope. Am. Heart J. 1982, 104, 1071–1075. [Google Scholar] [CrossRef]
- Baillie, G.S.; Sood, A.; McPhee, I.; Gall, I.; Perry, S.J.; Lefkowitz, R.J.; Houslay, M.D. β-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates β-adrenoceptor switching from Gs to Gi. Proc. Natl. Acad. Sci. USA 2003, 100, 940–945. [Google Scholar] [CrossRef]
- Mitro, P.; Rybárová, E.; Žemberová, E.; Tkáč, I. Enhanced plasma catecholamine and cAMP response during the head-up tilt test in patients with vasovagal syncope. Wien. Klin. Wochenschr. 2005, 117, 353–358. [Google Scholar] [CrossRef]
- Abe, H.; Kobayashi, H.; Nakashima, Y.; Izumi, F.; Kuroiwa, A. Plasma catecholamines and cyclic AMP response during head-up tilt test in patients with neurocardiogenic (vasodepressor) syncope. Pacing Clin. Electrophysiol. 1995, 18, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Kassimatis, T.; Lymperopoulos, A. Impaired desensitization of a human polymorphic alpha2B-adrenergic receptor variant enhances its sympatho-inhibitory activity in chromaffin cells. Cell Commun. Signal. CCS 2011, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Park, K.-I.; Choi, S.-Y.; Park, H.E.; Lee, H.; Bae, H.-M. Dynamic alterations in cerebral hemodynamics measured by portable near-infrared spectroscopy in orthostatic hypotension and intolerance. Am. J. Hypertens. 2023, 36, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Kochiadakis, G.E.; Kanoupakis, E.M.; Rombola, A.T.; Igoumenidis, N.E.; Chlouverakis, G.I.; Vardas, P.E. Reproducibility of tilt table testing in patients with vasovagal syncope and its relation to variations in autonomic nervous system activity. Pacing Clin. Electrophysiol. 1998, 21, 1069–1076. [Google Scholar] [CrossRef]
- Hosaka, H.; Takase, B.; Katsushika, S.; Ohsuzu, F.; Kurita, A. Altered fractal behavior and heart rate variability in daily life in neurally mediated syncope. Biomed. Pharmacother. 2003, 57, 77–82. [Google Scholar] [CrossRef]
- Yoshioka, K.; Amino, M.; Zareba, W.; Shima, M.; Matsuzaki, A.; Fujii, T.; Kanda, S.; Deguchi, Y.; Kobayashi, Y.; Ikari, Y. Identification of high-risk Brugada syndrome patients by combined analysis of late potential and T-wave amplitude variability on ambulatory electrocardiograms. Circ. J. 2013, 77, 610–618. [Google Scholar] [CrossRef]
- Amino, M.; Yoshioka, K.; Ichikawa, T.; Watanabe, E.; Kiyono, K.; Nakamura, M.; Sakama, S.; Ayabe, K.; Fujii, T.; Hashida, T. The presence of late potentials after percutaneous coronary intervention for the treatment of acute coronary syndrome as a predictor for future significant cardiac events resulting in re-hospitalization. J. Electrocardiol. 2019, 53, 71–78. [Google Scholar] [CrossRef]
- Amino, M.; Yoshioka, K.; Morita, S.; Iizuka, S.; Otsuka, H.; Yamamoto, R.; Aoki, H.; Aizawa, T.; Ikari, Y.; Nasu, S. One-year follow-up and convalescence evaluated by nuclear medicine studies and 24-hour Holter electrocardiogram in 11 patients with myocardial injury due to a blunt chest trauma. J. Trauma Acute Care Surg. 2009, 66, 1308–1310. [Google Scholar] [CrossRef]
- Sneddon, J.F.; Bashir, Y.; Murgatroyd, F.D.; Ward, D.E.; Camm, A.J.; Malik, M. Do patients with neurally mediated syncope have augmented vagal tone? Am. J. Cardiol. 1993, 72, 1314–1315. [Google Scholar] [CrossRef]
- Lazzeri, C.; La Villa, G.; Barletta, G.; Franchi, F. 24-hour heart rate variability in patients with vasovagal syncope. Pacing Clin. Electrophysiol. 2000, 23, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Foglia-Manzillo, G.; Giada, F.; Gaggioli, G.; Bartoletti, A.; Lolli, G.; Dinelli, M.; Del Rosso, A.; Santarone, M.; Raviele, A.; Brignole, M. Efficacy of tilt training in the treatment of neurally mediated syncope. A randomized study. EP Eur. 2004, 6, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.C.; Calkins, H.; DeBusk, K.; McKenzie, R.; Anand, R.; Sharma, G.; Cuccherini, B.A.; Soto, N.; Hohman, P.; Snader, S. Fludrocortisone acetate to treat neurally mediated hypotension in chronic fatigue syndrome: A randomized controlled trial. JAMA 2001, 285, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ungar, A.; Galizia, G.; Morrione, A.; Mussi, C.; Noro, G.; Ghirelli, L.; Masotti, G.; Rengo, F.; Marchionni, N.; Abete, P. Two-year morbidity and mortality in elderly patients with syncope. Age Ageing 2011, 40, 696–702. [Google Scholar] [CrossRef][Green Version]
- Di Girolamo, E.; Di Iorio, C.; Leonzio, L.; Sabatini, P.; Barsotti, A. Usefulness of a tilt training program for the prevention of refractory neurocardiogenic syncope in adolescents: A controlled study. Circulation 1999, 100, 1798–1801. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A. Practical Instructions for the 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, e43–e80. [Google Scholar] [CrossRef]
- Giancaterino, S.; Lupercio, F.; Nishimura, M.; Hsu, J.C. Current and future use of insertable cardiac monitors. JACC Clin. Electrophysiol. 2018, 4, 1383–1396. [Google Scholar] [CrossRef]
- Dalgaard, F.; Pallisgaard, J.L.; Numé, A.K.; Lindhardt, T.B.; Gislason, G.H.; Torp-Pedersen, C.; Ruwald, M.H. Rate or rhythm control in older atrial fibrillation patients: Risk of fall-related injuries and syncope. J. Am. Geriatr. Soc. 2019, 67, 2023–2030. [Google Scholar] [CrossRef]
- Park, J.; Jang, S.Y.; Yim, H.R.; On, Y.K.; Huh, J.; Shin, D.-H.; Kim, J.H.; Kim, J.S. Gender difference in patients with recurrent neurally mediated syncope. Yonsei Med. J. 2010, 51, 499–503. [Google Scholar] [CrossRef][Green Version]
- Brass, L.; Laposata, M.; Banga, H.; Rittenhouse, S. Regulation of the phosphoinositide hydrolysis pathway in thrombin-stimulated platelets by a pertussis toxin-sensitive guanine nucleotide-binding protein. Evaluation of its contribution to platelet activation and comparisons with the adenylate cyclase inhibitory protein, Gi. J. Biol. Chem. 1986, 261, 16838–16847. [Google Scholar]
- Calebiro, D.; Nikolaev, V.O.; Persani, L.; Lohse, M.J. Signaling by internalized G-protein-coupled receptors. Trends Pharmacol. Sci. 2010, 31, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Masuo, K. Roles of beta2-and beta3-adrenoceptor polymorphisms in hypertension and metabolic syndrome. Int. J. Hypertens. 2010, 2010, 832821. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Ostrom, R.S. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell. Mol. Neurobiol. 2003, 23, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Davare, M.A.; Avdonin, V.; Hall, D.D.; Peden, E.M.; Burette, A.; Weinberg, R.J.; Horne, M.C.; Hoshi, T.; Hell, J.W. A β2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1. 2. Science 2001, 293, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Torphy, T.J. β-Adrenoceptors, cAMP and airway smooth muscle relaxation: Challenges to the dogma. Trends Pharmacol. Sci. 1994, 15, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Billington, C.K.; Ojo, O.O.; Penn, R.B.; Ito, S. cAMP regulation of airway smooth muscle function. Pulm. Pharmacol. Ther. 2013, 26, 112–120. [Google Scholar] [CrossRef]
- Hong, F.; Brizendine, R.K.; Carter, M.S.; Alcala, D.B.; Brown, A.E.; Chattin, A.M.; Haldeman, B.D.; Walsh, M.P.; Facemyer, K.C.; Baker, J.E. Diffusion of myosin light chain kinase on actin: A mechanism to enhance myosin phosphorylation rates in smooth muscle. J. Gen. Physiol. 2015, 146, 267–280. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, X.; Yang, W.; Wang, H.; Zhao, H.; Yang, F.; Yang, Y.; Li, J.; Lv, X. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathway in rat hepatic stellate cells. Int. Immunopharmacol. 2015, 25, 340–352. [Google Scholar] [CrossRef]
- Huang, W.; Cane, M.C.; Mukherjee, R.; Szatmary, P.; Zhang, X.; Elliott, V.; Ouyang, Y.; Chvanov, M.; Latawiec, D.; Wen, L. Caffeine protects against experimental acute pancreatitis by inhibition of inositol 1, 4, 5-trisphosphate receptor-mediated Ca2+ release. Gut 2017, 66, 301–313. [Google Scholar] [CrossRef]
- Horrigan, L.A.; Kelly, J.P.; Connor, T.J. Immunomodulatory effects of caffeine: Friend or foe? Pharmacol. Ther. 2006, 111, 877–892. [Google Scholar] [CrossRef]

| Allele Type | Total | Glu12/12 | Glu12/9 | Glu9/9 |
|---|---|---|---|---|
| NMS | 9 | 1 | 8 | 0 |
| Healthy volunteers | 11 | 3 | 5 | 3 |
| Glu9 | Glu12 | |
|---|---|---|
| Binding energy of the Gi protein α-subunit a | −77.94 | −68.40 |
| Patients with NMS (VT) n = 11 | Average (%) | SD (%) | Healthy Volunteers n = 12 | Average (%) | SD (%) | p Value |
|---|---|---|---|---|---|---|
| Base | Base | |||||
| IP 50 µM | 0.560415 | 0.152698 | IP 50 µM | 0.404779 | 0.154299 | 0.01212 |
| IP 5 µM | 0.470369 | 0.170573 | IP 5 µM | 0.330279 | 0.15716 | 0.027111 |
| 70° | 70° | |||||
| IP 50 µM | 0.522241 | 0.148235 | IP 50 µM | 0.378662 | 0.156597 | 0.017344 |
| IP 5 µM | 0.435144 | 0.156784 | IP 5 µM | 0.304623 | 0.156206 | 0.029483 |
| 10 min | 10 min | |||||
| IP 50 µM | 0.586415 | 0.180438 | IP 50 µM | 0.396493 | 0.132411 | 0.00519 |
| IP 5 µM | 0.508118 | 0.204569 | IP 5 µM | 0.312684 | 0.134018 | 0.007836 |
| 20 min | 20 min | |||||
| IP 50 µM | 0.615201 | 0.187159 | IP 50 µM | 0.423144 | 0.119647 | 0.005006 |
| IP 5 µM | 0.542883 | 0.206041 | IP 5 µM | 0.331232 | 0.127212 | 0.004782 |
| Patients with NMS (VT) n = 11 | Average (%) | SD (%) | Healthy Volunteers n = 12 | Average (%) | SD (%) | p Value |
|---|---|---|---|---|---|---|
| Base | Base | |||||
| AD 100 µM | 0.653258 | 0.107929 | AD 100 µM | 0.541178 | 0.097983 | 0.008511 |
| AD 10 µM | 0.471868 | 0.168761 | AD 10 µM | 0.326923 | 0.149877 | 0.021068 |
| 70° | 70° | |||||
| AD 100 µM | 0.618596 | 0.104676 | AD 100 µM | 0.50587 | 0.118856 | 0.012396 |
| AD 10 µM | 0.430308 | 0.15054 | AD 10 µM | 0.287633 | 0.143551 | 0.015306 |
| 10 min | 10 min | |||||
| AD 100 µM | 0.658657 | 0.126682 | AD 100 µM | 0.496051 | 0.100559 | 0.00153 |
| AD 10 µM | 0.504208 | 0.186068 | AD 10 µM | 0.296339 | 0.112927 | 0.002731 |
| 20 min | 20 min | |||||
| AD 100 µM | 0.669466 | 0.184746 | AD 100 µM | 0.514371 | 0.089895 | 0.012058 |
| AD 10 µM | 0.544377 | 0.20292 | AD 10 µM | 0.334034 | 0.127794 | 0.004623 |
| Patients with NMS (MT) n = 6 | Average (%) | SD (%) | Healthy Volunteers n = 12 | Average (%) | SD (%) | p Value |
|---|---|---|---|---|---|---|
| Base | Base | |||||
| IP 50 µM | 0.311706 | 0.076293 | IP 50 µM | 0.404779 | 0.154299 | 0.053075 |
| IP 5 µM | 0.24143 | 0.069333 | IP 5 µM | 0.330279 | 0.15716 | 0.058083 |
| 70° | 70° | |||||
| IP 50 µM | 0.297053 | 0.091338 | IP 50 µM | 0.378662 | 0.156597 | 0.091763 |
| IP 5 µM | 0.217388 | 0.087596 | IP 5 µM | 0.304623 | 0.156206 | 0.074785 |
| 10 min | 10 min | |||||
| IP 50 µM | 0.444013 | 0.163987 | IP 50 µM | 0.396493 | 0.132411 | 0.276989 |
| IP 5 µM | 0.371646 | 0.199079 | IP 5 µM | 0.312684 | 0.134018 | 0.266197 |
| 20 min | 20 min | |||||
| IP 50 µM | 0.491652 | 0.167788 | IP 50 µM | 0.423144 | 0.119647 | 0.459322 |
| IP 5 µM | 0.408247 | 0.139467 | IP 5 µM | 0.331232 | 0.127212 | 0.421843 |
| Patients with NMS (MT) n = 6 | Average (%) | SD (%) | Healthy Volunteers n = 12 | Average (%) | SD (%) | p Value |
|---|---|---|---|---|---|---|
| Base | Base | |||||
| AD 100 µM | 0.493567 | 0.049775 | AD 100 µM | 0.541178 | 0.097983 | 0.095287 |
| AD 10 µM | 0.251196 | 0.048196 | AD 10 µM | 0.326923 | 0.149877 | 0.066226 |
| 70° | 70° | |||||
| AD 100 µM | 0.453367 | 0.04789 | AD 100 µM | 0.50587 | 0.118856 | 0.101355 |
| AD 10 µM | 0.22152 | 0.068808 | AD 10 µM | 0.287633 | 0.143551 | 0.102607 |
| 10 min | 10 min | |||||
| AD 100 µM | 0.554954 | 0.1411 | AD 100 µM | 0.496051 | 0.100559 | 0.194535 |
| AD 10 µM | 0.370648 | 0.18302 | AD 10 µM | 0.296339 | 0.112927 | 0.196221 |
| 20 min | 20 min | |||||
| AD 100 µM | 0.583728 | 0.10373 | AD 100 µM | 0.514371 | 0.089895 | 0.156635 |
| AD 10 µM | 0.409066 | 0.134564 | AD 10 µM | 0.334034 | 0.127794 | 0.349466 |
| Base | 70° | 10 min | 20 min | |
|---|---|---|---|---|
| Healthy volunteers n = 15 | 0.32 ± 0.14 | 0.27 ± 0.14 | 0.29 ± 0.10 | 0.34 ± 0.12 |
| patients with VT-NMS n = 27 | 0.47 ± 0.18 | 0.45 ± 0.19 | 0.52 ± 0.21 | 0.52 ± 0.21 |
| pvalue | 0.0051 | 0.0020 | 0.0002 | 0.0031 |
| a. SBP | Base | 70° | 3 min | 5 min | 10 min | 15 min | 20 min |
| Patients with VT-NMS | 116.4 ± 14.1 | 122.4 ± 14.0 | 123.9 ± 18.4 | 118.1 ± 16.6 | 111.8 ± 19.8 | 111.3 ± 21.4 | 112.3 ± 16.9 |
| Healthy volunteers | 107.9 ± 8.0 | 111.1 ± 15.5 | 108.8 ± 9.1 | 107.1 ± 12.6 | 101.9 ± 10.0 | 104.4 ± 10.7 | 101.9 ± 9.1 |
| p value | 0.036 | 0.020 | 0.019 | 0.032 | 0.090 | 0.264 | 0.037 |
| b. DBP | Base | 70° | 3 min | 5 min | 10 min | 15 min | 20 min |
| Patients with VT-NMS | 74.0 ± 12.0 | 80.6 ± 11.9 | 81.0 ± 11.6 | 78.6 ± 11.8 | 76.5 ± 13.5 | 74.3 ± 14.4 | 76.8 ± 15.1 |
| Healthy volunteers | 66.2 ± 8.8 | 79.7 ± 8.5 | 78.1 ± 7.8 | 74.5 ± 8.1 | 72.4 ± 10.6 | 73.6 ± 10.7 | 74.7 ± 8.7 |
| p value | 0.032 | 0.815 | 0.472 | 0.248 | 0.335 | 0.865 | 0.640 |
| c. HR | Base | 70° | 3 min | 5 min | 10 min | 15 min | 20 min |
| Patients with VT-NMS | 66.7 ± 14.1 | 78.5 ± 16.8 | 81.0 ± 15.8 | 82.1 ± 17.0 | 82.1 ± 18.1 | 84.3 ± 19.6 | 86.9 ± 21.6 |
| Healthy volunteers | 65.7 ± 8.9 | 74.3 ± 7.6 | 74.7 ± 6.8 | 77.1 ± 7.4 | 75.6 ± 7.4 | 78.3 ± 8.5 | 79.7 ± 8.0 |
| p value | 0.818 | 0.360 | 0.233 | 0.282 | 0.212 | 0.282 | 0.232 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komiyama, T.; Ayabe, K.; Hasegawa, M.; Yoshikawa, M.; Sakama, S.; Lee, K.-H.; Yagishita, A.; Amino, M.; Nagata, E.; Ikari, Y.; et al. Onset Mechanisms and Prognosis of Neurally Mediated Syncope. Reports 2023, 6, 56. https://doi.org/10.3390/reports6040056
Komiyama T, Ayabe K, Hasegawa M, Yoshikawa M, Sakama S, Lee K-H, Yagishita A, Amino M, Nagata E, Ikari Y, et al. Onset Mechanisms and Prognosis of Neurally Mediated Syncope. Reports. 2023; 6(4):56. https://doi.org/10.3390/reports6040056
Chicago/Turabian StyleKomiyama, Tomoyoshi, Kengo Ayabe, Misaki Hasegawa, Marie Yoshikawa, Susumu Sakama, Kyong-Hee Lee, Atsuhiko Yagishita, Mari Amino, Eiichiro Nagata, Yuji Ikari, and et al. 2023. "Onset Mechanisms and Prognosis of Neurally Mediated Syncope" Reports 6, no. 4: 56. https://doi.org/10.3390/reports6040056
APA StyleKomiyama, T., Ayabe, K., Hasegawa, M., Yoshikawa, M., Sakama, S., Lee, K.-H., Yagishita, A., Amino, M., Nagata, E., Ikari, Y., Yoshioka, K., & Kobayashi, H. (2023). Onset Mechanisms and Prognosis of Neurally Mediated Syncope. Reports, 6(4), 56. https://doi.org/10.3390/reports6040056








