Abstract
A Cryptococcus subspecies, neoformans, represents the most pathogenic infection for humans, particularly in immunocompromised hosts (e.g., cancer patients, drug users). In the present study, we described a 67-year old woman with non-Hodgkin lymphoma who developed an infectious disease sustained by Cryptococcus neoformans. Biochemical data documented a decrease in lymphocytes count while clinical evaluation was suggestive on meningeal infection. The microbiological analysis of the serum, using a dilution pattern through the CrAg lateral flow assay (Immy, Norman, OK 73069, USA) detected the antigen of Cryptococcus (dilution 1/1280), and a treatment with liposomal amphotericin B (3 mg/kg id) plus flucytosine (100 mg/kg per day orally in four divided doses) were started, showing an improvement of symptoms. This case report suggests that an antigen dilution can be used to perform a rapid diagnosis and to quickly start the pharmacological treatment.
1. Introduction
Cryptococcus is free-living encapsulated saprophytic yeast. Human infections are caused by Cryptococcus neoformans and cryptococcus gattii. The epidemiology and the clinical and molecular characteristics of these two species vary. C. neoformans is classified into C. neoformans var. grubii (serotype A) and C. neoformans var. neoformans (serotype D) [1].
Among the many species belonging to the genus Cryptococcus, the subspecies neoformans represents the most pathogenic infection for humans, particularly in immunocompromised hosts [2].
C. neoformans is a ubiquitous and opportunistic fungal pathogen encapsulated (30 μm thick) unicellular yeast, is about 5–12 μm in diameter, and is primarily responsible for cryptococcal meningitis [3]. The colonies are white to creamy and subsequently take on a sticky appearance.
Several studies suggest that impaired host cell-mediated immunity such as human immunodeficiency virus (HIV) infection autoimmune diseases, immunosuppressive therapy (e.g., corticosteroids, anticancer drugs), liver cirrhosis, lung diseases, lymphoproliferative malignancy and hematological malignancy, play a central role in the pathogenesis of cryptococcal infections [4,5,6,7]. In the present study, we describe a woman with non-Hodgkin lymphoma that developed a meningeal infection sustained by C. neoformans.
2. Case Presentation
A 67-year-old Caucasian woman, with an history of non-Hodgkin Lymphoma and previous positivity to COVID-19, presented to our ward on 14 May 2021, due to symptoms of 3 days of evolution of occipital headache that woke her up at night, fever (38 °C) and temporo-spatial disorientation. On exploration, she was hemodynamically stable, without respiratory dysfunctions, but with a disorientation in time, place, and person. She presented a stiff neck and Kernig and Brudzinski signs were positive. Biochemical analysis revealed a severe decrease in lymphocytes and platelet count, and an increase in C Reactive protein and neutrophils count (Table 1). A total body computed tomography (CT) scan with contrast showed a large (2.35 cm) hypodense cerebrospinal fluid-like lesion affecting the cerebral and cerebellar parenchyma, without associated edema (Figure 1), that was not suggestive of meningeal infectious disease.

Table 1.
Biochemical findings in enrolled woman, during the hospitalization.
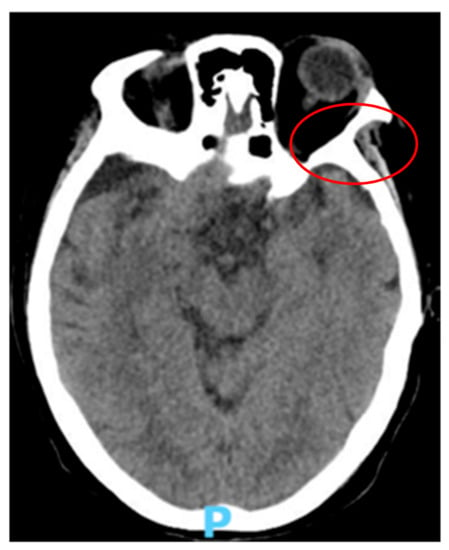
Figure 1.
Head computed tomography (CT) scan revealing a large (2.35 cm) hypodense cerebrospinal fluid-like lesion (red circle).
Considering clinical and laboratory data as well as the history of immunosuppression, the diagnosis of meningitis probably related to Cryptococcus infection was postulated and the patient was hospitalized.
Considering a possible fungal infection in immunosuppressed patients, Β-d glucan was requested, but it was referred as negative.
Blood samples were sent to microbiology operative unit for the analysis. In in blood samples of this woman, using a dilution pattern through the CrAg lateral flow assay (Immy, Norman, OK 73069, USA), we detected the antigen of Cryptococcus (dilution 1/1280). It is a dipstick sandwich immunochromatographic assay used for the qualitative or semi-quantitative detection of the capsular polysaccharide antigens of Cryptococcus species complex (Cryptococcus neoformans and Cryptococcus gattii), and the treatment with liposomal amphotericin B (3 mg/kg id) was started. The lumbar puncture was not performed because it was considered a high-risk procedure and the patient did not agree.
Two days later, the serological diagnosis was confirmed by broth microdilution plates of blood cultures coming back positive for C. neoformans with a high sensitivity to Amphotericin B (minimum inhibitory concentration, MIC: 0.5 μg/mL), Posaconazole (MIC: 0.25 µg/mL), Voriconazole (MIC: 0.25 µg/mL), and Itraconazole (MIC: 0.125 µg/mL) (Figure 2). The detection of C. neoformans was performed using the Maldi toff (Becton Dickinson, Four Oaks, NC 27524, USA) system, the automatic system for microbial identification using mass spectrometry (Figure 2), while the VitekS2 and out the Micronaut-AM system (Merlin broth test in microdilution system) was used for the sensitivity test (Figure 3 and Table 2).
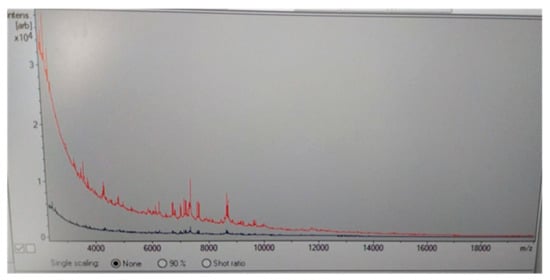
Figure 2.
Maldi Toff spectrometry of Cryptococcus neoformans colony (red line) respect to control line (blue line).
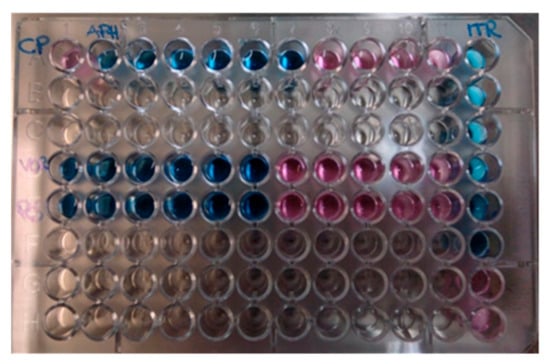
Figure 3.
Broth microdilution plates of blood cultures. In blue: sensible concentration. In red: resistant concentration.

Clinical evaluation revealed an improvement of clinical condition and, about 14 days later (June 3), a new evaluation of antigen, CrAg lateral flow assay (Immy, Norman, OK 73069, USA), revealed the persistence of the high value of dilution (1/1280), therefore, Flucytosine (100 mg/kg per day orally in four divided doses) was added to Amphotericin with a progressive improvement of clinical conditions and the patient was discharged after 8 days (11 June) on Fluconazole (400 mg per day). One month later, during the follow-up the patient does not show signs or symptoms of meningitis and the antigen in serum was not detected. Patients maintained close hematological surveillance under regular appointments at the Onco-Haematology Clinic of the same hospital.
3. Discussion and Conclusions
We reported a meningeal infection sustained by Cryptococcus neoformans in a woman with non-Hodgkin lymphoma and COVID-19.
Several authors described the development of meningitis sustained by C. neoformans in patients with immunocompromising conditions [7].
The Centers for Disease Control and Prevention USA documented in an epidemiological study that worldwide, there are almost one million new cryptococcal meningitis cases reported annually, suggesting that this infection represents an important global health concern.
In particular, it has been reported that patients with lymphoproliferative disorders, including malignant lymphoma, have a cell-mediated immunity suppression with intercurrent cryptococcal infections complicated with Acute Lymphocytic Leukemia, Adult T-cell Leukemia, Hairly Cell Leukemia, and Chronic Lymphocytic Leukemia [8,9,10,11].
In an Italian 10-year retrospective study, Pagano et al. [12] described 17 cases of cryptococcosis (8 with acute leukemia and 3 with non-Hodgkin lymphoma) in patients with hematological malignancies. More recently, Reisfeld-Zadok et al. [11] reported the development of Cryptococcal meningitis infection in 2 patients with chronic lymphocytic leukemia.
Hirai et al. [13], reviewed literature data for malignant lymphoma complicated by cryptococcal infection, reporting that 13 of 17 cases (76%) had a disseminated infection (e.g., meningitis or fungemia or intramuscular abscess), with a mortality of 41% in all cases and 54% in disseminated cases. The authors documented that several factors contributed to the etiology of disseminated cryptococcosis (e.g., non-Hodgkin’s lymphoma; cellular immunodeficiency associated with chemotherapy; cyclic hematopoietic disorders due to chemotherapeutic regimens including rituximab and corticosteroids).
Finally, Zhang et al. [14] described a 20-year-old male patient affected by Hodgkin lymphoma that developed eosinophilic meningitis that was probably related to cryptococcus infection responsive to antifungal therapy.
However, very few data have been published on the topic of non-Hodgkin and cryptococcus infection. In fact, when searching on PubMed “cryptococcus” and “lymphoma” there are 205 results, while searching “cryptococcus” and “non-Hodgkin lymphoma” shows that there have been 68 published papers. Of these, the most common infection involves the respiratory system, and only 10 reports (from 1958 to 2022) describe a cerebral infection sustained by cryptococcus in patients with non-Hodgkin lymphoma. In these reports, patients received a treatment with chemotherapy regimen [13,15] or after autologous stem cell transplant [16] or after monoclonal antibody treatment [17]. In contrast in our case, the patient has a history of non-Hodgkin lymphoma in follow-up and without chemotherapy treatment. However, in agreement to other studies [8,9,10,11], in our patient biochemical values documented a severe decrease in lymphocytes count and an increase in neutrophils one.
In a previous study, Korfel et al. [9], documented the development of a systemic mycosis caused by Cryptococcus species in two patients affected by Hodgkin’s lymphoma stage IVB (Ann Arbor). In this study, the authors cited 54 cases of cryptococcosis in patients with an history of Hodgkin’s lymphoma; in these patients, laboratory findings documented an absolute lymphopenia that probably played a role in the development systemic infectious disease.
In our case, the decrease of lymphocytes count could be related to the treatment with glucocorticoids for COVID-19, in agreement with current treatments [18]. In fact, in a case series of cryptococcal infections presented by Memorial Sloan-Kettering Cancer Center, it was reported that 38 of 46 patients infected by cryptococcus (from 1956 to 1972) were affected by leukemias or lymphomas, while 39 of these were treated with glucocorticoids [19]. In this group of patients, cryptococcal meningitis was commonly diagnosed. Moreover, all patients with cryptococcal infection were found to have lymphopenia. Similarly, a case series conducted from 1989 to 1999 at M D Anderson Cancer Center documented the presence of cryptococcal infection in 31 cancer patients (20 of whom with hematologic cancers); about 61% of these patients had lymphopenia and more than half (52%) were treated with glucocorticoids [20]. In order to reduce the time of diagnosis, we used the antigen dilution. Even if MALDI-TOF is necessary for an appropriate diagnosis of the species of pathogens, the antigen dilution can be used to perform a rapid diagnosis of the pathogen involved in the development of infection, so as to quickly start the pharmacological treatment.
To date there are three categories of methods that can be used to diagnose cryptococcal meningitis: India Ink microscopy, which can be used on cerebrospinal fluid (CSF); culture, which can be used on CSF or blood; and antigen detection.
Microscopy is a fundamental, easy-to-use technique but its sensitivity is dependent on the quality of the specimen and the experience of the laboratory personnel. However, microscopy has another limitation related to the stains used [21]. In fact, the India link is a commonly used stain and has a very low sensitivity (86%) that is decreased to 42% among persons with cerebrospinal fluid cultures with <1000 colony-forming units/mL [21].
In contrast, there are several methods to detect cryptococcal antigen in CSF or serum: latex agglutination (LA), enzyme immunoassay (EIA), and lateral flow assay (LFA) (Table 3).

Table 3.
Characteristics of the test commonly used for the diagnosis of Cryptococcal infections.
Lateral agglutination is the first test used in the clinical course for the diagnosis of fungal infection [1]. This technique uses antibodies raised in rabbits against whole cryptococcal cells and passively coated onto latex beads. This assay detected glucuronoxylomannan, the major capsular polysaccharide of C. neoformans, that is shed in large amounts into the blood and CSF during the cryptococcal meningitis infection [22].
The enzyme immunoassay is an automated spectrophotometric method commonly used in the management of biomarkers [23]
LFA is a semiquantitative test that can be used to measure disease burden by determining the titers for positive results [23]. This test uses gold-conjugated, monoclonal antibodies impregnated onto an immunochromatographic test strip to detect cryptococcal capsular polysaccharide glucuronoxylomannan antigen for all four C. neoformans serotypes (A–D) [24]. If cryptococcal antigen is present in a specimen, suspended, gold-conjugated antibodies bind to the antigen.
In a previous study Panackal et al. [25], the authors compared the sensitivity of the EIA and the latex agglutination test on 185 blood and 164 cerebrospinal fluid (CSF) samples obtained from patients with cryptococcosis without known immunocompromising conditions. In this study, the authors documented that the LA assay was more sensitive than the EIA [25].
Comparing these tests, we evaluated that the advantage of the LFA method is that it is simple to use, and the results are available in 10 min, with high sensitivity (95%) and low costs (about EUR 4) for each test.
In this infectious condition the time of the treatment is important to reducing the risk of severe disease and death. In fact, in this case after the clinical evaluation and the antigen test, a treatment with liposomal Amphotericin B improved clinical symptoms.
In conclusion our case supports the use of an antigen test (lateral flow assay) in cryptococcus infection in patients at high risk (cancer disease, chemotherapy, glucocorticoid treatment) to obtain a rapid diagnosis and to start a rapid treatment.
Author Contributions
Conceptualization, M.C., S.N. and F.Q.; data curation, M.C., S.N., F.Q. and A.D.L.; writing—original draft preparation, M.C. and S.N.; writing—review and editing, L.G.; supervision, P.M. and L.G.; project administration, P.M. and L.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declared that no grants were involved in supporting this work.
Institutional Review Board Statement
Ethical approval was not sought for the present study because it is only a presentation of an interesting case in a hospitalized patient treated in agreement with normal protocols. Moreover, the manuscript does not concern drug use but concerns diagnostic methods.
Informed Consent Statement
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kozel, T.R.; Wickes, B. Fungal diagnostics. Cold Spring Harb. Perspect. Med. 2014, 4, a019299. [Google Scholar] [CrossRef] [PubMed]
- Srichatrapimuk, S.; Sungkanuparph, S. Integrated therapy for HIV and cryptococcosis. AIDS Res. Ther. 2016, 13, 42. [Google Scholar] [CrossRef]
- Negroni, R. Cryptococcosis. Clin. Regul. Dermatol. 2012, 30, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Idnurm, A.; Lin, X. Morphology and its underlying genetic regulation impact the interaction between Cryptococcus neoformans and its hosts. Med. Mycol. 2015, 53, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Kiertiburanakul, S.; Wirojtananugoon, S.; Pracharktam, R.; Sungkanuparph, S. Cryptococcosis in human immunodeficiency virus-negative patients. Int. J. Infect. Dis. 2006, 10, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Schmalzle, S.A.; Buchwald, U.K.; Gilliam, B.L.; Riedel, D.J. Cryptococcus neoformansinfection in malignancy. Mycoses 2020, 63, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Cunha Pereira, T.; Rb-Silva, R.; Félix Soares, R.; Domingues, N.; Mariz, J. Case Report: Cryptococcal meningitis in Hodgkin’s Lymphoma patient receiving brentuximab-vedotin therapy. F1000Research 2020, 9, 687. [Google Scholar] [CrossRef]
- Rhew, D.C.; Gaultier, C.R.; Daar, E.S.; Zakowski, P.C.; Said, J. Infections in Patients with Chronic Adult T-Cell Leukemia/Lymphoma: Case Report and Review. Clin. Infect. Dis. 1995, 21, 1014–1016. [Google Scholar] [CrossRef]
- Korfel, A.; Menssen, H.D.; Schwartz, S.; Thiel, E. Cryptococcosis in Hodgkin’s disease: Description of two cases and review of the literature. Ann. Hematol. 1998, 76, 283–286. [Google Scholar] [CrossRef]
- Dincol, G.; Kahraman, R. Cryptococcus neoformans meningitis in a patient with hairy cell Leukmia. Am. J. Hematol. 2006, 81, 387. [Google Scholar] [CrossRef]
- Reisfeld-Zadok, S.; Elis, A.; Szyper-Kravitz, M.; Chowers, M.; Lishner, M. Cryptococcal meningitis in chronic lymphocytic leukemia patients. Isr. Med. Assoc. J. IMAJ 2009, 11, 437–439. [Google Scholar] [PubMed]
- Pagano, L.; Fianchi, L.; Caramatti, C.; D’Antonio, D.; Melillo, L.; Caira, M.; Masini, L.; Todeschini, G.; Girmenia, C.; Martino, B.; et al. Cryptococcosis in patients with hematologic malignancies. A report from GIMEMA-infection. Haematologica 2004, 89, 852–856. [Google Scholar] [PubMed]
- Hirai, Y.; Ainoda, Y.; Shoji, T.; Fujita, T.; Yoshinaga, K.; Shiseki, M.; Mori, N.; Teramura, M.; Totsuka, K.; Motoji, T. Disseminated Cryptococcosis in a Non-Hodgkin’s Lymphoma Patient with Late-Onset Neutropenia Following Rituximab-CHOP Chemotherapy: A Case Report and Literature Review. Mycopathologia 2011, 172, 227–232. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Shen, H.; Tao, J.; Wang, J. Case Report: Cryptococcal eosinophilic meningitis in a patient with Hodgkin lymphoma. Front. Neurol. 2022, 13, 898525. [Google Scholar] [CrossRef]
- A To, C.; Hsieh, R.W.; McClellan, J.S.; Howard, W.; Fischbein, N.J.; Brown, J.M.Y.; Felsher, D.W.; Fan, A.C. Cryptococcal osteomyelitis and meningitis in a patient with non-hodgkin’s lymphoma treated with PEP-C. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef]
- Chaaban, S.; Wheat, L.; Assi, M. Cryptococcal meningitis post autologous stem cell transplantation. Transpl. Infect. Dis. 2014, 16, 473–476. [Google Scholar] [CrossRef]
- Cruz, D.; Costa, P.; Sagüés, M. Meningeal cryptococcosis in a patient with angioimmunoblastic lymphoma treated with alemtuzumab. Med. Clin. 2019, 152, e19–e20. [Google Scholar] [CrossRef]
- Marcianò, G.; Roberti, R.; Palleria, C.; Mirra, D.; Rania, V.; Casarella, A.; De Sarro, G.; Gallelli, L. SARS-CoV-2 Treatment: Current Therapeutic Options and the Pursuit of Tailored Therapy. Appl. Sci. 2021, 11, 7457. [Google Scholar] [CrossRef]
- Kaplan, M.H.; Rosen, P.P.; Armstrong, D. Cryptococcosis in a cancer hospital.Clinical and pathological correlates in forty-six patients. Cancer 1977, 39, 2265–2274. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Peitsch, W.K.; Reddy, B.T.; Whimbey, E.E.; Han, X.Y.; Bodey, G.P.; Rolston, K.V.I. Cryptococcosis in Patients with Cancer. Clin. Infect. Dis. 2001, 32, e145–e150. [Google Scholar] [CrossRef]
- Bloomfield, N.; Gordon, M.A.; Elmendorf, D.F.; Elmendorf, J.D.F. Detection of Cryptococcus neoformans Antigen in Body Fluids by Latex Particle Agglutination. Exp. Biol. Med. 1963, 114, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Marcos, J.Y.; Pincus, D.H. Fungal diagnostics: Review of commercially available methods. In Fungal Diagnostics: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2013; pp. 25–54. [Google Scholar]
- Tanner, D.C.; Weinstein, M.P.; Fedorciw, B.; Joho, K.L.; Thorpe, J.J.; Reller, L. Comparison of commercial kits for detection of cryptococcal antigen. J. Clin. Microbiol. 1994, 32, 1680–1684. [Google Scholar] [CrossRef] [PubMed]
- Gates-Hollingsworth, M.A.; Kozel, T.R. Serotype Sensitivity of a Lateral Flow Immunoassay for Cryptococcal Antigen. Clin. Vaccine Immunol. 2013, 20, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A.; Dekker, J.P.; Proschan, M.; Beri, A.; Williamsona, P.R. Enzyme Immunoassay versus Latex Agglutination Cryptococcal Antigen Assays in Adults with Non-HIV-Related Cryptococcosis. J. Clin. Microbiol. 2014, 52, 4356–4358. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).