Abstract
Background aims: The possibility of permanent neurological sequels after surgery of benign lesions affecting the spinal cord is well known. Frequently, they are irreversible, with no effective treatment other than rehabilitation. However, in recent years, intrathecal cell therapy with autologous bone marrow stromal cells (MSCs) in patients with incomplete paraplegia has shown benefits for diverse sequels of spinal cord injury (SCI). Methods: We present two patients with chronic spinal cord sequels after a surgery, who underwent cell therapy treatment with NC1 medicament (repeated intrathecal administrations of MSCs). Results: In both cases, cell therapy achieved a clear improvement in neurological sequels, such as recovery of gait disturbances, bowel dysfunction, or neuropathic pain. Conclusion: Intrathecal cell therapy with autologous MSCs offers a new approach for neurological sequels after spinal cord surgery.
1. Introduction
The most common causes of SCI are road accidents, closely followed by falls. Violence and sports injuries are also relatively common causes [1,2,3]. Regardless, it is well known that one of the problems of neurosurgery is the possibility of neurological sequelae after resection of benign lesions. Medical/surgical complications affect approximately 4% of SCI cases worldwide [2,4].
In spinal cord pathology, the removal of an intramedullary benign ependymoma is a characteristic example in which tumor removal can achieve complete healing of the patient, but is often associated with variable sequels, which may be irreversible. It is accepted that approximately 70% of patients undergoing resection of an intramedullary ependymoma will experience postoperative worsening, and although 62% improve or remain without deficit at follow-up, neurological sequels persist in approximately 25% of patients [5]. Thoracic disc herniation is another example of a benign entity in which it is common to observe the appearance of sequelae after surgery, which are estimated in up to 15% of patients, regardless of the surgical approach [6,7]. In these conditions, permanent neurological complications are generally considered justifiable and unavoidable, without therapeutic strategies other than rehabilitation.
On the other hand, in recent years, cell therapy using intrathecal administrations of autologous bone marrow mesenchymal stromal cells (MSCs) has proven useful for improving chronic established sequels of patients with incomplete spinal cord injury (SCI) [8,9]. However, when the recent literature on clinical trials with cell therapy in the field of spinal cord injury was reviewed in depth, it was clear that in many cases the type and origin of spinal cord injury was not defined [10,11,12,13], or they referred fundamentally to traffic accidents or other type of accidents [14,15,16]. On ClinicalTrials.gov [NCT03505034, NCT02688049, NCT03521336 and NCT03521323] and in the recent literature [17], some clinical trials exclude patients with spinal cord injuries derived from tumors or other types of non-traumatic spinal cord injuries. Such studies leave out many patients whose quality of life has been greatly affected after resection of benign lesions. There is no documented scientific evidence regarding the usefulness of cell therapy in neurosurgical sequels of the spinal cord. Although there are no clear data showing that therapy with MSCs is superior to other types of therapy, it is considered that the advantages that can be derived from the said treatment outweigh the lack of therapies to which these patients are referred.
In our case, the choice of cell type was based not only on the experience developed with the treatment with MSCs of patients with traumatic spinal cord injury [8,9], but also on the ease of access to this therapy as a possible new therapeutic route for these patients who otherwise would not be able to access any type of treatment. At that time, our Unit had an authorized and validated GMP Room for the manufacture of a medication based on MSCs (medicament NC1) for the treatment of patients with traumatic SCI, which opened the possibility of bringing this treatment closer to other types of patients.
This paper aimed to demonstrate that this type of cell therapy may also be useful in chronic and established sequels after spinal cord surgery. Here, we reported the successful cases of two patients with neurological sequels after the resection of benign lesions that received intrathecal injection of medicament NC1, which represents a new approach for the treatment of neurosurgery sequels in spinal cord injury.
2. Cases
2.1. Patient Selection
Therapy with NC1 was authorized by the Spanish Agency for Medicaments and Health Products (EAMPS) and the Medical Management of Puerta de Hierro–Majadahonda Hospital for the treatment of two patients with a diagnosis of SCI. The therapy consisted of an intrathecal administration of the NC1 medicament into the subarachnoid space through lumbar puncture. The two patients were selected due to neurological sequels, which had persisted after spinal cord surgery, like gait disturbances, bowel dysfunction, or neuropathic pain. Ethical aspects and possible benefits were considered. Previously to the therapy being administered, informed consent was obtained. The experimental treatment with the cell medicament was explained based on our experience in treatment with NC1 of patients with SCI [8,9,18].
The NC1 is a cell therapy medicament developed by Cell Therapy Unit of the Puerta de Hierro–Majadahonda Hospital, and at the time of these treatments, NC1 was approved as a medicament under clinical investigation (PEI number 12-141), and later, in 2019, it was approved for hospital use (registration number 83796). It was elaborated in our cleanroom after obtaining the bone marrow of the patients. It consists of expanded autologous MSCs and autologous plasma as its excipient. The culture and purification of MSCs have been previously described [9,18], as well as its phenotypic characterization, formulation, and packaging. All the materials are provided as Supplementary Materials File S1.
2.2. Cell Therapy Treatment
Prior to cell therapy treatment, a peripheral blood sample was obtained from each patient for genomic studies (to rule out chromosomal abnormalities after cell expansion and to obtain a genetic fingerprint, KaryoNIM Stem Cells and KaryoNIM STR test, respectively; NIMGenetics; additional information is provided like Supplementary Materials File S1). After the genetic studies, 500 cc of peripheral blood sample were obtained from each patient to purify the plasma fraction before NC1 production. Approximately 2 weeks later, 50 mL of bone marrow was aspirated under aseptic conditions from the iliac bones of each patient and processed in our GMP cleanroom for culture and expansion of MSCs. Mononuclear cells (MNCs) were separated by an automated cell-processing system (SEPAX, BioSafe) and plated in 175 cm2 flasks. When the cultures approached confluency (90–100%), the cells were subcultured in four floor factory farms.
After obtaining the MSCs necessary for treatment, the NC1 medicament was formulated at a concentration of 100,000 cells/µL resuspended in the autologous plasma of the patients. After formulation, the NC1 medicament was packaged in sterile and endotoxin-free 1 mL Hamilton micro-syringes. The micro-syringes with NC1 medicament were placed inside a sterile box, which was double bagged before being transported to the operating room for cell trans-plantation.
The second and third doses of medicament NC1 were prepared using cryopreserved MSCs, maintaining the same formulation and packaging conditions. The phenotypic characterization of the MSCs and genomic and sterility studies were performed at all patient doses. All materials are provided as Supplementary Materials File S1.
The patients received a total dose of 300 × 106 MSCs in two or three administrations (two administration in Case 1, and three administrations in Case 2, see below) over the course of 6 months.
3. Description of Cases
3.1. Case 1
A 49-year-old female patient wished to explore the potential of cell therapy for chronic neurological sequels established after the resection of a dorso-lumbar grade II spinal ependymoma that was performed 10 years earlier (Figure 1).
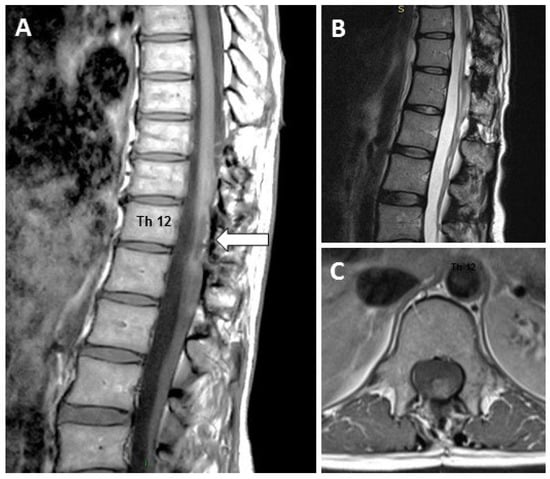
Figure 1.
Magnetic resonance showing the residual lesion (arrow) due to the surgery, which occurred 10 years before cell therapy: (A) Sagittal T2 weighted, (B) Sagittal T1 weighted and (C) Axial T2 weighted MRI scans showing the residual spinal cord injury.
The main symptoms were significant neuropathic pain, with a score of 9/10 in the Visual Analog Scale (VAS) [19], and a significant alteration of gait, with enlargement of the support base. She reported a minimal bowel dysfunction, with a score of 2 in the Krogh et al. scale [20] and gave a score of 4 in bladder function according to the Geffner scale [21]. According to the ASIA assessment provided by the American Spinal Injury Association [22], she had an ASIA grade D and a motor score (MS) of 37/50 in the lower extremities. The punctuation in pin prick score (PPS) was 94/112, and the light touch score (LTS) was 97/112.
After obtaining authorization from our Clinical Trials Committee and the Spanish Agency of Medicament and Health Products (AEMPS) (authorization code TRT256600482463), the patient received lumbar punction, and with a gap of six months, two intrathecal doses of 150 × 106 autologous expanded MSCs, supported in autologous plasma (NC1 medicament). The patient was followed until month 12 from the first administration.
After the start of cell therapy, the patient experienced a progressive reduction in neuropathic pain (VAS = 6 prior to the second MSCs administration, and VAS = 4 at the end of follow-up). This score was maintained 2 years after finishing cell therapy.
Gait analysis was conducted by assessment of the amplitude of the step (width and length), symmetry, and gait speed (expressed in cm/s) using the NeuroCom Balance Master® platform and analyzed by NeuroCom Balance Manager System version 9.3, Middleton, Wisconsin, USA. Improvement was observed, with an increase in the amplitude of the step from an average of 26 cm prior to the treatment to 30.5 cm at the end of follow-up. An increase in the average speed was also observed, from 27.2 cm/s prior to treatment to 32.1 cm/s at the end of follow-up. Figure 2 shows the evolution of these parameters.
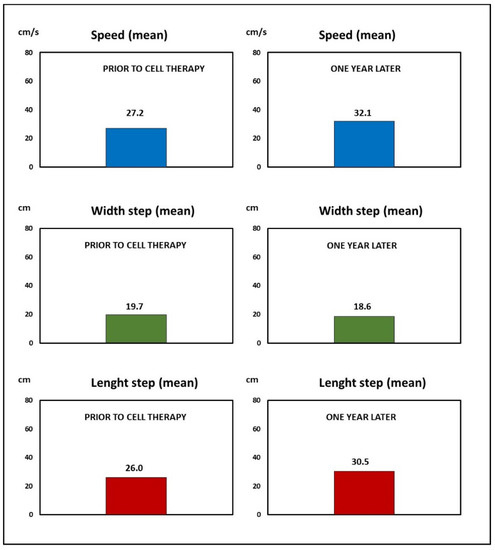
Figure 2.
Case 1: The improvement obtained by cell therapy in the gait analysis.
Scores in the Kroght [20] and Geffner [21] scales showed no changes at the end of follow-up. In the ASIA assessment [22], modifications in sensitivity or motor scores were also not observed at the end of the study, but in neurophysiological studies, improvements in parameters of motor evoked responses after transcranial magnetic stimulation were recorded (Table 1).

Table 1.
Mean values of the motor responses obtained after transcranial magnetic stimulation. After cell therapy, improvements in latency and amplitude in motor evoked potentials were observed.
3.2. Case 2
A 60-year-old male with incomplete paraplegia was considered for cell therapy in 2017. Four years before, he suffered complete paraplegia after resection of a thoracic herniation at level Th11–Th12 (Figure 3). He improved with rehabilitation until he was catalogued as an ASIA D. Chronic and established sequels that remained unchanged in the last few years consisted of gait disturbance and bowel dysfunction (score of 10 on the Krogh scale) [20].
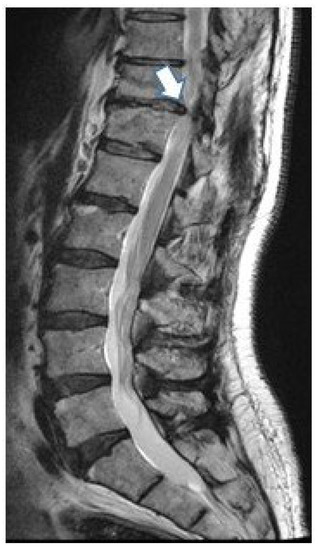
Figure 3.
MR showing the disc herniation (arrow) that was removed in this patient.
After authorization from our Clinical Trials Committee and the AEMPS (authorization code TRT945500494563), the patient received three intrathecal administrations of 100 × 106 MSCs by punction lumbar between December 2017 and June 2018, according to the 100/3 guideline for intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury [9] (ClinicalTrials gov NCT02570932).
When the status prior to cell therapy and the situation one year later were compared, clear modifications were observed, which have been maintained at three years follow-up after the start of cell therapy. Bowel dysfunction improved from a 10 on the Krogh scale [20] (moderate dysfunction) to a score of 2 at the end of cell therapy (very minor dysfunction).
In the gait, a clear improvement was observed by the patient. The observation was objectively confirmed, with an increase in the length of the step from an average of 50.80 cm prior to the treatment to 55.80 cm at the end of follow-up. An increase in the average speed was recorded, from 63.30 cm/s prior to treatment to 69.20 cm/s at the last follow-up (Figure 4).
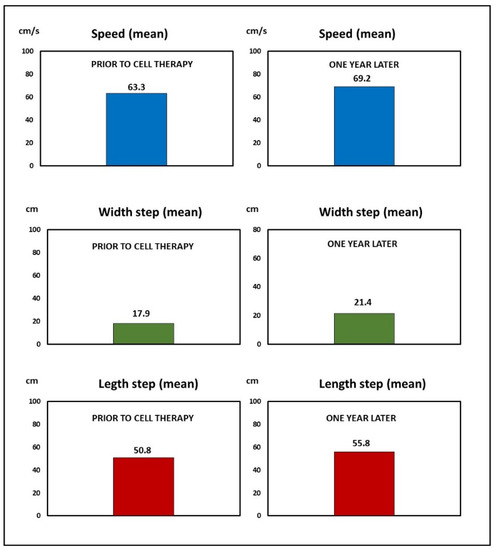
Figure 4.
Case 2: Improvement obtained by cell therapy in the gait analysis.
4. Discussion
Currently, there is no documented scientific evidence on the usefulness of cell therapy in neurosurgical sequels of the spinal cord. In the recent literature, there are many reviews on the usefulness of cell therapy to treat neurological sequels produced after a spinal cord injury [23,24,25], but none specifically consider the benefits that this type of therapy can have in patients with neurological lesions in the spinal cord derived from a neurosurgical intervention. The reviews have mainly considered lesions produced by trauma from falls, traffic accidents, and sports injuries, among others [1,2,3]. To explain the increase of sensory and motor function after cell therapy in patients with SCI, reviewers have mainly analyzed the use of appropriate cell types, transplantation timing, and delivery route with larger sample sizes and suitable control groups [24,25], but with little attention paid to the origin of the trauma.
We can also find studies in patients with SCI in which cell therapy has been shown to be safe, and scientific evidence of sensory and motor improvement has also been demonstrated. Among these studies, we can find both the combined use of Mesenchymal stem cells (MSCs) and Schwann cells (SCs) [16] as well as the use of other types of cells such as olfactory ensheathing cells [26,27]. In all cases, cell therapy is safe in patients, even in studies with a long follow-up period [27]. The authors consider that we should be cautious, even if neurological improvement is obtained, because a larger number of patients is required for a definitive evaluation of the therapy.
In our study, the cases presented demonstrated that intrathecal cell therapy with NC1 medicament may be useful in chronic neurologic sequels due to spinal cord surgery. Disturbance in gait is one of the most frequent sequels of spinal cord surgery. After cell therapy, its improvement can be perceived early by patients, and can be documented objectively. Persistence of the improvement supports a maintained benefit of cell therapy.
In Case 1, neuropathic pain was a permanent sequel for more than 10 years after surgery and was decreased significantly by cell therapy. This supports previous observations by us showing that intrathecal cell therapy with MSCs can be useful for the control of neuropathic pain, possibly through the release of Transforming Growth Factor-beta 1 (TGF-β1) by transplanted MSCs [28].
Improvement in bowel dysfunction has been consistently reported in chronic incomplete paraplegic patients who have received repeated intrathecal administrations of MSCs [8,9], a finding recently documented by objective studies [29]. The improvement in bowel dysfunction was confirmed in Case 2, supporting again the long-term benefit of cell therapy.
With the results obtained, it seems clear that intrathecal administration of MSCs may be useful in neurological sequels arising as complications of spinal surgery, even when these sequels have had a long time for their evolution. In addition, the administered therapy with NC1 medicament appears to have permanent benefit, at least three years after cell therapy treatment.
Sometimes, trials in cell therapy do not include a suitable number of participants. As has already been described in the literature, many variables may contribute to the low number; for example, a small target population and the need to establish homogeneous groups to try to avoid allocation bias and tendency results [25].
Other essential questions to clarify for the clinical application of MSCs therapy include the heterogeneity of MSCs and their susceptibility in different culture conditions in vitro [23], as well as the heterogeneity of the types and origins of spinal cord injuries.
From the preliminary results obtained in this study, it is necessary to develop a clinical trial in a homogeneous group of patients with spinal cord injuries because of surgery, with established and solid criteria to help us verify the benefit of this type of therapy.
5. Conclusions
Neurological sequels persist in many patients following surgery for benign lesions affecting the spinal cord. Complications are often incurable and are generally considered justifiable and unavoidable, without therapeutic strategies other than rehabilitation. Translational research is considered key to bringing investigational treatments closer to availability for patients who otherwise would not be able to access therapies for their diseases. Treatment with autologous mesenchymal stromal cells, through administration of NC1 medicament, could be a new therapeutic tool to provide considerable improvements in the quality of life of patients with incurable neurological complications after spinal cord surgery.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/reports5040043/s1. File S1: Data on the NC1 medicament.
Author Contributions
M.Z. coordinated the research, conceived, and designed the study, and elaborated the manuscript with final decisions about editing. C.B. actively participated in the research and in the elaboration and editing of the manuscript. C.A., P.M., S.D.L.C., N.R. and E.M. carried out the manufacture of the drug and the quality controls associated with the drug NC1. M.Z. performed the evaluation of patients as candidates to receive cell therapy. C.F.-M. performed the administration of the NC1 drug by lumbar puncture and evaluation of patients. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with Cell Therapy Unit of the Puerta de Hierro–Majadahonda Hospital, it was approved for hospital use (registration number 83796).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, and written informed consent was obtained from the patients to publish this paper.
Acknowledgments
The present study was supported by the development of our cell therapy program, in particular, the Mapfre Foundation and Rafael del Pino Foundation.
Conflicts of Interest
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- Furlan, J.C.; Sakakibara, B.M.; Miller, W.C.; Krassioukov, A.V. Global incidence and prevalence of traumatic spinal cord injury. Can. J. Neurol. Sci. 2013, 40, 456–464. [Google Scholar] [CrossRef]
- National Spinal Cord Injury Statistical Center. Traumatic Spinal Cord Injury Facts and Figures at a Glance; University of Alabama at Birmingham: Birmingham, AL, USA, 2022. [Google Scholar]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, Y.; Vogel, L.C.; Devivo, M.J. Causes of spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2013, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prokopienko, M.; Kunert, P.; Podgórska, A.; Marchel, A. Surgical treatment of intramedullary ependymomas. Neurol. Neurochir. Pol. 2017, 51, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Stillerman, C.B.; Chen, T.C.; Couldwell, W.T.; Zhang, W.; Weiss, M.H. Experience in the surgical management of 82 symptomatic herniated thoracic discs and review of the literature. J. Neurosurg. 1998, 88, 623–633. [Google Scholar] [CrossRef]
- Börm, W.; Bäzner, U.; König, R.W.; Kretschmer, T.; Antoniadis, G.; Kandenwein, J. Surgical treatment of thoracic disc herniations via tailored posterior approaches. Eur. Spine J. 2011, 20, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Fernández, C.; Tapiador, N.; Sevilla, M.; Morejón, C.; Montilla, J.; et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy 2017, 19, 349–359. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Bonilla, C.; Marin, E.; Tapiador, N.; Sevilla, M.; Vazquez, D.; Carballido, J.; et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy 2018, 20, 806–819. [Google Scholar] [CrossRef]
- Saini, R.; Pahwa, B.; Agrawal, D.; Singh, P.K.; Gujjar, H.; Mishra, S.; Jagdevan, A.; Misra, M.C. Efficacy and outcome of bone marrow derived stem cells transplanted via intramedullary route in acute complete spinal cord injury—A randomized placebo controlled trial. J. Clin. Neurosci. 2022, 100, 7–14. [Google Scholar] [CrossRef]
- Smirnov, V.A.; Radaev, S.M.; Morozova, Y.V.; Ryabov, S.I.; Yadgarov, M.Y.; Bazanovich, S.A.; Lvov, I.S.; Talypov, A.E.; Grin’, A.A. Systemic Administration of Allogeneic Cord Blood Mononuclear Cells in Adults with Severe Acute Contusion Spinal Cord Injury: Phase 1/2a Pilot Clinical Study-Safety and Primary Efficacy Evaluation. World Neurosurg. 2022, 161, e319–e338. [Google Scholar] [CrossRef]
- Tang, Q.R.; Xue, H.; Zhang, Q.; Guo, Y.; Xu, H.; Liu, Y.; Liu, J.M. Evaluation of the Clinical Efficacy of Stem Cell Transplantation in the Treatment of Spinal Cord Injury: A Systematic Review and Meta-Analysis. Cell Transplant. 2021, 30, 9636897211067804. [Google Scholar] [CrossRef] [PubMed]
- Larocca, T.F.; Macêdo, C.T.; Souza, B.; Andrade-Souza, Y.M.; Villarreal, C.F.; Matos, A.C.; Silva, D.N.; da Silva, K.N.; de Souza, C.; Paixão, D.; et al. Image-guided percutaneous intralesional administration of mesenchymal stromal cells in subjects with chronic complete spinal cord injury: A pilot study. Cytotherapy 2017, 19, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Tang, F.; Zhao, Y.; Han, G.; Yin, N.; Li, X.; Chen, B.; Han, S.; Jiang, X.; Yun, C.; et al. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transplant. 2018, 27, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Oraee-Yazdani, S.; Akhlaghpasand, M.; Golmohammadi, M.; Hafizi, M.; Zomorrod, M.S.; Kabir, N.M.; Oraee-Yazdani, M.; Ashrafi, F.; Zali, A.; Soleimani, M. Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: Safety considerations and possible outcomes. Stem Cell Res. Ther. 2021, 12, 445. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pang, M.; Du, C.; Liu, Z.Y.; Chen, Z.H.; Wang, N.X.; Zhang, L.M.; Chen, Y.Y.; Mo, J.; Dong, J.W.; et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: A phase 1/2 pilot study. Cytotherapy 2021, 23, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Montilla, J.; Bustamante, S.; Carballido, J.; Marin, E.; Martinez, F.; et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016, 18, 1025–1036. [Google Scholar] [CrossRef]
- Woodforde, J.M.; Merskey, H. Some relationships between subjective measures of pain. J. Psychosom. Res. 1972, 16, 173–178. [Google Scholar] [CrossRef]
- Krogh, K.; Christensen, P.; Sabroe, S.; Laurberg, S. Neurogenic bowel dysfunction score. Spinal Cord. 2006, 44, 625–631. [Google Scholar] [CrossRef]
- Geffner, L.F.; Santacruz, P.; Izurieta, M.; Flor, L.; Maldonado, B.; Auad, A.H.; Montenegro, X.; Gonzalez, R.; Silva, F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transplant. 2008, 17, 1277–1293. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Wang, X.R.; Li, M.M.; Tao, Z.H.; Teng, W.W.; Saijilaf. Mesenchymal Stromal Cell Therapy in Spinal Cord Injury: Mechanisms and Prospects. Front. Cell. Neurosci. 2022, 16, 862673. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, F.; Barati, S. Effects of mesenchymal stem cell transplantation on spinal cord injury patients. Cell Tissue Res. 2022. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, L.T.; Macêdo, C.T.; Damasceno, P.; das Neves, Í.; de Lima, C.S.; Santos, G.C.; de Santana, T.A.; Sampaio, G.; Silva, D.N.; Villarreal, C.F.; et al. Clinical Trials Using Mesenchymal Stem Cells for Spinal Cord Injury: Challenges in Generating Evidence. Cells 2022, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Tabakow, P.; Jarmundowicz, W.; Czapiga, B.; Fortuna, W.; Miedzybrodzki, R.; Czyz, M.; Huber, J.; Szarek, D.; Okurowski, S.; Szewczyk, P.; et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013, 22, 1591–1612. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sim, A.; Féron, F.; Cochrane, J.; Bassingthwaighte, L.; Bayliss, C.; Davies, W.; Fronek, P.; Gray, C.; Kerr, G.; Licina, P.; et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain 2008, 131, 2376–2386. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Aguayo, C.; Fernández, C.; Gutiérrez, R.; Rodríguez-Boto, G.; Saab, A.; Hassan, R.; Ortega, C. Intrathecal administration of autologous bone marrow stromal cells improves neuropathic pain in patients with spinal cord injury. Neurosci. Lett. 2018, 670, 14–18. [Google Scholar] [CrossRef]
- Guadalajara Labajo, H.; León Arellano, M.; Vaquero Crespo, J.; Valverde Núñez, I.; García-Olmo, D. Objective demonstration of improvement of neurogenic bowel dysfunction in a case of spinal cord injury following stem cell therapy. J. Surg. Case Rep. 2018, 2018, rjy300. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).