Abstract
Strongyloides stercoralis is an intestinal nematode that can induce disseminated infection in immunocompromised patients. It is most commonly acquired in tropical and subtropical countries; however, foci of the infection have also been reported in temperate geographic areas. In non-endemic areas, the diagnosis of an S. stercoralis infection is challenging due to the variety of clinical symptoms. Herein, we report the case of a patient, born and raised in the Calabria region of Southern Italy, who presented with melanoma and S. stercoralis hyperinfection, which is characterized by dyspnea, productive cough, inappetence, marked asthenia, weight loss, and Klebsiella pneumoniae bacteremia. He worked as a farmer and never traveled to another country known to be endemic for S. stercoralis. Despite the prompt identification of the parasite with sputum microscopy and the initiation of therapy with ivermectin and piperacillin–tazobactam, the patient later died. This case underscores the continued risk for S. stercoralis infection even in geographic areas that were previously considered non-endemic for the nematode and indicates that the geographic distribution of S. stercoralis may be expanding in Italy.
1. Introduction
Strongyloidiasis is a parasitic infection caused by the soil-transmitted helminth Strongyloides stercoralis. The disease affects about 70 million people worldwide and is endemic in many tropical and subtropical countries, accounting for 76.1% of global infections [1]. However, foci of the infection have also been documented in temperate geographic areas, such as Japan, Australia, and the USA [2,3,4]. In Italy, a prevalence of about 0.03% has been estimated [1], with a higher value of about 7% for migrant populations from Africa and Latin America [5]. Low socio-economic status [6], alcoholism [7], Caucasian race [8], and male gender [6] have been associated with a higher prevalence of strongyloidiasis. Occupations such as farming [9,10] and coal mining [11] also enhance the risk of infection since transmission occurs through a percutaneous route by infective (filariform) larvae free-living in contaminated soil. In this frame, an open question is the zoonotic potential of S. stercoralis. Several reports have shown that the nematode can adapt from humans to dogs [12], suggesting a possible cross-transmission of the infection. Recent phylogenetic studies [13,14] further strengthen this hypothesis, even though the transmission between the two hosts is still debated [12]. Of epidemiologic relevance is the worldwide distribution of canine S. stercoralis infections and the evidence that Italy is the European country where the highest number of cases has been recorded [12]. A peculiarity of Strongyloides stercoralis is its complex life cycle, which alternates between free-living and parasitic cycles and has three developmental stages (adult, rhabditiform larva, and filariform larva). An additional feature of Strongyloides stercoralis is its replication within the bowels of human hosts, allowing for cycles of autoinfection (Figure 1).
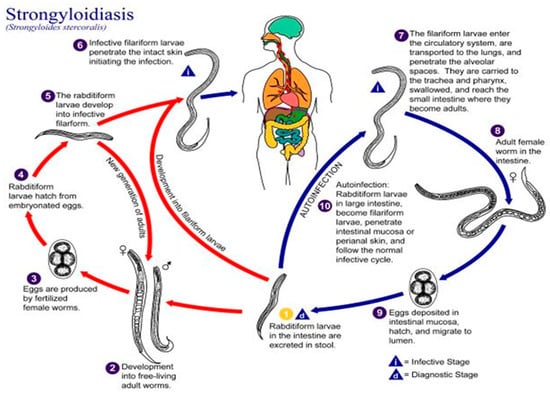
Figure 1.
The life cycle of Strongyloides stercoralis, from the Centre of Disease Control (CDC). https://www.cdc.gov/parasites/strongyloides/biology.html (accessed on 10 July 2022).
This phenomenon yields, in turn, a chronic infection lasting several decades even in the absence of re-exposure to an external infective source [15]. Autoinfection is also the pathogenic mechanism underlying hyperinfection syndrome (HS) and disseminated strongyloidiasis (DS), in which the intestinal production of intestinal infective larvae and a huge number of S. stercoralis fusiform larvae disseminate to all organs and systems, especially in immunocompromised subjects [16]. The detection of Strongyloides stercoralis larvae in sputum specimens and in feces categorizes HS, while a systemic infection encompassing the central nervous system, liver, peritoneum, and kidneys is observed in DS. Gram-negative sepsis, pneumonia, or meningitis can also occur, thus, affecting the clinical outcome of this parasitic infection [16]. In non-endemic areas, strongyloidiasis is still a diagnostic challenge, mainly due to the variety of its clinical manifestations, which results in an underestimation of the disease prevalence [17,18]. Even in Italy, the phenomenon is underestimated, although cases of strongyloidiasis have been repeatedly reported in this country [19,20,21,22,23,24,25]. Herein, we describe for the first time a fatal case of autochthonous S. stercoralis HS in an immunocompromised patient from the Calabria region of Southern Italy.
2. Case Presentation Section
A 61-year-old Caucasian male was admitted to the Emergency Medicine Unit at the Pugliese-Ciaccio Hospital on 13 November 2021 with a complaint of dyspnea, a productive cough, and constipation; the latter had lasted for 3 days. His respiratory symptoms were associated with a loss of appetite, marked asthenia, and weight loss. At the time of his admission, he worked as a farmer and had never traveled outside the Calabria region.
2.1. Medical History
The patient had a history of hypertension, colon polyposis, removed by endoscopic surgery, and migraines. In July 2019, he was diagnosed with BRAF+ acromial melanoma associated with lung involvement and underwent targeted therapy cycles with dabrafenib (150 mg twice a day), plus trametinib (2 mg/day) at first. A total body computerized tomography (TBTC) in the follow-up period revealed the presence of brain lesions, which is suggestive of cerebral metastatic processes; thus, the targeted therapy was withdrawn and replaced with external beam radiotherapy (a total dose of 3000 Gy), followed by immunotherapy with nivolumab, a 240-mg flat dose (6 cycles), as a second-line therapy. Dexamethasone, 4 mg once a day, and Mannitol were also added as a supportive therapy. Three months after the beginning of the second-line therapy, the TBTC revealed a volumetric increase in encephalic lesions; thus, the second-line therapy was withdrawn, and the patient underwent a temozolomide treatment, 200 mg a day (6 cycles), plus external beam radiotherapy, followed by stereotaxic surgery at the level of the fronto-parietal brain region. Maintenance therapy with temozolomide, 120 mg once a day, was undertaken after the surgical procedure.
2.2. Physical Examination
During the physical examination, the patient showed the following: weak vesicular breath sounds across the lung, with wheezing and crackles at the level of the middle and basilar regions; regular heart activity and murmurs; the liver edge and spleen were not palpable; a positive Murphy’s sign; peripheral edema.
2.3. Chest Radiograph
A chest X-ray revealed the presence of bilateral lung interstitial thickening with a combination of reticular and nodular patterns (Figure 2).
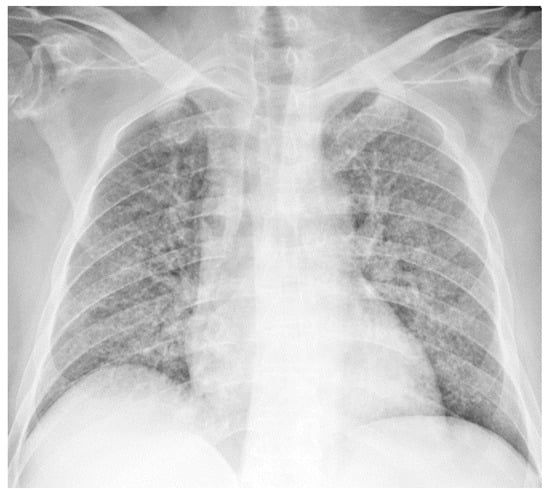
Figure 2.
Chest X-ray showing bilateral lung interstitial thickening; the cardiac shadow is within normal limits; clear costophrenic angles.
2.4. Laboratory Diagnosis and Therapy
On admission, the laboratory test revealed the presence of hyperglycemia and anemia, while a differential white cell count showed the occurrence of eosinopenia, lymphopenia, and neutrophil values within the normal range. A treatment with calcium N5-methyltetrahydrofolate pentahydrate, 15 mg once a day, and vitamin B12, 500 µg/mL once a day, was then started. The patient also received a continuous IV furosemide infusion (10 mg/mL vial diluted in 500 mL of physiological solution), enoxaparin, 4000 IU once a day, and insulin lispro. The laboratory tests performed from the second to the fifth day of admission revealed a moderate increase in the neutrophil absolute count with concomitant eosinopenia and lymphopenia. On the second day of admission, a decrease in the hemoglobin value (7.3 g/dL) was recorded, which required blood transfusion. A sharp rise in the serum total IgE levels was detected on the third day of admission. Other abnormal test results included a steady increase in the procalcitonin (PCT) values from 1.00 μg/L to 15.92 μg/L on the second day of admission; thus, an empirical antimicrobial therapy with piperacillin–tazobactam, 4.5 mg three times a day, and levofloxacin, 500 mg a day, was started. The clinical biochemistry profile, recorded during hospitalization, is summarized in Table 1; the timeline of the differential white cell counts is reported in Figure 3.

Table 1.
Clinical biochemistry profile, recorded during hospitalization (13 November 2021, admission; 18 November 2021, exit; Hb, hemoglobin; HCT, hematocrit test; MCV, mean cell volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width).
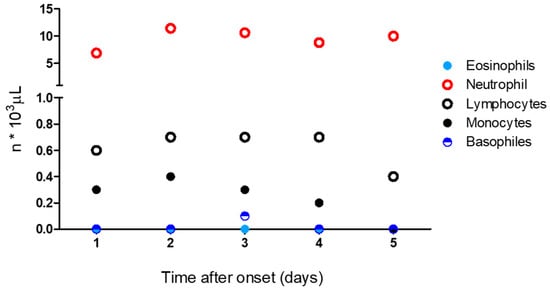
Figure 3.
Timeline of the differential white cell count from admission (1st day) to outcome (5th day).
On the first day of admission, expectorated sputum and hemoculture examinations were performed. The results from the direct microscopic examination of the expectorated sputum showed numerous S. stercoralis larvae. The larvae were elongated with a notched tail and were identified as third-stage larvae (L3). They measured from 430 to 500 µm in length and from 14 to 16 µm in width, with a prominent esophagus. It measured from 200 to 250 µm. All of the measurements were performed using an optical microscope, equipped with a micrometric eyepiece (Figure 4, A–C panels). Papanicolaou staining of the sputum specimen confirmed the light microscopy observations (Figure 4, B panel).
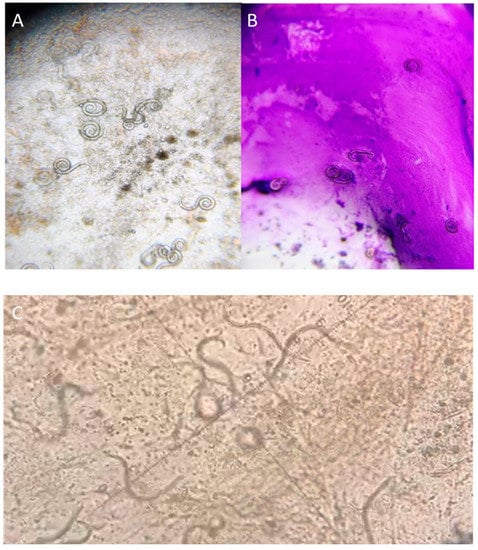
Figure 4.
Direct microscopic examination of the expectorated sputum, showing numerous larvae of Strongyloides stercoralis (A–C panels). Papanicolau staining (B panel).
The Strongyloides stercoralis HS syndrome was, therefore, diagnosed, and subcutaneous treatment with ivermectin, 200 µg/kg/day on alternate days, was started. Due to the severe constipation, a stool examination was not performed. The hemoculture revealed the presence of Klebsiella pneumoniae. The antimicrobial susceptibility testing results (Figure 5) confirmed the susceptibility to the chosen empirical antimicrobial agents. Despite ivermectin and other therapeutic approaches, the patient died on the sixth day of his admission because of a cardio-respiratory arrest.
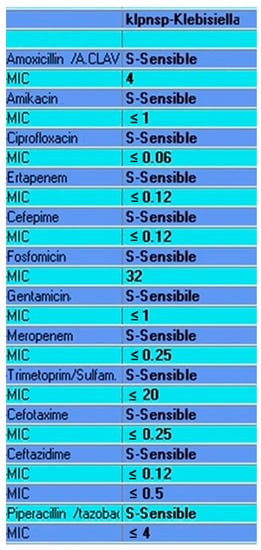
Figure 5.
Klebsiella Pneumoniae antimicrobial susceptibility test results.
3. Discussion
The symptoms and clinical outcomes of strongyloidiasis depend upon the interplay between the helminth and the host cell-mediated immune response, leading to a wide spectrum of disease presentation. Usually, only HS and DS are considered life-threatening conditions, while acute and chronic S. stercoralis infections are both categorized as “uncomplicated forms” of the disease, occurring with mild and non-specific symptoms, or asymptomatically. HS recognized as the main pathogenetic mechanism an increased rate of autoinfection processes and is often related to immunosuppression [16]. In this condition, the unbalanced immune response mainly involves T-cell subsets, and among these are the regulatory T cells (Tregs), which are now recognized as potential new players in the HS pathogenetic framework [26]. This cell population controls the immune response through several mechanisms, such as cell-to-cell contact, the production of inhibitory cytokines, and the modulation of cytokine effects [27]. In patients with S. stercoralis and HTLV-1 co-infections, an increased proportion of CD4+CD25+Foxp3+ Tregs was observed. The expansion of lymphocytes expressing this phenotype is correlated with a decreased antigen-driven production of IL-5 and lower eosinophil counts, thus, allowing for the occurrence of HS in these patients [26]. Pertaining to the case, our patient had intrinsic immunological abnormalities due to the presence of metastatic melanoma and the temozolomide and dexamethasone therapeutic regimens over the protracted period. From all of the prescribed drugs, the use of corticosteroids represents the most frequent risk factor for the development of HS due to their capability of inducing acute suppression of eosinophilia and lymphocyte activation [28], irrespective of the dose or route of administration [29,30,31,32]. Corticosteroids may also have a direct effect on S. stercoralis, fostering the transformation of rhabditiform larvae to invasive filariform larvae or rejuvenating the reproductively latent adult females [33]. Of note, temozolomide therapy has been shown to increase the proportion of the CD4+CD25+Foxp3+ Tregs cell population [34], which, in turn, could contribute to the occurrence of eosinopenia. Concomitantly with eosinopenia, we observed a marked increase in serum total IgE levels in our patient. Mounting evidence supports the notion that the IgE-mediated activation of accessory cells has a crucial role in the resistance against parasitic infections [35]. However, the increase in serum IgE, commonly seen in up to 75% of people with chronic strongyloidiasis, cannot be beneficial since most of it is non-specific and may negatively affect the development of the host defense mechanisms by saturating the IgE receptors on the effector cells [35]. Clinically, the diagnosis of HS is based on the presence of signs and symptoms related to increased larval migration and development, as well as the exacerbation of pulmonary symptoms, which is a common observation. Our patient showed severe respiratory symptoms, including a productive cough, a loss of appetite, marked asthenia, and weight loss; thus, further examinations were performed. The chest X-ray revealed the presence of bilateral lung interstitial thickening with a combination of reticular and nodular patterns, while the identification of S. stercoralis third-stage (L3) larvae in the sputum specimen by microscopic evaluation and special staining allowed for the diagnosis of pulmonary strongyloidiasis [36,37,38]. Therefore, subcutaneous treatment with ivermectin, 200 µg/kg/day on alternate days, was started [37]. Although several therapeutic options, such as thiobendazole, mebendazole, and albendazole, are available, ivermectin has become the drug of choice for the treatment of severe forms of strongyloidiasis [39]. Ivermectin in monotherapy regimens offers several advantages, such as better tolerance, various dosages, and various routes of administration that can be tailored to the patient’s clinical conditions. Of note, the death rate of infections from the severe form of S. stercoralis is dependent mainly on the patient’s overall status and not solely on the clearance of the parasite [39]. Since HS is often complicated by bacterial and fungal infections [33,40], a hemoculture was also carried out, which showed the presence of Klebsiella pneumoniae. As expected, the Klebsiella pneumoniae bacteremia was associated with a marked increase in the serum PCT level; thus, antibiotic therapy was started. Despite the prompt identification of the S. stercoralis larvae with sputum microscopy and the initiation of the therapeutic regimen, the patient showed an unfavorable outcome because of the late diagnosis at the stage of the HS disease, which was complicated by a bacterial infection. Our patient underscores the continued risk for transmission of S. stercoralis in Italy, even in non-endemic geographic areas. He worked as a farmer and spent his entire life in the Calabrian region, as well as he never traveled to another country known to be endemic for S. stercoralis. However, it remains to be determined when our patient was infected because the infection can last for decades. He had numerous risk factors for developing HS, including his job and the cancer that required treatment with steroids and chemotherapy; thus, screening before the immunosuppressive therapy should have been taken into consideration. At present, there is no gold-standard technique for diagnosing S. stercoralis infections, and the available parasitological and serological diagnostic tools still possess limitations [41]. However, serial and specific stool examinations through several methodological approaches (i.e., the Baermann technique, the Horada–Mori filter paper culture, the quantitative acetate concentration technique, or nutrient agar plate cultures) are recommended as the laboratory investigations of choice for the diagnosis of strongyloidiasis in chronically infected patients [38]. Particular attention should also be paid to the kind of job and the environmental and social risk factors for S. stercoralis infections, especially for subjects with long-lasting gastrointestinal discomfort or skin manifestations, such as urticaria and recurrent maculopapular rashes [38], regardless of the blood eosinophil counts. In conclusion, as a large number of strongyloidiasis cases have been reported in areas of Northern Italy [19], this autochthonous case report suggests that the geographic distribution of S. stercoralis may be expanding in Italy, including areas of Southern Italy. Our evidence further emphasizes the importance of vigilant screenings to prevent severe forms of strongyloidiasis in immunocompromised patients. With that concern, routine empiric treatments for strongyloidiasis with ivermectin have been recommended [38], even though no standard regimens or significant evidence-based studies have been reported [42,43].
Author Contributions
A.C., G.T., V.V., S.N., and G.D.S. have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design, M.C. and M.C.C.; data acquisition, P.M. and. E.C.; data analysis and data interpretation, M.C., F.L., and M.C.C.; drafting of the manuscript, M.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
We did not receive funding for this study.
Institutional Review Board Statement
The study was approved by the Pugliese-Ciaccio Hospital Ethical Committee (protocol number: 259-2022/9/15).
Informed Consent Statement
Patient consent was waived due to exitus. Therefore, the consent was obtained from the patient’s son.
Data Availability Statement
Data are available in the medical records present in the Pugliese Ciaccio hospital archives.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buonfrate, D.; Bisanzio, D.; Giorli, G.; Odermatt, P.; Fürst, T.; Greenaway, C.; French, M.; Reithinger, R.; Gobbi, F.; Montresor, A.; et al. The Global Prevalence of Strongyloides stercoralis Infection. Pathogens 2020, 9, 468. [Google Scholar] [CrossRef]
- Genta, R.M. Global prevalence of strongyloidiasis: Critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 1989, 11, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; McCormick, M.; Ribes, J.A. Invasive enteric infections in hospitalized patients with underlying strongyloidiasis. Am. J. Clin. Pathol. 2007, 128, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Schar, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Marrone, R.; Silva, R.; Mirisola, C.; Ragusa, A.; Mistretta, M.; Perandin, F.; Bisoffi, Z. Prevalence of Strongyloidiasis in a Cohort of Migrants in Italy and Accuracy of a Novel ELISA Assay for S. stercoralis Infection, a Cross-Sectional Study. Microorganisms 2021, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Walzer, P.D.; Milder, J.E.; Banwell, J.G.; Kilgore, G.; Klein, M.; Parker, R. Epidemiologic features of Strongyloides stercoralis infection in an endemic area of the United States. Am. J. Trop. Med. Hyg. 1982, 31, 313–319. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.C.; Ribeiro, C.T.; Mendes Dde, M.; Oliveira, T.C.; Costa-Cruz, J.M. Frequency of Strongyloides stercoralis infection in alcoholics. Memórias Do Inst. Oswaldo Cruz 2002, 97, 119–121. [Google Scholar] [CrossRef]
- Davidson, R.A.; Fletcher, R.H.; Chapman, L.E. Risk factors for strongyloidiasis. A case-control study. Arch. Intern. Med. 1984, 144, 321–324. [Google Scholar] [CrossRef]
- Marnell, F.; Guillet, A.; Holland, C. A survey of the intestinal helminths of refugees in Juba, Sudan. Ann. Trop. Med. Parasitol. 1992, 86, 387–393. [Google Scholar] [CrossRef]
- Sanchez, P.R.; Guzman, A.P.; Guillen, S.M.; Adell, R.I.; Estruch, A.M.; Gonzalo, I.N.; Olmos, C.R. Endemic strongyloidiasis on the Spanish Mediterranean coast. Q. J. Med. 2001, 94, 357–363. [Google Scholar] [CrossRef]
- Wagenvoort, J.H.; Houben, H.G.; Boonstra, G.L.; Scherpbier, J. Pulmonary superinfection with Strongyloides stercoralis in an immunocompromised retired coal miner. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 518–519. [Google Scholar] [CrossRef]
- Ottino, L.; Buonfrate, D.; Paradies, P.; Bisoffi, Z.; Antonelli, A.; Rossolini, G.M.; Gabrielli, S.; Bartoloni, A.; Zammarchi, L. Autochthonous Human and Canine Strongyloides stercoralis Infection in Europe: Report of a Human Case in An Italian Teen and Systematic Review of the Literature. Pathogens 2020, 9, 439. [Google Scholar] [CrossRef] [PubMed]
- Nagayasu, E.; Aung, M.P.P.T.H.H.; Hortiwakul, T.; Hino, A.; Tanaka, T.; Higashiarakawa, M.; Olia, A.; Taniguchi, T.; Win, S.M.T.; Ohashi, I.; et al. A possible origin population of pathogenic intestinal nematodes, Strongyloides stercoralis, unveiled by molecular phylogeny. Sci. Rep. 2017, 7, 4844. [Google Scholar] [CrossRef] [PubMed]
- Jaleta, T.G.; Zhou, S.; Bemm, F.M.; Schär, F.; Khieu, V.; Muth, S.; Odermatt, P.; Lok, J.B.; Streit, A. Different but overlapping populations of Strongyloides stercoralis in dogs and humans-Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl. Trop. Dis. 2017, 11, e0005752. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Weller, P.F. Strongyloidiasis and other intestinal nematode infections. Infect. Dis. Clin. N. Am. 1993, 7, 655–682. [Google Scholar] [CrossRef]
- Corti, M. Strongyloides stercoralis in Immunosuppressed Patients. Arch. Clin. Infect. Dis. 2016, 11, e27510. [Google Scholar] [CrossRef]
- Olsen, A.; van Lieshout, L.; Marti, H.; Polderman, T.; Polman, K.; Steinmann, P.; Stothard, R.; Thybo, S.; Verweij, J.J.; Magnussen, P. Strongyloidiasis—The most neglected of the neglected tropical diseases? Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Toledo, R.; Muñoz-Antoli, C.; José-Guillermo, E. Strongyloidiasis with Emphasis on Human Infections and Its Different Clinical Forms. Adv. Parasitol. 2015, 88, 165–241. [Google Scholar] [PubMed]
- Buonfrate, D.; Baldissera, M.; Abrescia, F.; Bassetti, M.; Caramaschi, G.; Giobbia, M.; Mascarello, M.; Rodari, P.; Scattolo, N.; Napoletano, G.; et al. Epidemiology of Strongyloides stercoralis in northern Italy: Results of a multicenter case–control study, February 2013 to July 2014. Eurosurveillance 2016, 21, 30310. [Google Scholar] [CrossRef]
- Abrescia, F.F.; Falda, A.; Caramaschi, G.; Scalzini, A.; Gobbi, F.; Angheben, A.; Gobbo, M.; Schiavon, R.; Rovere, P.; Bisoffi, Z. Reemergence of Strongyloidiasis, Northern Italy. Emerg. Infect. Dis. 2009, 15, 1531–1533. [Google Scholar] [CrossRef]
- Venturini, E.; Fusani, L.; Mantella, A.; Bianchi, L.; Antonelli, A.; Montagnani, C.; Chiappini, E.; Spinicci, M.; Bartoloni, A.; Rossolini, G.M.; et al. Strongyloidiasis in Children Outside the Tropics: Do We Need to Increase Awareness? Microorganisms 2021, 9, 1905. [Google Scholar] [CrossRef] [PubMed]
- Marchese, V.; Crosato, V.; Gulletta, M.; Castelnuovo, F.; Cristini, G.; Mattelli, A.; Castelli, F. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection 2021, 49, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, A.; Bortesi, L.; Benini, M.; Bisoffi, Z. A case of chronic strongyloidiasis diagnosed by histopathological study. Int. J. Infect. Dis. 2018, 77, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Scaglia, M.; Brusita, R.; Gatti, S.; Bernuzzi, A.M.; Strosselli, M.; Malfitano, A.; Capelli, D. Autochtonous strongyloidiasis in Italy: An epidemiological and clinical review of 150 cases. Bull. Soc. Path. Ex. 1984, 77, 328–332. [Google Scholar]
- Angheben, A.; Mistretta, M.; Gobbo, M.; Bonafini, S.; Iacovazzi, T.; Sepe, A.; Gobbi, F.; Marocco, S.; Rossanese, A.; Bisoffi, Z. Acute strongyloidiasis in Italian tourists returning from Southeast Asia. J. Travel Med. 2011, 18, 138–140. [Google Scholar] [CrossRef][Green Version]
- Montes, M.; Sanchez, C.; Verdonck, K.; Lake, J.E.; Gonzalez, E.; Lopez, G.; Terashima, A.; Nolan, T.; Lewis, D.E.; Gotuzzo, E.; et al. Regulatory T Cell Expansion in HTLV-1 and Strongyloidiasis Co-infection Is Associated with Reduced IL-5 Responses to Strongyloides stercoralis Antigen. PLoS Negl. Trop. Dis. 2009, 3, e456. [Google Scholar] [CrossRef]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef]
- Concha, R.; Harrington, W.J.; Rogers, A.I. Intestinal strongyloidiasis: Recognition, management, and determinants of outcome. J. Clin. Gastroenterol. 2005, 39, 203–211. [Google Scholar] [CrossRef]
- Suvajdzic, N.; Kranjcic-Zec, I.; Jovanovic, V.; Popovic, D.; Colovic, M. Fatal strongyloidosis following corticosteroid therapy in a patient with chronic idiopathic thrombocytopenia. Haematologia 1999, 29, 323–326. [Google Scholar]
- Kaslow, J.E.; Novey, H.S.; Zuch, R.H.; Spear, G.S. Disseminated strongyloidiasis: An unheralded risk of corticosteroid therapy. J. Allergy Clin. Immunol. 1990, 86, 138–142. [Google Scholar] [CrossRef]
- Thomas, M.C.; Costello, S.A. Disseminated strongyloidiasis arising from a single dose of dexamethasone before stereotactic radiosurgery. Int. J. Clin. Pract. 1998, 52, 520–521. [Google Scholar] [PubMed]
- Siddiqui, A.A.; Berk, S.L.; Genta, R.M. Strongyladiasis. In Tropical Infections Diseases; Elsevier: Philadelphia, PA, USA, 2005; pp. 1274–1285. [Google Scholar]
- Keiser, P.B.; Nutman, T.B. Strongyloides stercoralis in the Immunocompromised Population. Clin. Microbiol. Rev. 2004, 17, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Karachi, A.; Dastmalchi, F.; Mitchell, D.A.; Rahman, M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro-Oncology 2018, 20, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Negrao-Correa, D.; Taylor & Francis Group. Importance of immunoglobulin E (IgE) in the protective mechanism against gastrointestinal nematode infection: Looking at the intestinal mucosae. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 291–299. [Google Scholar]
- Siddiqui, A.A.; Berk, S.L. Diagnosis of Strongioloides stercoralis Infection. Clin. Infect. Dis. 2001, 33, 1040–1047. [Google Scholar] [CrossRef]
- Newnham, M.S. Manifestations, diagnosis, and treatment of Strongioloides stercoralis infection. Ann. Pharmacother. 2007, 41, 1992–2001. [Google Scholar] [CrossRef]
- Luvira, V.; Siripoon, T.; Phiboonbanakit, D.; Somsri, K.; Watthanakulpanich, D.; Dekumyoy, P. Strongyloides stercoralis: A Neglected but Fatal Parasite. Trop. Med. Infect. Dis. 2022, 7, 310. [Google Scholar] [CrossRef]
- Luvira, V.; Watthanakulpanich, D.; Pittisuttithum, P. Management of Strongyloides stercoralis: Puzzling parasite. Int. Health 2014, 6, 273–281. [Google Scholar] [CrossRef]
- Newberry, A.M.; Williams, D.N.; Stauffer, W.M.; Boulware, D.R.; Hendel-Paterson, B.R.; Walker, P.F. Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis. Chest 2005, 128, 3681–3684. [Google Scholar] [CrossRef]
- Mendes, T.; Minori, K.; Ueta, M.; Miguel, D.C.; Allegretti, S.M. Strongyloidiasis Current Status with Emphasis in Diagnosis and Drug Research. J. Parasitol. Res. 2017, 2017, 5056314. [Google Scholar] [CrossRef]
- Santiago, M.; Leitao, B. Prevention of Strongyloides hyperinfection syndrome: A rheumatological point of view. Eur. J. Intern. Med. 2009, 20, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Nellore, A. Management of Strongyloides in Solid Organ Transplant Recipients. Infect. Dis. Clin. N. Am. 2018, 32, 749–763. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).