Abstract
Actinomyces odontolyticus is a strictly anaerobic species, a member of the Actinomyces genus and of the commensal flora, especially oral flora, which can trigger severe infections through breaches in healthy tissue or necrotic tissue that are often hard to diagnose clinically and microbiologically. Most infections with this species are pulmonary or pleural, which might hint at a connection with poor dental hygiene, but other locations have been documented. We present a case of a tubo-ovarian abscess with a difficult identification of the etiological agent in a woman with multiple admissions, no significant comorbidities, and a longstanding use of an IUD (intrauterine device). To our knowledge, no previous case of tubo-ovarian abscess with an accurate A. odontolyticus microbiological species identification has been reported so far. This case also highlights the importance of considering an anaerobic species as an etiologic agent in an infectious process concerning a previously damaged tissue and the importance of appropriate harvesting and culturing in the accurate diagnosis of such species.
1. Introduction
Actinomyces genus belongs to a family called Actinomycetaceae. They are facultative anaerobic or strictly anaerobic, non-motile, non-spore-forming, unencapsulated, straight to slightly curved bacilli of various lengths. The short rods can be seen forming small clusters or diphtheroid arrangements. Longer rods can be straight, wavy, or branched, the branched pattern being the most specific one, also called Actinomyces-like. Although Actinomyces are Gram-positive, irregular staining can cause a beaded or banded appearance similar to Nocardia spp. [1]. Actinomyces spp. mainly belong to the commensal flora of the oropharynx, gastrointestinal tract, and urogenital tract [2] but acquire pathogenicity through invasion of breached or necrotic tissue [3], causing actinomycosis.
Actinomycosis is a chronic, granulomatous, infectious disease characterized by the development of fistulous tracts through which the infection can drain pus into another cavity or to the surface. The most frequent genus incriminated for this type of infection is Actinomyces, out of which Actinomyces israelii is the most frequently isolated species, although actinomycosis can be caused by other genera such as Propionibacterium or Bifidobacterium [1].
The most common presentation of actinomycosis is the oral-cervicofacial one, accounting for more than half of all actinomycosis cases. Pelvic actinomycosis is usually linked to the prolonged use of IUDs, promoting the ascension of the microorganisms through wires left in the exocervix. In addition, the IUD alters the metabolism of endometrial cells, thus favoring persistent local inflammation [4]. Another probable route of infection is through oral sex practices since Actinomyces odontolyticus is a predominant species of the tongue flora and, more importantly, represents an important factor in early plaque development [5]. Infections with Actinomyces odontolyticus are rare, with the first case being reported by Batty in 1958 in a patient with advanced dental decay. We hereby present the case of a 48-years old patient with a reported 10-year use of an IUD and delayed treatment due to the patient’s refusal of admission.
2. Case Report
A 48-years old woman (gravida 4, term 2, abortion 2) with her last menstrual period started on 10 December 2021 (6 weeks of amenorrhea) presented on 15 January 2022, accusing moderate pain in the lower left quadrant. The pain started 7 days ago with an intensification 3 days prior to presentation. Anamnesis did not reveal any significant comorbidities, no previous dental conditions, recent dental extractions, or other major surgical intervention, and no gynecological condition either. Clinical examination reveals nothing significant other than tenderness in the lower left quadrant and the wires of an IUD at the vaginal speculum examination, which the patient claims she had for 10 years. Ultrasound examination highlights a heterogenous, septate, 61/56 mm cystic process in the left fallopian tube without any signs of intra-cystic vegetation and without a Doppler signal. Abdominal-pelvic computer tomography confirmed the presence of an IUD and described the aforementioned process as being thick-walled and iodophilic, with heterogenous and moderately hyperdense content, suggesting the possibility of a pyosalpinx. Blood work showed leukocytosis (WBC/mm3) and high C-reactive protein levels (28.3 mg/dL). Extraction of the IUD was decided. The patient denied admission after the extraction even though she had been well informed of her medical condition and the further investigation required for it.
The patient presents for a second time two weeks later, accusing pain in the lower left quadrant and fever. She had been following antibiotic therapy prior to the admission (Cefuroxime and Ampicillin), which reportedly ameliorated the symptomatology. Ultrasound reveals an enlargement of the cystic process. Blood work shows a milder leukocytosis (WBC/mm3) and C-reactive protein levels (12.96 mg/dL). Bacteriological exam from cervical secretions (collected with a swab and transported in Amies medium) came back negative at 48 h. The diagnosis of left pyosalpinx is confirmed, and therapy with antibiotics (Ceftamil, Clindamycin) and NSAIDS is established, with remission of symptomatology. Leukocytosis and C-reactive protein levels normalized. Stationary evolution of the cystic process alongside a 25 mm fluid mass in the Douglas pouch was discovered by an ultrasound conducted 5 days after the admission. The patient is discharged with NSAIDS and a 7-day course of 500 mg orally every 8 h of amoxicillin + clavulanic acid antibiotic therapy.
The patient came back the third time a month later with the same pain in the lower left quadrant, which reappeared 7 days prior to the admission. After an ultrasound confirming the findings from the previous ultrasound, the decision to perform a left salpingo-oophorectomy was taken. Surgery was complicated by the presence of multiple adhesions between the uterus, the left fallopian tube, and left ovary, and the sigmoid colon, causing the cystic fallopian mass to rupture. Green-yellow pus was extracted from the ruptured mass using a sterile syringe and sent for bacteriological examination, and the left fallopian tube alongside the left ovary was sent for histopathological examination. The pus was cultured in both aerobic and anaerobic conditions on Columbia blood agar and on MacConkey agar, mannitol salt agar, and Sabouraud agar in aerobic conditions. Anaerobiosis was provided using an anaerobic jar and anaerobic atmosphere-generating sachet (Thermo Scientific AnaeroGenTM, MA, USA). Aerobic media showed no growth at 24 h, 48 h, and 72 h. The blood agar plate placed in anaerobiosis showed significant growth at 72 h of small, grey colonies with a brownish background (Figure 1). Gram stain from those colonies indicated Gram-negative short bacilli disposed of in a branched pattern, characteristic of Actinomyces spp. (Figure 2). Further identification using Vitek 2 Compact by bioMerieux revealed excellent identification (98% accuracy) for Actinomyces odontolyticus. The species was deemed resistant to Clindamycin but sensible to Penicillin and Metronidazole. Histopathological examination of the surgical piece highlighted large areas of the fallopian tube with densely populated neutrophilic infiltrate and partial cystic transformation of the left ovary with granulation tissue, but no bacterial colonies or sulfur granules present. The patient was started on 250 mg 3 times a day of Metronidazole antibiotic therapy until she was discharged 5 days post-operation. The patient’s status improved post-surgery, and she was discharged with complete remission of symptoms. On follow-up, the patient showed no significant symptoms or clinical signs, a good healing of the surgical scar, and normal WBC, C-reactive protein levels and bacteriological exam.
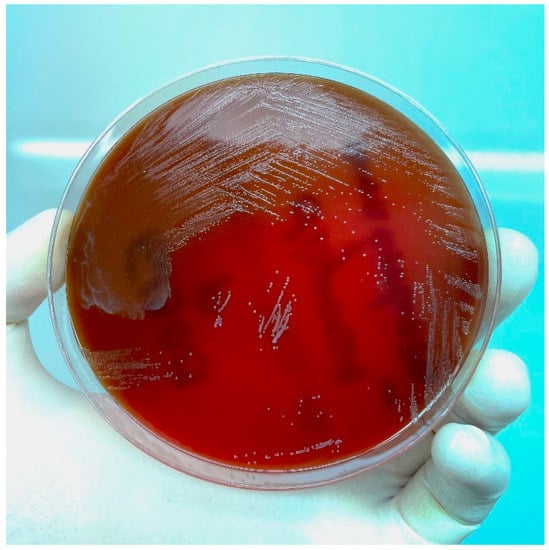
Figure 1.
Actinomyces odontolyticus growth at 72 h on blood agar culture media in anaerobic conditions.
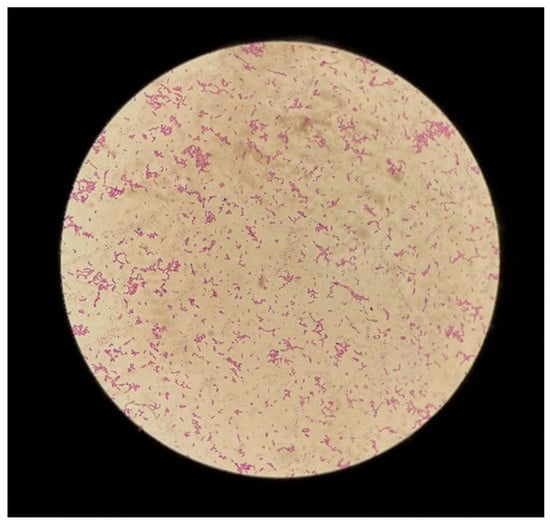
Figure 2.
Gram stain from culture media showing branched Actinomyces-like Gram-positive bacilli.
3. Review of Literature
For the review concerning actinomycosis with Actinomyces odontolyticus and tubo-ovarian abscesses with the above-mentioned species, we used the electronic databases of PubMed and Cochrane for our review, with the following terms for the search: “Actinomyces odontolyticus” combined with the Boolean operator “AND” along with “case report” “tubo-ovarian”, “pelvic”, and “abscess”. Only studies written in English, Spanish, French, or German were selected for this review. The review was conducted with data available up to 26 June 2022. We found a total of 54 reported cases of actinomycosis with Actinomyces spp., with no previously documented case of tubo-ovarian abscess with A. odontolyticus, one case of ovarian cyst actinomycosis and one previously documented case regarding pelvic actinomycosis with Actinomyces odontolyticus at a patient who simultaneously presented toxic shock syndrome with group A Streptococcus [6]. We also aimed to discover whether actinomycosis is more frequent in immunocompromised patients or not—out of the 54 reported cases, only 16 cases were confirmed to be immunocompromised from various causes (malignancy with chemo- or radiotherapy, chronic liver disease, longstanding corticosteroid therapy, HIV positive serology, post-transplant status, type 2 diabetes). The extended results of our review are displayed in Table 1.

Table 1.
Review of literature by reference, patient age, gender, site of infection, laboratory technique used for the identification and confirmation of A. odontolyticus, relevant comorbidities or invasive procedures connected to the Actinomyces infection, immune status, and outcome of the infectious episode.
4. Discussion
We present the first case of a tubo-ovarian abscess caused by Actinomyces odontolyticus in a 48-year-old woman from Cluj-Napoca, Romania. Gynecological involvement of A. odontolyticus remains a mystery, with only two other case reports existing in the world, one that involved an ovarian cyst and one case of pelvic actinomycosis. This might be due to the difficulty in correctly harvesting pus and other body fluids for the preservation of anaerobic species, culturing this aforementioned class of microorganisms, and also in accurately diagnosing them prior to the MALDI-TOF and VITEK era. In all existing gynecological cases, the outcome of the patient remains positive. Regarding the risk factors, in our case report, we incriminated the IUD due to the place where the infections are localized; however, in other instances, poor oral hygiene or immunosuppression might be responsible too. Further studies are needed for a proper understanding of the pathogenicity, risk factors, and antimicrobial resistance of this enigmatic microorganism.
In our brief review, we tackled the issue of human actinomycosis and included 54 case reports that present different types of infections caused by Actinomyces. In order to be able to draw some conclusions, we grouped them by infection type as follows.
Out of the 54 case reports, almost half of them, 23 to be precise, are either pulmonary or pleural. Male-to-female ratio of these patients is 13:10, with males slightly edging the females. Out of all these cases, only one patient died, and in that case, actinomycosis occurred in an immunosuppressed patient with sarcoidosis, lymphoma, and undertreatment with corticosteroids. Four cases present a disseminated infection with pulmonary or pleural involvement suggesting the invasiveness of this microorganism as well as its capacity to produce secondary distal infections. Considering the risk factors presented in these cases, poor oral hygiene, periodontal disease, and tooth extraction are the most prevalent, with different types of immunosuppression coming in a close second. Based on these observations, it seems that pulmonary infections with Actinomyces are in close contingency with oral health issues, and they should be traced together in the future to properly unlock the circumstances of this infection occurrence.
Gastroenterological involvement of Actinomyces is much more heterogenous. We found seven case reports that describe infections in this medical field, four case reports of hepatic infections, with two being disseminated infections with hepatic involvement and two only with hepatic involvement. The other three case reports are from infections that occurred in the gall bladder, esophagus, and sigmoid colon. Regarding the outcome, six out of the seven case reports are with a favorable outcome; only one patient died who was also immunodeficient and suffering from an HIV infection. In addition, all cases with hepatic involvement had associated oral health issues. This observation adds another hypothesis regarding Actinomyces infections that needs to be further studied, that oral health issues might produce intermittent bacteremia that could lead to organ seedings. The rest of the reported cases have a much closer relationship with the underlying condition (recurrent cholecystitis, refractory GERD, or colon cancer). Interestingly, in this group of infections, males seem to be more affected, with a ratio of 6 males–1 female. However, this might be due to the relatively small sample size, only seven case reports, and definitely more data are needed to properly assess the gender differences in GI Actinomycosis.
Regarding the cardiovascular system and Actinomyces infections, we found nine case reports of cardiac, pericardial, or mediastinal involvement with a similar dominance of male patients (a ratio of 7 males–2 females). Additionally, the same risk factors concerning oral health were found in four of them. The rest of them presented either an invasive procedure such as a cardioverter defibrillator recently implanted, gastric surgery, and heart-lung transplant or drug abuse (alcohol and cocaine). Interestingly, only one of these cases had a poor outcome in a transplant patient with immunosuppression. Analyzing the bacteriemia and sepsis reported in five of the cases can argue that systemic isolation of this microorganism is linked to immunosuppression since all these cases are from patients with immunosuppression. This only adds to the idea presented before that Actinomyces remains a threat only in high-risk patients.
Neurological involvement of Actinomyces remains rare, similar to the genital one. We found only three cases of cerebral or meningeal actinomycosis. Thus, it is difficult to assess the role of this bacteria in neurological infections. All of them presented a favorable outcome, which raises the suspicion of the pathogenicity of this bacterium, contamination versus infection, since, in two cases, there is no report of the method used for microbiologic identification.
The rest of the case reports are much more heterogeneous, with Actinomycesbeing reported from renal, vertebral, cervicofacial, laryngeal, breast, soft tissue, and osseous infections, and thus difficult to assess the relevance of the infections in the overall outcome of each case. However, all of these cases had a favorable outcome and similar risk factors as discussed so far.
Actinomyces odontoliticus has been isolated in 12 cases from infections with more than one microorganism, highlighting the risk that may be associated with this type of infection. Some of the associated microorganisms included yeasts and bacteria, both Gram-positive and Gram-negative, such as L. rhamnosus, S. mitis, A. schaalii, P. asaccarolyticus, C. albicans, S. constelatus, V. atypica, S. anginosus, P. denticola, E. coli, group A streptococcus, E. corrodens, K. pneumoniae, H. influenzae, S. milieri, and H. aprophilus. Treatment in these cases may prove to be a challenge in the context of antimicrobial resistance. However, based on the existing data, only one patient died from an infection that involved Actinomyces odontolyticus and another microorganism, and thus, so far, this seems not to be an issue.
Diagnosis of Actinomyces odontolyticus in the microbiology laboratory is often a challenge, and for a long time, medical professionals had to rely only on microscopy and bacterial morphology. In 11 instances out of 54, authors reported the use of Gram stain, colony morphology, or biochemical tests. Another important observation is that, in many cases, the diagnosis methods are not presented. In 29 case reports, authors failed to present how this bacterium was diagnosed, and thus their findings come into question. However, the most popular and accurate diagnosis tools that were presented are MALDI-TOF, Vitek, or 16 s RNA sequencing. The technological revolution in the microbiology laboratory has the potential to further explore the involvement of this bacterium in human infections by facilitating diagnosis and, in the long term, analyzing the possible risk factors. The preliminary data of this review show that males seem to be more affected by Actinomyces odontolyticus, and oral health issues are the dominant risk factor evaluated. This is available for all types of infections analyzed in this paper. It is of utmost importance to keep a close eye on this understudied pathogen in order to properly assess its involvement in human infections.
5. Strengths and Limitations
Strengths: This study is a case report associated with a brief review of literature—this association enables the integration of the data within this case report into a larger context, thus establishing connections between the case report and the currently available data in order to highlight the prevalence and incidence of female genital tract actinomycosis with Actinomyces odontolyticus amongst other cases of actinomycosis with the above-mentioned species and to possibly establish a correlation between the infection, the comorbidities of the patients and their immune status. Moreover, this case report features the optimal process of harvesting bodily fluids and pus in order to preserve anaerobic species, the correct way of culturing said species, and a good method of accurately identifying the species, all three stages being essential for a precise diagnosis. Last but not least, the case report includes the histopathological exam as a certification of the presence of inflammatory modifications, suggesting an infectious process, but without the presence of characteristic sulfur granules, which can be seen as another particularity of the case.
Limitations: The available data on the patient do not contain pictures from the CT scans and the histopathological exam. We also could not find any gynecological tumoral markers as part of the differential diagnosis of actinomycosis (such as CA-125, alpha feto-protein (AFP), carcinoembryonic antigen (CEA), human chorionic gonadotropin (hCG), human epididymis protein 4 (HE4), etc.) considering the fact that actinomycosis can mimic neoplastic processes from a clinical and imagistic standpoint [65,66,67]. Despite their low specificity, their varying degrees of prognostic and predictive value, and the relatively low detection rate of early-stage malignancies, tumoral markers can be a useful tool for the appreciation of the progression of the disease. Fortunately, the histopathological exam is a certification of the absence of any dysplastic or neoplastic modifications. Another limitation is the use of Vitek 2 Compact by bioMerieux system for the identification of the microorganism instead of a MALDI-TOF or 16S RNA analysis, which proved to be more accurate. [68,69]. Ultimately, the literature review was made using only PubMed as a database—a more accurate review could have been established had we had access to larger subscription-based databases.
6. Conclusions
Actinomyces odontolyticus and Actinomyces spp. infections are a rare encounter in the current clinical practice; however, that may be caused not only by the many conditions required by this commensal microorganism in order to become pathogenic but also by the difficult diagnosis of this species, requiring correct pre-analytical sample collection in order to preserve an anaerobic environment during transportation and correct and detailed microbiological examination for anaerobic species. This case report and brief literature review, tackling the actinomycosis sphere of infectious diseases, will hopefully provide a solid ground for further, more detailed research into this species.
Author Contributions
Conceptualization, A.G.P. and D.A.T.; methodology, A.G.P. and D.A.T.; validation, C.C., A.G.P. and D.A.T.; formal analysis, A.G.P. and V.N.; investigation, A.G.P., V.N., G.D.S. and M.C.R.; resources, C.C., G.D.S. and M.C.R.; data curation, A.G.P. and V.N.; writing—original draft preparation, A.G.P. and D.A.T.; writing—review and editing, C.C. and D.A.T.; visualization, C.C., A.G.P. and D.A.T.; supervision, C.C.; project administration, C.C.; funding acquisition, C.C. and A.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. Ethical approval was not sought for the present study because research involving secondary use of data which is provided without any identifier or group of identifiers which would allow attribution of private information to an individual and research based on review of published literature do not require ethics review.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hall, V.; Copsey, S. Propionibacterium, Lactobacillus, Actinomyces, and other non-spore-forming anaerobic gram-positive rods. In Manual of Clinical Microbiology, 11th ed.; Jorgensen, J., Carroll, C.J., Funke, G., Pfaller, A.M., Landry, L.M., Richter, S.S., Warnock, W.D., Eds.; ASM Press: Washington, DC, USA, 2015; p. 920. [Google Scholar]
- Valour, F.; Sénéchal, A.; Dupieux, C.; Karsenty, J.; Lustig, S.; Breton, P.; Gleizal, A.; Boussel, L.; Laurent, F.; Braun, E.; et al. Actinomycosis: Etiology, clinical features, diagnosis, treatment and management. Infect. Drug Resist. 2014, 7, 183. [Google Scholar]
- Cintron, J.R.; Del Pino, A.; Duarte, B.; Wood, D. Abdominal actinomycosis. Dis. Colon Rectum 1996, 39, 105. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Croxatto, H.B.; Bardin, C.W. Mechanisms of Action of Intrauterine Devices. Obstet. Gynecol. Surv. 1996, 51 (Suppl. S12), 42S–51S. [Google Scholar] [CrossRef]
- Liljemark, W.F.; Bloomquist, C.G.; Bandt, C.L.; Pihlstrom, B.L.; Hinrichs, J.E.; Wolff, L.F. Comparison of the distribution of Actinomyces in dental plaque on inserted enamel and natural tooth surfaces in periodontal health and disease. Oral Microbiol. Immunol. 1993, 8, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Noska, A. Intrauterine device infection causing concomitant streptococcal toxic shock syndrome and pelvic abscess with Actinomyces odontolyticus bacteraemia. BMJ Case Rep. 2016, 2016, bcr2015213236. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Beck, P.P.; Vaznitsel, M.; Bran-Acevedo, A.; Hunter, M.; Ang, J.R.; Roland, W. Acute disseminated actinomycosis presenting as pneumonia with bilateral pulmonary nodules and pelvic osteomyelitis in an immunocompetent patient. IDCases 2022, 29, e01540. [Google Scholar] [CrossRef] [PubMed]
- Furuya, K.; Ito, K.; Sugiyama, K.; Fujita, A.; Kanemoto, H.; Shimada, T. A case of recurrent acute cholecystitis caused by Actinomyces odontolyticus, rare actinomycosis. BMC Infect. Dis. 2022, 22, 518. [Google Scholar] [CrossRef]
- Tu, J.; MacDonald, M.; Mansfield, D. Pulmonary actinomycosis and polymicrobial empyema in a patient with ABPA and bronchocoele. Respirol. Case Rep. 2022, 10, e0954. [Google Scholar] [CrossRef]
- Deltenre, M.; Thimmesch, M.; Creuven, M.; Pierart, F. Pulmonary actinomycosis caused by Actinomyces odontolyticus in a two-year-old child. Rev. Mal. Respir. 2022, 39, 270–274. [Google Scholar] [CrossRef]
- Razok, A.; Ali, M.; Aker, L.; Ziglam, H. Actinomyces odontolyticus bacteraemia associated with cervical and mediastinal abscesses in an immunocompetent patient: First reported case in Qatar. New Microbes New Infect. 2022, 45, 100956. [Google Scholar] [CrossRef]
- Kitano, H.; Hieda, K.; Kitagawa, H.; Nakaoka, Y.; Koba, Y.; Ota, K.; Shigemoto, N.; Hayashi, T.; Kashiyama, S.; Teishima, J.; et al. Case Report: Emphysematous Pyelonephritis With a Congenital Giant Ureterocele. Front. Pediatr. 2021, 9, 775468. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-L.; Wu, C.-T.; Chang, Y.-C.; Fan, C.-K.; Lee, Y.-J. Case report of an unusual hepatic abscess caused by Actinomyces odontolyticus in a patient with human immunodeficiency virus infection. BMC Infect. Dis. 2021, 21, 998. [Google Scholar] [CrossRef] [PubMed]
- Farah Khoury, M.; Perek, S.; Raz-Pasteur, A. Implantable cardioverter defibrillator related Actinomyces Odontolyticus endocarditis and bacteremia-First reported case. IDCases 2021, 25, e01228. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.M.; Quaresma, M.M.; Haghighi, E.; Barata, J.A. Radiation proctitis-related lumbar spondylodiscitis due to Actinomyces odontolyticus: A rare finding. BMJ Case Rep. 2021, 14, e237047. [Google Scholar] [CrossRef]
- Jain, H.; Singh, G.; Eranki, A. Actinomyces odontolyticus causing meningitis and cervical abscess. Bayl. Univ. Med. Cent. Proc. 2021, 34, 492–493. [Google Scholar] [CrossRef]
- Patel, K.; MacDonald, M.; Hmoud, H.; Czinn, E.; Wutawunashe, C.; Fisher, P. Aortic valve endocarditis by Actinomyces odontolyticus and Gemella morbillorum oral pathogens. IDCases 2021, 24, e01079. [Google Scholar] [CrossRef]
- Massey, M.; Barney, J. Pulmonary actinomycosis and marijuana vaping. BMJ Case Rep. 2021, 14, e240973. [Google Scholar] [CrossRef]
- Khiatah, B.; Shah, K.; Belikova, A.; Saeed, M. Sepsis due to Actinomyces odontolyticus as a Rare Complication of Neobladder. Case Rep. Infect. Dis. 2021, 2021, 6699046. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.; Wu, J.; Wang, J.; Tung, C. Suppurative mediastinal lymphadenitis caused by Actinomyces odontolyticus: Successfully diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. J. Postgrad. Med. 2021, 67, 46–48. [Google Scholar] [CrossRef]
- Yesilbas, O.; Yozgat, C.Y.; Nizam, O.G.; Duramaz, B.B.; Turel, O. Life-threatening multiple brain abscesses secondary to Actinomyces odontolyticus. Pediatr. Int. 2020, 62, 1307–1308. [Google Scholar] [CrossRef]
- Rueda, M.S.; Hefter, Y.; Stone, B.; Hahn, A.; Jantausch, B. A Premature Infant with Neonatal Actinomyces odontolyticus Sepsis. J. Pediatr. Infect. Dis. Soc. 2021, 10, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.J.; Shupak, R.P. Cervicofacial actinomycosis of the mandible in a paediatric patient. BMJ Case Rep. 2020, 13, e233681. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Wu, D.; Feng, M.; Yang, P.; Hu, X.; Tattevin, P.; Hong, G.; Chen, R.; Qiu, C. Pulmonary lesions associated with sputum culture-positive actinomycetes: Report of one case. Ann. Transl. Med. 2019, 7, 793. [Google Scholar] [CrossRef]
- Diab, C.; Almarzouq, A.; Ajise, O.; Barkati, S.; Tchervenkov, J.; Andonian, S. Renal actinomycosis presenting as uro-cutaneous fistula. Urol. Case Rep. 2019, 28, 101054. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kusakabe, Y.; Enomoto, M.; Yamamoto, N.; Aihara, K.; Yamaoka, S.; Mishima, M. Drastically progressive lung cavity lesion caused by Actinomyces odontolyticus in a patient undergoing chemoradiotherapy: A case report and literature review. Respir. Med. Case Rep. 2019, 28, 100950. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, E.; Bernardinello, N.; Alfieri, V.; Pellegrino, F.; Lazzari, C.; Gnetti, L.; Chetta, A. A pulmonary infection by Actinomyces odontolyticus and Veillonella atypica in an immunocompetent patient with dental caries. Respirol. Case Rep. 2019, 7, e00493. [Google Scholar] [CrossRef]
- Yun, S.S.; Cho, H.S.; Heo, M.; Jeong, J.H.; Lee, H.R.; Ju, S.; Kim, J.Y.; You, J.W.; Cho, Y.J.; Jeong, Y.Y.; et al. Lung abscess by Actinomyces odontolyticus and Parvimonas micra co-infection presenting as acute respiratory failure: A case report. Medicine 2019, 98, e16911. [Google Scholar] [CrossRef]
- Schimmel, T.; Trawinski, H.; Karlas, T.; Wendt, S.; Lübbert, C. Polymicrobial liver abscesses and pleural empyema in a 40-year-old male after tooth extraction and closed periodontal treatment: A case report. Z Gastroenterol. 2019, 57, 600–605. [Google Scholar]
- Clyde, M.; McAllister, J.; Obeidallah, A.; Ahmad, I. Actinomyces odontolyticus infection 3 months post-robotic-assisted laparoscopic prostatectomy. BMJ Case Rep. 2019, 12, e228184. [Google Scholar] [CrossRef]
- Palmitessa, V.; Cuppone, R.; Monno, R.; Fumarola, L.; Lippolis, A. A case report of esophageal actinomycosis in an immunocompetent patient and review of the literature. New Microbiol. 2019, 42, 55–60. [Google Scholar]
- Gray, A.; Do, P. The case of the unwanted crystal: A case of pediatric pulmonary Actinomyces odonolyticus. Clin. Case Rep. 2018, 6, 1230–1231. [Google Scholar] [CrossRef] [PubMed]
- Prashant, N.; Azuhairy, A. Actinomycosis of Distal Phalanx Twenty Years after Flap Reconstruction of Index Finger: A Case Report. Malays. Orthop. J. 2018, 12, 48–50. [Google Scholar] [PubMed]
- Yanagisawa, R.; Minami, K.; Kubota, N.; Iwade, T.; Ogiso, Y. Asymptomatic subcutaneous cervical mass due to Actinomyces odontolyticus infection in a pyriform sinus fistula. Pediatr. Int. 2017, 59, 941–942. [Google Scholar] [CrossRef]
- Broly, E.; Risse, J.; Maschino, F.; Wahl, D. Cardiac Tamponade Due to Actinomyces odontolyticus Originating from a Dentigerous Cyst. J. Oral Maxillofac. Surg. 2016, 74, 2453–2456. [Google Scholar] [CrossRef]
- Weiand, D.; Barlow, G. The rising tide of bloodstream infections with Actinomyces species: Bimicrobial infection with Actinomyces odontolyticus and Escherichia coli in an intravenous drug user. Oxf. Med. Case Rep. 2014, 2014, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Nebrera Navarro, F.; Ramirez Portero, C. Actinomyces odontolyticus pneumonia in a patient with iatrogenic A-hypogammaglobulinemia. Med. Clin. (Barc.) 2015, 145, 458. [Google Scholar] [CrossRef] [PubMed]
- Lensing, F.; Abele, T.; Wiggins, R., 3rd; Quigley, E. Laryngeal actinomycosis. Proc. Bayl. Univ. Med. Cent. 2014, 27, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Rich, B.S.; Angeles, C.; Barie, P.S. Actinomyces odontolyticus Breast Abscess. Surg. Infect. 2013, 14, 331–332. [Google Scholar] [CrossRef]
- Chao, C.-T.; Liao, C.-H.; Lai, C.-C.; Hsueh, P.-R. Liver abscess due to Actinomyces odontolyticus in an immunocompetent patient. Infection 2011, 39, 77–79. [Google Scholar] [CrossRef]
- Antony, B.; Shivakumarappa, G.M.; Mohan, D.R. Empyema thoracis due to actinomyces odontolyticus. Indian J. Pathol. Microbiol. 2009, 52, 120. [Google Scholar] [CrossRef]
- Davanos, E.; Rahman, S.M.; Nogid, B. Treatment of Eikenellacorrodens and Actinomyces odontolyticus foot abscess in a penicillin-allergic patient. Ann. Pharmacother. 2008, 42, 1706–1710. [Google Scholar] [CrossRef] [PubMed]
- Pant, R.; Marshall, T.L.; Crosher, R.F. Facial actinomycosis mimicking a desmoid tumour: Case report. Br. J. Oral Maxillofac. Surg. 2008, 46, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Delarbre, X.; Auzary, C.; Bahnini, A.; Nordmann, P.; Delfraissy, J.F. Actinomyces odontolyticus isolation during prosthetic aortic graft infection with paraprosthetic duodenal fistula. Rev. Med. Interne 2007, 28, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Louerat, C.; Depagne, C.; Nesme, P.; Biron, F.; Guerin, J.C. Disseminated actinomycosis. Rev. Mal. Respir. 2005, 22, 473–476. [Google Scholar] [CrossRef]
- Cone, L.A.; Leung, M.M.; Hirschberg, J. Actinomyces odontolyticus bacteremia. Emerg. Infect. Dis. 2003, 9, 1629–1632. [Google Scholar] [CrossRef]
- Sofianou, D.; Avgoustinakis, E.; Dilopoulou, A.; Pournaras, S.; Tsirakidis, G.; Tsakris, A. Soft-tissue abscess involving Actinomyces odontolyticus and two Prevotella species in an intravenous drug abuser. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 75–79. [Google Scholar] [CrossRef]
- Takiguchi, Y.; Terano, T.; Hirai, A. Lung Abscess Caused by Actinomyces odontolyticus. Intern. Med. 2003, 42, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Alamillos-Granados, F.; Dean-Ferrer, A.; Garcίa-López, A.; López-Rubio, F. Actinomycotic ulcer of the oral mucosa: An unusual presentation of oral actinomycosis. Br. J. Oral Maxillofac. Surg. 2000, 38, 121–123. [Google Scholar] [CrossRef]
- Iancu, D.; Chua, A.; Schoch, P.E.; Cunha, B.A. Actinomyces odontolyticus pulmonary infection. Am. J. Med. 1999, 107, 293–294. [Google Scholar]
- Litwin, K.A.; Jadbabaie, F.; Villanueva, M. Case of Pleuropericardial Disease Caused by Actinomyces odontolyticus that Resulted in Cardiac Tamponade. Clin. Infect. Dis. 1999, 29, 219–220. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Gonzalez-Castaneda, C.; del Campo-Matias, F.; Bellido-Casado, J.; Diaz, G. Empyema Necessitatis due to Actinomyces odontolyticus. Chest 1997, 111, 1144. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Das, S.S.; Mitchelmore, I.J. Polymicrobial brain abscess involving Haemophilus paraphrophilus and Actinomyces odontolyticus. Postgrad. Med. J. 1996, 72, 297–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bassiri, A.G.; Girgis, R.E.; Theodore, J. Actinomyces odontolyticus thoracopulmonary infections. Two cases in lung and heart-lung transplant recipients and a review of the literature. Chest 1996, 109, 1109–1111. [Google Scholar] [PubMed]
- Dontfraid, F.; Ramphal, R. Bilateral Pulmonary Infiltrates in Association with Disseminated Actinomycosis. Clin. Infect. Dis. 1994, 19, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.; Bayardelle, P.; Bélanger, R.; Fortin, L. Sacroiliitis and septicemia caused by Campylobacter rectus and Actinomyces odontolyticus. Can. J. Infect. Dis. 1994, 5, 133–136. [Google Scholar] [PubMed]
- Civen, R.; Väisänen, M.-L.; Finegold, S.M. Peritonsillar Abscess, Retropharyngeal Abscess, Mediastinitis, and Nonclostridial Anaerobic Myonecrosis: A Case Report. Clin. Infect. Dis. 1993, 16 (Suppl. S4), S299–S303. [Google Scholar] [CrossRef]
- Verrot, D.; Disdier, P.; Harlé, J.R.; Peloux, Y.; Garbes, L.; Arnaud, A.; Weiller, P.J. Pulmonary actinomycosis: Caused by Actinomyces odontolyticus? Rev. Med. Interne 1993, 14, 179–181. [Google Scholar] [CrossRef]
- Hooi, L.N.; Na, B.S.; Sin, K.S. A case of empyema thoracis caused by actinomycosis. Med. J. Malays. 1992, 47, 311–315. [Google Scholar]
- Peloux, Y.; Raoult, D.; Chardon, H.; Escarguel, J. Actinomyces odontolyticus infections: Review of six patients. J. Infect. 1985, 11, 125–129. [Google Scholar] [CrossRef]
- Klaaborg, K.-E.; Kronborg, O.; Olsen, H. Enterocutaneous fistulization due to Actinomyces odontolyticus. Report of a case. Dis. Colon Rectum 1985, 28, 526–527. [Google Scholar] [CrossRef]
- Ruutu, P.; Pentikäinen, P.J.; Larinkari, U.; Lempinen, M. Hepatic actinomycosis presenting as repeated cholestatic reactions. Scand. J. Infect. Dis. 1982, 14, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.J.; Angevine, J.M.; Sundstrom, W. Actinomycotic pulmonary abscess in an immunosuppressed patient. Am. J. Clin. Pathol. 1979, 72, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.D.; Hintz, C.S.; Haselby, R.C. Malar mass due to Actinomyces odontolyticus. J. Clin. Microbiol. 1977, 5, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, P.; Jiao, G.; Lv, J.; Ma, C.; Song, X.; Zhang, J.; Wu, C.; Li, R.; Zhu, H. Mimicking uterine malignancy: Pelvic actinomycosis with giant uterine leiomyoma. IDCases 2020, 23, e00878. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Ramírez-Durán, N.; Sandoval-Trujillo, H.; Romero-Figueroa, M.D.S. Pelvic actinomycosis. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 9428650. [Google Scholar] [CrossRef]
- Liu, Y. Actinomycosis-induced adnexal and uterine masses mimicking malignancy on FDG PET/CT. Am. J. Obstet. Gynecol. 2019, 220, 281. [Google Scholar] [CrossRef]
- Guo, L.; Ye, L.; Zhao, Q.; Ma, Y.; Yang, J.; Luo, Y. Comparative study of MALDI-TOF MS and VITEK 2 in bacteria identification. J. Thorac. Dis. 2014, 6, 534–538. [Google Scholar]
- Rudolph, W.W.; Gunzer, F.; Trauth, M.; Bunk, B.; Bigge, R.; Schröttner, P. Comparison of VITEK 2, MALDI-TOF MS, 16S rRNA gene sequencing, and whole-genome sequencing for identification of Roseomonas mucosa. Microb. Pathog. 2019, 134, 103576. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).