Enhanced Gait Recovery in Chronic Post-COVID-19 Stroke: The Role of Combined Physical Rehabilitation
Abstract
:1. Introduction
1.1. Post-Stroke Rehabilitation Strategies
1.2. Use of Manual Myofascial Release Therapy in Stroke Rehabilitation
1.3. Adaptive Ballistic Strength Training Exercises in Neurorehabilitation
1.4. Aerobic Training in Stroke Recovery
2. Detailed Case Description
2.1. Patient Characteristics: De-Identified Patient-Specific Information
2.2. Patient Characteristics: Primary Concerns and Symptoms of the Patient
2.3. Medical, Family, and Psychosocial History, including Relevant Genetic Information
2.4. Experimental Design
2.5. Physiotherapy Assessment
2.6. Therapeutic Interventions
2.7. Statistical Methods
2.8. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stroke Unit Trialists’ Collaboration. Organised Inpatient (Stroke Unit) Care for Stroke. Cochrane Database Syst. Rev. 2013, 2013, CD000197. [Google Scholar]
- Li, Y.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Mao, L.; Hong, C.; Chen, S.; Wang, Y.; Wang, H.; et al. Acute Cerebrovascular Disease following COVID-19: A Single Center, Retrospective, Observational Study. SSRN Electron. J. 2020, 5, 279–284. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Smith, C.J.; Roffe, C.; Simister, R.; Narayanamoorthi, S.; Marigold, R.; Willmot, M.; Dixit, A.; Hassan, A.; Quinn, T.J.; et al. Characteristics and Outcomes of COVID-19 Associated Stroke: A UK Multicentre Case-Control Study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Ishida, K.; Torres, J.; Grory, B.M.; Raz, E.; Humbert, K.; Henninger, N.; Trivedi, T.; Lillemoe, K.; Alam, S.; et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke 2020, 51, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Keith, K.A.; Huang, J.H. COVID-19 and Stroke: A Review. Brain Hemorrhages 2021, 2, 76–83. [Google Scholar] [CrossRef]
- Foged, F.; Rasmussen, I.E.; Bjørn Budde, J.; Rasmussen, R.S.; Rasmussen, V.; Lyngbæk, M.; Jønck, S.; Krogh-Madsen, R.; Lindegaard, B.; Ried-Larsen, M.; et al. Fidelity, Tolerability and Safety of Acute High-Intensity Interval Training after Hospitalisation for COVID-19: A Randomised Cross-over Trial. BMJ Open Sport Exerc. Med. 2021, 7, e001156. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Recovery from Stroke: Current Concepts and Future Perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef]
- Li, S. Spasticity, Motor Recovery, and Neural Plasticity after Stroke. Front. Neurol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Maier, M.; Ballester, B.R.; Verschure, P.F.M.J. Principles of Neurorehabilitation after Stroke Based on Motor Learning and Brain Plasticity Mechanisms. Front. Syst. Neurosci. 2019, 13, 74. [Google Scholar] [CrossRef]
- Kleim, J.A.; Jones, T.A. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation after Brain Damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A. Neural Plasticity and Neurorehabilitation: Teaching the New Brain Old Tricks. J. Commun. Disord. 2011, 44, 521–528. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Need to Scale up Rehabilitation; World Health Organization: Geneva, Switzerland, 2017.
- Eldar, R.; Kullmann, L.; Marincek, C.; Sekelj-Kauzlarić, K.; Svestkova, O.; Palat, M. Rehabilitation Medicine in Countries of Central/Eastern Europe. Disabil. Rehabil. 2008, 30, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Ivanhoe, C.B.; Reistetter, T.A. Spasticity: The Misunderstood Part of the Upper Motor Neuron Syndrome. Am. J. Phys. Med. Rehabil. 2004, 83, S3–S9. [Google Scholar] [CrossRef]
- Mehrholz, J.; Carr, J.H. Physical Therapy for the Stroke Patient: Early Stage Rehabilitation; Thieme: New York, NY, USA, 2012. [Google Scholar]
- Mehrholz, J. Neurorehabilitation Practice for Stroke Patients. Textb. Stroke Med. 2019, 426–448. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, S.-J. Effects of Joint Mobilization and Stretching on the Range of Motion for Ankle Joint and Spatiotemporal Gait Variables in Stroke Patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104933. [Google Scholar] [CrossRef]
- Bunketorp-Käll, L.; Pekna, M.; Pekny, M.; Samuelsson, H.; Blomstrand, C.; Nilsson, M. Motor Function in the Late Phase after Stroke: Stroke Survivors’ Perspective. Ann. Rehabil. Med. 2020, 44, 362–369. [Google Scholar] [CrossRef]
- Lord, S.; McPherson, K.M.; McNaughton, H.K.; Rochester, L.; Weatherall, M. How Feasible Is the Attainment of Community Ambulation after Stroke? A Pilot Randomized Controlled Trial to Evaluate Community-Based Physiotherapy in Subacute Stroke. Clin. Rehabil. 2008, 22, 215–225. [Google Scholar] [CrossRef]
- McNaughton, H.; Gommans, J.; McPherson, K.; Harwood, M.; Fu, V. A Cohesive, Person-Centric Evidence-Based Model for Successful Rehabilitation after Stroke and Other Disabling Conditions. Clin. Rehabil. 2023, 37, 975–985. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil. Neural Repair 2017, 31, 793–799. [Google Scholar] [CrossRef]
- Lee, S.-H. Stroke Revisited: Diagnosis and Treatment of Ischemic Stroke; Springer: Singapore, 2017; ISBN 9789811014246. [Google Scholar]
- DiGiovanna, E.L.; Schiowitz, S.; Dowling, D.J. An Osteopathic Approach to Diagnosis and Treatment; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; ISBN 9780781742931. [Google Scholar]
- Smith, J. Structural Bodywork: An Overview; Churchill Livingstone: London, UK, 2005; pp. 7–12. [Google Scholar]
- Duncan, B.; McDonough-Means, S.; Worden, K.; Schnyer, R.; Andrews, J.; Meaney, F.J. Effectiveness of Osteopathy in the Cranial Field and Myofascial Release versus Acupuncture as Complementary Treatment for Children with Spastic Cerebral Palsy: A Pilot Study. J. Am. Osteopath. Assoc. 2008, 108, 559–570. [Google Scholar] [PubMed]
- Marathe, D.N.; Kunde, C.; Ganvir, S.S. A Comparative Study Between the Immediate Effects of Tendinous Pressure Technique versus Myofascial Release in the Reduction of Spasticity: A Cross over Study. Vims J. Phys. Ther. 2020, 2, 21–27. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Calvo-Sanz, J.; Serra-Llobet, P.; Alcoba-Kait, J.; González-Rueda, V.; Rodríguez-Rubio, P.R. The Effectiveness of Massage Therapy for Improving Sequelae in Post-Stroke Survivors. A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Zhang, J.; Hou, Y.; Jiang, B.-Y.; Pan, H.-F.; Wang, J.; Zhong, D.-Y.; Guo, H.-Y.; Zhu, Y.; Cheng, J. Effectiveness and Safety of Chinese Massage Therapy (Tui Na) on Post-Stroke Spasticity: A Prospective Multicenter Randomized Controlled Trial. Clin. Rehabil. 2017, 31, 904–912. [Google Scholar] [CrossRef]
- Hansen, A.B.; Price, K.S.; Feldman, H.M. Myofascial Structural Integration: A Promising Complementary Therapy for Young Children with Spastic Cerebral Palsy. J. Evid. Based Complement. Altern. Med. 2012, 17, 131–135. [Google Scholar] [CrossRef]
- Park, D.-J.; Hwang, Y.-I. A Pilot Study of Balance Performance Benefit of Myofascial Release, with a Tennis Ball, in Chronic Stroke Patients. J. Bodyw. Mov. Ther. 2016, 20, 98–103. [Google Scholar] [CrossRef]
- Parikh, R.J.; Sutaria, J.M.; Ahsan, M.; Nuhmani, S.; Alghadir, A.H.; Khan, M. Effects of Myofascial Release with Tennis Ball on Spasticity and Motor Functions of Upper Limb in Patients with Chronic Stroke: A Randomized Controlled Trial. Medicine 2022, 101, e29926. [Google Scholar] [CrossRef]
- Kumar, C.; Vaidya, S.N. Effectiveness of Myofascial Release on Spasticity and Lower Extremity Function in Diplegic Cerebral Palsy: Randomized Controlled Trial. Int. J. Phys. Med. Rehabil. 2014, 3, 2. [Google Scholar] [CrossRef]
- Paul, J.; Nathan, S.; Kumar, P. Remya Effectiveness of Myofascial Release in Reduction of Hamstrings Spasticity among Diplegic Cerebral Palsy Children. Int. J. Med. Exerc. Sci. 2018, 4, 453–458. [Google Scholar] [CrossRef]
- Mewada, V.; Barot, K. Effect of Myofascial Release on Spastic Muscles and Functional Outcome in Chronic Stroke Subject—A Randomized Control Trial. Int. J. Sci. Res. 2021, 10, 69–71. [Google Scholar] [CrossRef]
- Intanon, S.; Khamwong, P.; Nantakool, S. A Preliminary Study of Myofascial Release Technique Effect on the Range of Hip Flexion, Knee Flexion, and Ankle Dorsiflexion Motion at Affected Lower Extremity in Individuals with Chronic Stroke. JAMS 2021, 54, 29–34. [Google Scholar]
- Nadeau, S.; Bertrand Arsenault, A.; Gravel, D.; Bourbonnais, D. Analysis of The Clinical Factors Determining Natural and Maximal Gait Speeds in Adults with a Stroke. Am. J. Phys. Med. Rehabil. 1999, 78, 123–130. [Google Scholar] [CrossRef]
- Morris, S.L.; Dodd, K.J.; Morris, M.E. Outcomes of Progressive Resistance Strength Training following Stroke: A Systematic Review. Clin. Rehabil. 2004, 18, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Ada, L.; Dorsch, S.; Canning, C.G. Strengthening Interventions Increase Strength and Improve Activity after Stroke: A Systematic Review. Aust. J. Physiother. 2006, 52, 241–248. [Google Scholar] [CrossRef]
- Williams, G.; Kahn, M.; Randall, A. Strength Training for Walking in Neurologic Rehabilitation Is Not Task Specific: A Focused Review. Am. J. Phys. Med. Rehabil. 2014, 93, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Veldema, J.; Jansen, P. Resistance Training in Stroke Rehabilitation: Systematic Review and Meta-Analysis. Clin. Rehabil. 2020, 34, 1173–1197. [Google Scholar] [CrossRef]
- Macko, R.F.; Ivey, F.M.; Forrester, L.W.; Hanley, D.; Sorkin, J.D.; Katzel, L.I.; Goldberg, A. Treadmill Exercise Rehabilitation Improves Ambulatory Function and Cardiovascular Fitness in Patients with Chronic Stroke a Randomized, Controlled Trial. Stroke 2005, 36, 2206–2211. [Google Scholar] [CrossRef]
- Marsden, D.L.; Dunn, A.; Callister, R.; Levi, C.; Spratt, N.J. Characteristics of Exercise Training Interventions to Improve Cardiorespiratory Fitness after Stroke: A Systematic Review with Meta-Analysis. Neurorehabilit. Neural Repair 2013, 27, 775–788. [Google Scholar] [CrossRef]
- Yamin, G.; Shahid, Z.; Ghafoor, F.; Idress, H.; Babur, N.; Hayat, K. Comparison of the Effectiveness of Aerobic Training with Conventional Physical Therapy Treatment in Improving the Quality of Life in Chronic Stroke Patients. Preprint 2022. [Google Scholar] [CrossRef]
- Constans, A.; Pin-barre, C.; Temprado, J.-J.; Decherchi, P.; Laurin, J. Influence of Aerobic Training and Combinations of Interventions on Cognition and Neuroplasticity after Stroke. Front. Aging Neurosci. 2016, 8, 164. [Google Scholar] [CrossRef]
- Canadian Partnership for Stroke Recovery. Clinician’s Guide Aerobic Exercise after Stroke; Canadian Partnership for Stroke Recovery: Ottawa, ON, Canada, 2013. [Google Scholar]
- MacKay-Lyons, M.; Billinger, S.A.; Eng, J.J.; Dromerick, A.; Giacomantonio, N.; Hafer-Macko, C.; Macko, R.; Nguyen, E.; Prior, P.; Suskin, N.; et al. Aerobic Exercise Recommendations to Optimize Best Practices in Care after Stroke: AEROBICS 2019 Update. Phys. Ther. 2020, 100, 149–156. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P., Jr.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of Acute Cerebral Infarction: A Clinical Examination Scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Fi, M.; Dw, B. Functional evaluation: The barthel index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Hislop, H.J.; Montgomery, J. Daniels and Worthingham’s Muscle Testing: Techniques of Manual Examination; Saunders: Philadelphia, PA, USA, 2007; ISBN 9781416023500. [Google Scholar]
- Norkin, C.C.; Joyce White, D. Measurement of Joint Motion: A Guide to Goniometry; F.A. Davis Company: Philadelphia, PA, USA, 2009. [Google Scholar]
- Cheng, D.K.-Y.; Dagenais, M.; Alsbury-Nealy, K.; Legasto, J.M.; Scodras, S.; Aravind, G.; Takhar, P.; Nekolaichuk, E.; Salbach, N.M. Distance-Limited Walk Tests Post-Stroke: A Systematic Review of Measurement Properties. NeuroRehabilitation 2021, 48, 413. [Google Scholar] [CrossRef] [PubMed]
- Enright, P.L. The Six-Minute Walk Test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Portney, L.G. Single-Subject Designs. In Foundations of Clinical Research: Applications to Evidence-Based Practice, 4th ed.; F.A. Davis Company: New York, NY, USA, 2020. [Google Scholar]
- Crozier, J.; Roig, M.; Eng, J.J.; MacKay-Lyons, M.; Fung, J.; Ploughman, M.; Bailey, D.M.; Sweet, S.N.; Giacomantonio, N.; Thiel, A.; et al. High-Intensity Interval Training after Stroke: An Opportunity to Promote Functional Recovery, Cardiovascular Health, and Neuroplasticity. Neurorehabil. Neural Repair 2018, 32, 543–556. [Google Scholar] [CrossRef]
- Gjellesvik, T.I.; Brurok, B.; Hoff, J.; Tørhaug, T.; Helgerud, J. Effect of High Aerobic Intensity Interval Treadmill Walking in People with Chronic Stroke: A Pilot Study with One Year Follow-Up. Top. Stroke Rehabil. 2012, 19, 353–360. [Google Scholar] [CrossRef]
- Lau, K.W.K.; Mak, M.K.Y. Speed-Dependent Treadmill Training Is Effective to Improve Gait and Balance Performance in Patients with Sub-Acute Stroke. J. Rehabil. Med. 2011, 43, 709–713. [Google Scholar]
- Boyne, P.; Dunning, K.; Carl, D.; Gerson, M.; Khoury, J.; Kissela, B. High-Intensity Interval Training in Stroke Rehabilitation. Top. Stroke Rehabil. 2013, 20, 317–330. [Google Scholar] [CrossRef]
- Grozdek Čovčić, G.; Jurak, I.; Telebuh, M.; Maček, Z.; Bertić, Ž.; Žura, N.; Grubišić, M.; Matić, H.; Tišlar, M.H.; Jakuš, L. Effects of Bobath Treatment and Specific Mobilizations on Gait in Stroke Patients: A Randomized Clinical Trial. NeuroRehabilitation 2022, 50, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Laimi, K.; Mäkilä, A.; Bärlund, E.; Katajapuu, N.; Oksanen, A.; Seikkula, V.; Karppinen, J.; Saltychev, M. Effectiveness of Myofascial Release in Treatment of Chronic Musculoskeletal Pain: A Systematic Review. Clin. Rehabil. 2018, 32, 440–450. [Google Scholar] [CrossRef] [PubMed]
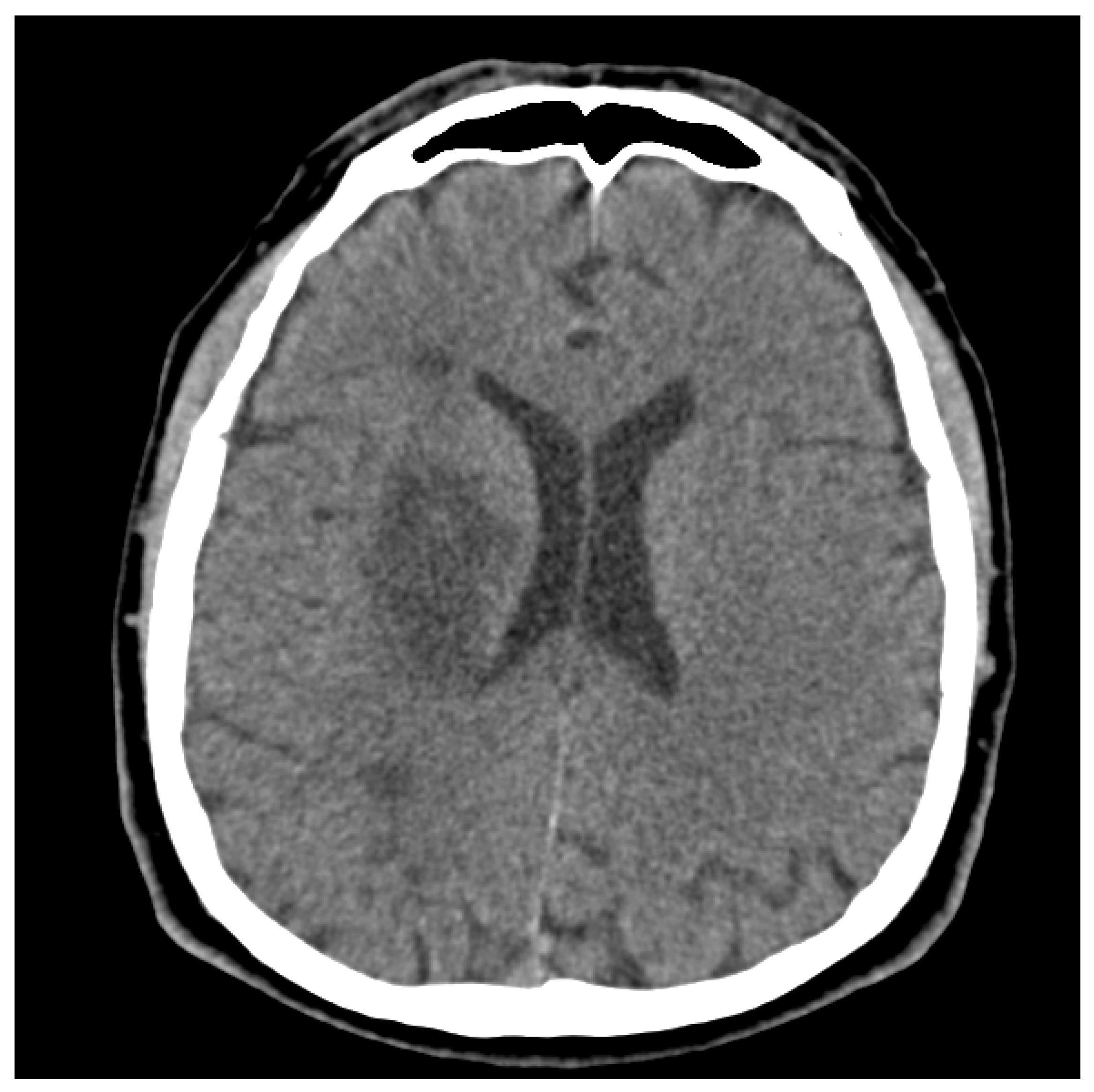
| Phase | I. (Week 2–8) |
|---|---|
| Patient’s status | Left motor hypotonia; incapable of walking or changing body position; National Institutes of Health Stroke Scale (NIHSS) score 14; Barthel index score 15 |
| Dosing | 4 times/week for 40 min (week 3–5); 3 times/week for 50 min (week 6–8) |
| Goals | Preventing the development of movement patterns resulting from abnormal muscle tone; establishing controlled movement of the upper and lower trunk; bed mobility: scooting up, scooting down, scooting laterally, rolling in bed, supine to sitting, sitting to supine; transfers (bed to chair and back) |
| Exercises | Circulatory and breathing exercises; passive movement (particular attention to the hip and shoulder joints); assisted active movement; rhythmic initiation, Proprioceptive Neuromuscular Facilitation (PNF) technique; active internal and external rotation of the hip; hip adduction exercises; “bridging” (hip extension); sequence of exercise progression following the patterns of motor development acquired during the infant’s life (rolling, sitting); adapted progressive resistance and isometric strengthening exercises to strengthen the trunk muscles to stabilize sitting position; “rhythmic stabilization” and “alternating isometrics” PNF techniques to fix sitting |
| Outcome | The goals set for the first phase of our rehabilitation were achieved. |
| Phase | II. (Week 9–16) |
| Patient’s status | Spasticity had already developed on the affected side, pronounced in the upper limb; shoulder drawn backward, arm turned inward, elbow bent with fisted hand and palm down, pelvis drawn backward with the leg turned inward, hip, knee and ankle straightened, foot stiffened downward |
| Dosing | 3 × 50 min per week |
| Goals | Reduction in spasticity, normalization of muscle tone; improvement in static and dynamic balance; independent toileting; mobility in the apartment with a 4-point cane |
| Exercises | “hold and relax” and “rhythmic rotation” PNF technique for increased muscle tone; stretching under load for tone reduction; range of motion (ROM) exercises; adapted progressive resistance training and isometric exercises for strength training of the trunk and lower limbs; static and dynamic balance exercises, and “rhythmic stabilization” PNF technique; assisted walking exercises with weight reduction by therapist, step variations, tandem walking, side-step walking, backward walking |
| Outcome | The goals of the second phase of our neurorehabilitation were achieved. Reduced spasticity, improved lower limb strength, and static and dynamic balance resulted in walking with a cane in the apartment. |
| Phase | III. (Week 17–24) |
| Patient’s status | Hypertonia on the left side, shortened lateral trunk. |
| Dosing | 3 × 50 min per week |
| Goals | Reducing muscle stiffness; maintaining ROM; improving gait quality by lowering proximal compensation and improving distal power generation; grooming, dressing, stair use (16 steps); achieving medium-distance safe walks of 500 m |
| Exercises | Spasticity reduction, muscle tone normalization with stretching under load, PNF techniques and ROM exercises; gait training with static and dynamic balance exercises; 15 min of adapted progressive resistance training for strengthening lower limb muscle groups: leg extension, leg curl, sit to stand, step ups |
| Outcome | The goals of the third phase were achieved to a limited extent: our patient has learned how to dress and wash himself in an adapted way; goes up and down the stairs by step to step; his endurance has not improved, medium-distance walks (500 m) were not feasible; the quality of gait has not improved, there remains proximal compensation and reduced distal power generation |
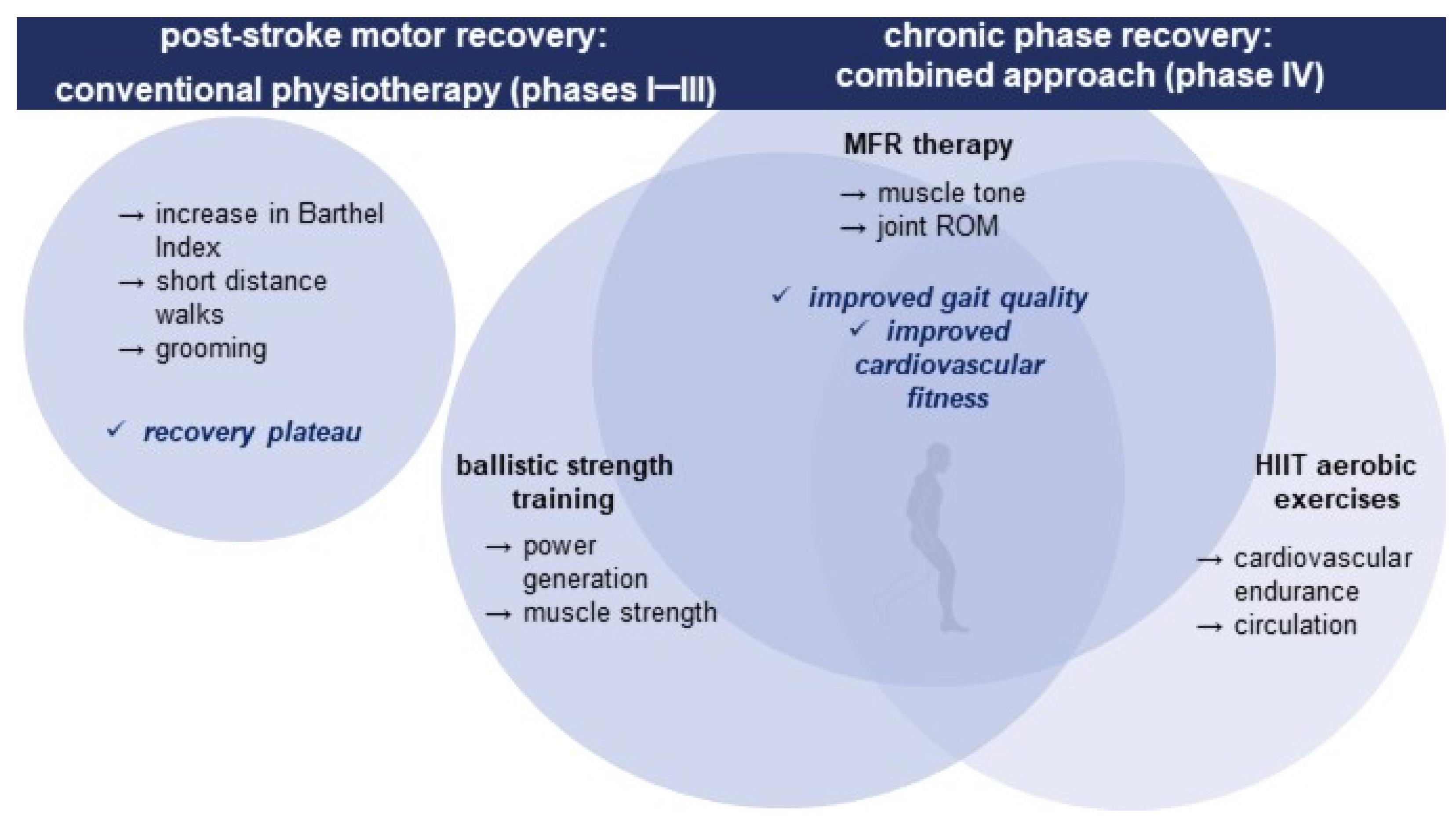
| Phase | IV. (Week 25–32) | ||
|---|---|---|---|
| Therapeutic intervention | Myofascial release (MFR) | Ballistic strength training (BST) | High-Intensity Interval Training (HIIT) |
| Dosing | Once a week for 8 weeks, 50 min/session | 4 times a week, 15–20 min/session | 4 times per week 20 min/session |
| Goals | Breaking down tissue adhesions; improving ROM; reducing spasticity | Improving the three main areas of power generation for propulsion; improving lower limb strength | Improving cardiovascular endurance; improving gait speed |
| Exercises | 30 s of superficial myofascial spreading and 3–5 min of slow strokes (with fingers, loose fists, elbow or forearm adjusted to local myofascial tissue barriers and relaxing ability) on plantar fascia; calf muscles; hamstrings; superior and inferior extensor retinaculum; tibialis anterior/extensor hallucis longus | Exercise 1: ankle plantar flexion, maximum power and velocity 2 × 8 repetition 1 min interserial recovery, 3 s intraserial recovery (0–4 week), same exercise with elastic band resistance (5–8 week) Exercise 2: patient in standing position in hip flexion 90 grade, hip; extension with maximum power and velocity, 2 × 8 repetition. 1 min; interserial recovery, 3 s intraserial recovery (0–4 week), same exercise; with elastic band resistance (5–8 weeks) Exercise 3: patient in standing position, hip flexion over 90 grade, maximum power and velocity, 2 × 8 repetition, 1 min interserial recovery, 3 s intraserial recovery (0–4 week), same exercise with elastic band resistance (5–8 week); 3 min recovery between exercises | Maximum safe speed walking (0.33–0.40 m/s) for 30 s followed by 2 min of recovery, a total session time of 20 min |
| Outcome | Improved ROM, reduced spasticity; improved lower limb strength and power generation; improved gait speed; improved cardiovascular endurance | ||
| Acute Phase | Early Subacute Phase | Late Subacute Phase | Chronic Phase | |||
|---|---|---|---|---|---|---|
| Hospital assessment | Baseline | End of Phase I | End of Phase II | End of Phase III | End of Phase IV | |
| Weeks 0–2 | Weeks 3–8 | Weeks 9–16 | Weeks 17–24 | Weeks 25–32 | ||
| National Institutes of Health Stroke Scale (NIHSS) | 14 | 9 | 6 | 4 | 4 | 4 |
| untestable limb ataxia | ||||||
| Barthel index | 15 | 15 | 25 | 60 | 70 | 80 |
| Early Subacute Phase | Late Subacute Phase | Chronic Phase | ||||
|---|---|---|---|---|---|---|
| Baseline | End of Phase I | End of Phase II | End of Phase III | End of Phase IV | ||
| Weeks 0–2 | Weeks 3–8 | Weeks 9–16 | Weeks 17–24 | Weeks 25–32 | ||
| Manual Muscle Testing on affected side (MMT) | Hip flexors | 0 | 3− | 3+ | 3+ | 4+ |
| Hip extensors | 0 | 3 | 4− | 4− | 4 | |
| Knee flexors | 0 | 2 | 2+ | 2+ | 3+ | |
| Knee extensors | 0 | 2 | 3 | 3 | 4− | |
| Ankle plantar flexors | 0 | 3− | 3+ | 3+ | 4 | |
| Ankle dorsal flexors | 0 | 0 | 2− | 2+ | 2+ | |
| Modified Ashworth Scale on affected side | Hip internal rotator | - | 2 | 1+ | 1+ | 1 |
| Knee extensors | - | 2 | 1+ | 1+ | 1 | |
| Ankle plantar flexors | - | 3 | 2 | 2 | 1+ | |
| Active range of motion (AROM) via goniometry | Hip flexion | 0° | 88° | 96° | 98° | 108° |
| Hip extension | 0° | 10° | 14° | 15° | 20° | |
| Knee flexion | 0° | 15° | 88° | 90° | 120° | |
| Ankle plantar flexion | 0° | 22° | 35° | 36° | 45° | |
| Ankle dorsal flexion | 0° | 0° | 2° | 6° | 8° | |
| Early Subacute Phase | Late Subacute Phase | Chronic Phase | |||
|---|---|---|---|---|---|
| Baseline | End of Phase I | End of Phase II | End of Phase III | End of Phase IV | |
| Weeks 0–2 | Weeks 3–8 | Weeks 9–16 | Weeks 17–24 | Weeks 25–32 | |
| 10-meter walk test (10MWT) fast walking speed with 4-point cane | 0 m/s | 0 m/s | 0.28 m/s | 0.31 m/s | 0.43 m/s |
| 6-minute walk test with 4-point cane (with supervision) | - | - | - | 85 m, VO2 peak: 15.8 | 127 m, VO2 peak: 16.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodor, H.P.; Dávid, H.; Czont, A.; Miklóssy, I.; Orbán, K.-C.; Tar, G.; Fodor, A.; Kovács, Z.; Albert, B.; Salamon, P. Enhanced Gait Recovery in Chronic Post-COVID-19 Stroke: The Role of Combined Physical Rehabilitation. Reports 2023, 6, 51. https://doi.org/10.3390/reports6040051
Fodor HP, Dávid H, Czont A, Miklóssy I, Orbán K-C, Tar G, Fodor A, Kovács Z, Albert B, Salamon P. Enhanced Gait Recovery in Chronic Post-COVID-19 Stroke: The Role of Combined Physical Rehabilitation. Reports. 2023; 6(4):51. https://doi.org/10.3390/reports6040051
Chicago/Turabian StyleFodor, Hunor Pál, Hunor Dávid, Attila Czont, Ildikó Miklóssy, Kálmán-Csongor Orbán, Gyöngyi Tar, Abony Fodor, Zita Kovács, Beáta Albert, and Pál Salamon. 2023. "Enhanced Gait Recovery in Chronic Post-COVID-19 Stroke: The Role of Combined Physical Rehabilitation" Reports 6, no. 4: 51. https://doi.org/10.3390/reports6040051
APA StyleFodor, H. P., Dávid, H., Czont, A., Miklóssy, I., Orbán, K.-C., Tar, G., Fodor, A., Kovács, Z., Albert, B., & Salamon, P. (2023). Enhanced Gait Recovery in Chronic Post-COVID-19 Stroke: The Role of Combined Physical Rehabilitation. Reports, 6(4), 51. https://doi.org/10.3390/reports6040051







