Abstract
A high risk of developing insulin resistance (IR) and, eventually, type 2 diabetes mellitus (T2DM) is associated with chronic hepatitis C virus (HCV). Multiple mechanisms can account for the development of IR in chronic HCV patients, steatosis or fatty liver that can lead to metabolic syndrome, and the inflammatory process associated with the presence of HCV infection. In this article, we analyze the reported values of homoeostasis model assessment (HOMA-IR) before and after successful direct-acting agents (DAAs) treatment in the literature (23 studies) at certain intervals, respectively 12, 24, and 52 weeks depending on the presence of T2DM among patients. The meta-analysis showed improvement of IR in most cases except for three studies that presented a minimal increase in HOMA-IR value for the non-T2DM group at the 12- and 24-week check-ups possibly hinting at a prediabetes group. All other studies showed an important decrease in HOMA-IR post-DAA treatment specifically for the T2DM group. The most significant change in HOMA-IR values was noticed after 24 weeks in all categories. Our meta-analysis showed that clearance of HCV leads to improvement of IR, especially in the case of patients with T2DM.
1. Introduction
A group of metabolic risk factors known as metabolic syndrome raises the risk of both type 2 diabetes mellitus (T2DM) and atherosclerotic cardiovascular disease. It is believed that insulin resistance (IR) contributes to the onset of metabolic syndrome [1,2,3]. Central obesity, high blood pressure, reduced high-density lipoprotein (HDL) cholesterol, elevated fasting glucose, and increased triglyceride levels are all included in a recently proposed description of metabolic syndrome [1].
According to clinical definitions, IR is a disease that makes cells less responsive to an established amount of insulin, necessitating larger doses of insulin to produce the same cellular response [4]. IR is evaluated using insulin’s capacity to regulate blood sugar levels. IR is influenced by both environmental and genetic variables, and it is crucial for the pathophysiology of diabetes. One approach for calculating IR, which is computed as insulin (U/m) [fasting glucose (mmol/L)/22.5], is the homoeostasis model assessment (HOMA-IR). For IR examination, a 12-h overnight fast is necessary [5].
Chronic hepatitis C (HCV) infection is one of the major global health problems. Metabolic disturbances are frequent in HCV-infected patients. Epidemiological studies underlined the concept that HCV infection is an independent predictor of insulin resistance (IR) and diabetes mellitus (DM). There are enough pieces of evidence that type 2 diabetes mellitus (T2DM) are widespread among chronic HCV-infected patients compared to patients with other liver disease aetiology [6,7]. One plausible explanation for this observation is that HCV infection, or the inflammatory response to infection, contributes to the development of IR and, as a result, to an increased risk of T2DM [8].
Multiple pathogenic mechanisms can play a contributing factor in producing and exacerbating IR in chronic HCV patients. Steatosis of the liver, pre-existing disorders including metabolic syndrome, and chronic inflammatory processes are some of the variables that can affect the emergence and severity of IR in HCV-infected patients [9]. Steatosis affects more than half of chronic HCV-infected patients, and many studies linked it to IR [10,11]. High HCV RNA levels are associated with the rapid progression of IR over time [12]. Moreover, HCV clearance after antiviral therapy improves IR. Furthermore, it was shown that IR is genotype-dependent (especially 1 and 4) [13,14].
HOMA-IR is the homeostatic model assessment of insulin resistance (HOMA-IR) and is one of the most frequently used methods of determining insulin resistance in large population-based studies since it is mathematically derived from single fasting glucose and insulin measurements.
HOMA-IR was widely used to monitor longitudinal changes in IR in individuals with type 2 diabetes. The index is also useful in non-diabetics for comparison of IR among individuals with abnormal glucose tolerance and to assess longitudinally the development of impaired glucose tolerance [15].
HOMA-IR is based on the feedback loop of glucose and insulin after it had been assimilated by the cells and therefore is more representative of hepatic glucose output and hepatic insulin resistance.
Longitudinal kinetics of the HOMA-IR after HCV clearance show a descendent trend over time in concordance with a decrease in liver stiffness and extrahepatic manifestations, and improvement in liver histology and biochemical liver markers [16,17]. Therefore, the current meta-analysis aims to demonstrate the improvement of IR in patients with HCV treated with direct-acting agents (DAAs) that attained HCV clearance and to further analyze how the HOMA-IR levels modify in a time-dependent manner after HCV clearance in diabetic and non-diabetic patients.
2. Materials and Methods
We performed a search in the online journal databases Embase, PUBMED, and Google Scholar with several search strings (using the keywords “Hepatitis C”, “DAA”, “HOMA-IR”, “Diabetes”) to find studies that report the changes in insulin resistance as depicted by HOMA-IR in hepatitis C patients, that had undergone DAA treatment, with or without type 2 diabetes mellitus (T2DM). The initial search yielded 390 results. Of the 390 studies, 18 were not written in English, and 57 were excluded by design (45 were reviews, 4 were editorials, 3 were meta-analyses, 2 were trial protocols, and 3 were conference abstracts). After screening study titles for retrieval, we excluded 32 studies as a duplicate and 283 abstracts were retrieved. After the abstracts were analyzed, we excluded 230 studies as they do not mention HOMA-IR levels. We further read the full text and excluded 30 studies as they did not report HOMA-IR levels. The workflow of the study selection followed PRISMA guidelines and is presented in Figure 1. We selected 23 studies that combined and evaluated 6073 patients that had undergone DAA treatment and obtained SVR (Table 1).
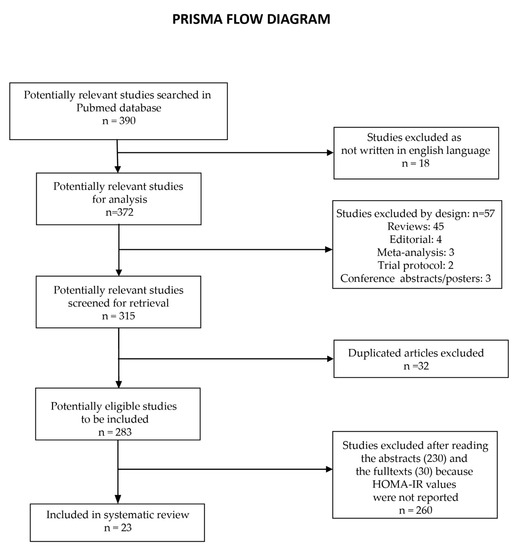
Figure 1.
PRISMA workflow of the study selection process.

Table 1.
Studies included in the meta-analysis.
2.1. Quality Assessment
This meta-analysis assumes a bias risk and is based on observational and experimental studies that may be clinical or preclinical. The transferability of study results, which is linked to study comparability, can be hampered by several problems.
The comparability of the initial investigations was weakened by the inclusion of cell lines in addition to human subjects. Second, varied observation durations, research methodologies, and treatment approaches were used depending on the population examined as well as the sample size. Additionally, we did not include any non-English papers that might have had crucial data. Furthermore, we only searched the PUBMED Central database for relevant articles; however, other databases may have additional studies that were not retrieved. Due to all of these factors, this meta-analysis cannot be completely devoid of bias; therefore, the interpretations of the meta-analysis findings are constrained.
2.2. Data Extraction
The following information was extracted from each study: Publication data, year of publication, number of cases, treatment type, weeks passed after treatment when HOMA-IR was determined, type of study design (non-T2DM, T2DM, Mixed), HOMA-IR values before and after DAA treatment. The data were imported in STATA statistical software (StataCorp. LLC, Texas, TX, USA) and we used the meta-analysis procedure to compute effect sizes of HOMA-IR decrease after DAA treatment as expressed by Hedges’s g and heterogeneity between the studies. The same procedure generated the forest plots showing individual studies and pooled effect sizes overall and for various subgroups (based on the time passed from DAA treatment and diabetic status).
3. Results
Sixty studies were included in the meta-analysis. Most of the studies showed a decrease in HOMA-IR after DAA treatment, even if a few showed a weak increase in non-T2DM patients [18,19,20]. We stratified the studies according to the time passed after DAA treatment to use a significant amount of information as possible.
The subgroup analysis showed that overall the HOMA-IR decrease was significant after DAA treatment (Hedges’s g = 0.983). A fairly important decrease in HOMA-IR was observed after 12 weeks after DAA treatment; even if the maximum decrease was observed after 24 weeks, it was not statistically significant. Depending on the diabetic status it is a clear difference between T2DM patients, in which the HOMA-IR decrease (Hedges’s g = 1.055) was significantly greater than in non-T2DM patients (Hedges’s g = 0.658) (Table 2).

Table 2.
Subgroup meta-analysis summary, using the random-effects model of HOMA-IR decrease after DAA treatment.
After 12 weeks after DAA treatment, the decrease in HOMA-IR was weak in non-T2DM patients (Hedges’s g = 0.40) but strong in T2DM patients (Hedges’s g = 1.05). We only had three studies that enrolled only T2DM patients and they were assessed at 12 weeks after DAA treatment (Figure 2). After 24 weeks after DAA treatment, the decrease was still significantly greater in mixed patients (diabetic and non-diabetic) studies (Hedges’s g = 1.73) compared with non-T2DM patients (Hedges’s g = 0.92) (Figure 3). After 52 weeks after DAA treatment, we observe a lower difference in HOMA-IR decrease between mixed patients (Hedges’s g = 1.70) and non-T2DM patients (Hedges’s g = 0.70) (Figure 4). Of note, studies with mixed patients had a significantly greater heterogeneity than studies with only non-T2DM patients.
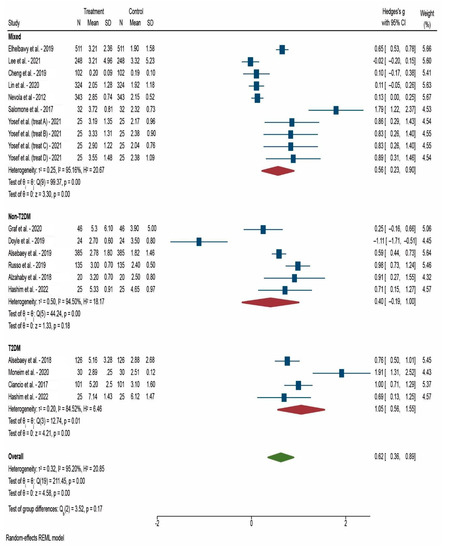
Figure 2.
Forest plot of meta-analysis of studies that reported HOMA-IR at 12 weeks after DAA treatment [24,26,29,31,33,34,35,36,38,39,40]. N: Number of participants in the study; SD: Standard deviation.
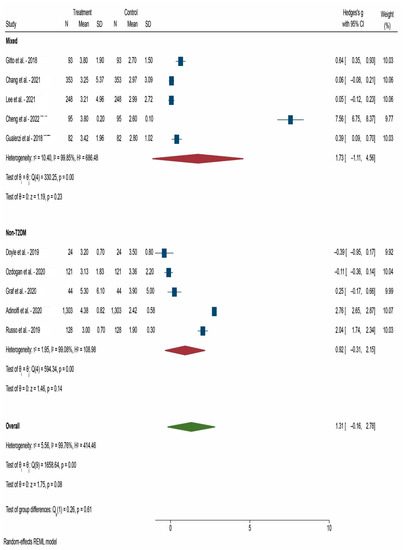
Figure 3.
Forest plot of meta-analysis of studies that reported HOMA-IR at 24 weeks after DAA treatment [18,23,24,25,26,27,28,31,33,37]. N: Number of participants in the study; SD: Standard deviation.
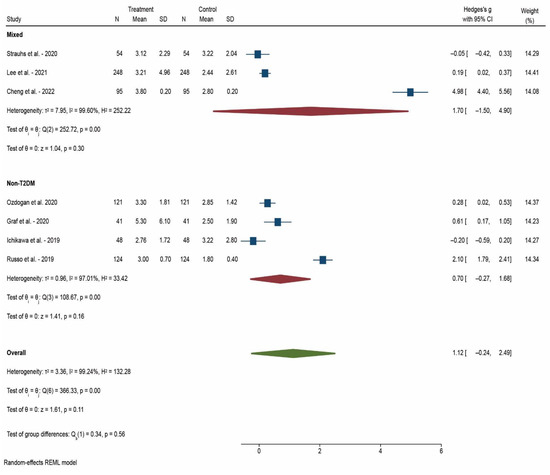
Figure 4.
Forest plot of meta-analysis of studies that reported HOMA-IR at 52 weeks after DAA treatment [19,20,22,23,30,31,33]. N: Number of participants in the study; SD: Standard deviation.
4. Discussion
The present meta-analysis included 23 studies of changes in IR, reflected by HOMA-IR levels, after successful clearance of HCV infection by DAA treatments. The pooled analysis showed a higher HOMA-IR reduction after DAA treatment in diabetic compared with non-diabetic patients.
HCV-infected patients are more likely to develop IR and, as a result, T2DM. The mechanisms underlying insulin resistance are considered multifactorial. The development of IR involves glucose consumption in skeletal muscle and glucose production in liver cells.
Recent research has revealed some of the mechanisms underlying HCV-induced IR, including liver steatosis, chronic inflammation, and metabolic disorders, such as metabolic syndrome. It was shown that increased intrahepatic TNF-α that occurs in HCV infection causes insulin resistance by suppressing insulin-induced tyrosine phosphorylation of insulin receptor substrate 1 in liver cells [7]. Moreover, TNF-α inhibits phosphatidylinositol 3 kinase, which results in a reduction in the body cell’s ability to use glucose.
According to a few studies, the reported prevalence of IR in HCV-infected individuals has increased from 30% to 70%, regardless of the severity of hepatic disease [30]. The HCV core protein blocks the glucose transporter 2. Tumor necrosis factor, which is released by HCV and inhibits phosphatidylinositol 3 kinase and the insulin receptor substrate, results in a reduction in the cell’s ability to use glucose. Moreover, IR development has been connected to stress and malfunction in the endoplasmic reticulum, and a consequence of IR is mitochondrial dysfunction. Steatosis, fibrosis, cardiovascular consequences (such as atherosclerosis), type 2 diabetes, and hepatocellular cancer are some of the clinical results of IR [41].
Hepatogenous diabetes is T2DM caused by cirrhosis. To better determine the effect of HCV eradication, classification according to hepatogenous or hereditary T2DM would be beneficial [42], but this is not always possible since hepatogenous diabetes does not have a standard definition, even in international guidelines.
HCV infection clearance has been shown to improve IR, with HOMA-IR levels significantly improving in antiviral therapy responders compared to non-responders. Later findings suggest that HCV infection directly promotes IR by reducing insulin signaling mechanisms and that DAA therapy has a beneficial effect in restoring insulin sensitivity in HCV-infected patients [33].
Recently, it was reported in patients with liver carcinoma that viral clearance improved IR in patients who had baseline IR, whilst it increased HOMA-IR in those without baseline IR [43]. One possible explanation is that HCV induces the baseline IR by impairing glucose metabolism [44].
The improvement of HOMA-IR levels was dependent on the time that passed after the successful clearance of HCV infection, the values were decreasing even more over time. The explanation of this descendent trend can be that even if the fasting glucose levels did not significantly decrease compared with the baseline through 1 year of monitoring, insulin levels progressively decreased after the treatment. Increased peripheral resistance to insulin activity, which is induced by the proinflammatory cytokine milieu caused by chronic hepatic injury, is one of the main drivers of IR in HCV-infected patients [45]. As a result, the considerable decrease in IR post-SVR may be explained by the decrease in hepatic inflammation that happens after HCV eradication.
Insulin sensitivity improves independently of weight loss, confirming HCV-related chronic hepatitis as a separate risk factor for the development of IR [14]. High BMI significantly decreased the likelihood of IR improvement. Obesity is a hallmark of metabolic syndrome and has been linked to IR [46,47]. In the DAA era, longer follow-up studies are necessary to elucidate this problem. Patients with HCV who achieve SVR but remain overweight and/or insulin resistant may be at an increased risk of long-term liver and non-liver complications. Therefore, in these patients, IR should be managed by dietary intervention (a combination between calorie restriction and reduction in carbohydrates with high glycemic index) [48] and in selected cases by pharmacological interventions [49].
The benefits of obtaining SVR were observed in our meta-analysis, particularly in patients with T2DM, which presented that HOMA-IR values decrease significantly greater than in non-T2DM patients (Hedges’s g = 1.055) vs. (Hedges’s g = 0.658). Some studies reported an insignificant reduction in HOMA-IR after HCV clearance or even an increase. These results can be confounded by the distribution of HCV genotypes in the studied populations and by the lack of subgroup analysis of diabetic and non-diabetic patients. Furthermore, we used only HOMA-IR to measure IR, while other markers of IR were not analyzed (e.g., insulin-like growth factor-1) [50].
Our meta-analysis has some limitations. First, there are factors which were not taken into consideration, such as age, gender, and body mass index primarily due to the unavailability of these data in the original studies. Second, the limited information in the case of the mixed cohort lots with T2DM and non-T2DM patients were not individually evaluated.
Third, the measurement of the HOMA-IR index itself, similar to any laboratory test is prone to pre-analytical and analytical laboratory errors. Moreover, in real populations, some individuals can secrete more insulin regardless of an insulin sensitivity level and can have lower rates of insulin clearance [51]. Therefore, for these individuals, fasting indices can overestimate insulin resistance. To confirm and clarify our results, further studies are required. There is a need for a more complete understanding of the impact of IR on DAA treatments’ success rate and the dynamics of IR after successful HCV clearance.
5. Conclusions
The meta-analysis showed the improvement of IR in the majority of studies except for a few non-T2DM studies that could contain a pre-diabetic subgroup, noted by the slight increase in HOMA-IR value. Unlike the non-T2DM and mixed studies, T2DM presented an important decrease in HOMA-IR post-DAA treatment specifically. The most significant change in HOMA-IR values was noticed after 24 weeks in all categories after DAA treatment had successfully ended.
Author Contributions
M.-S.P. and P.M. contributed to the conceptualization, methodology, investigation, and validation; D.-C.P., A.I.D., A.O., D.-M.F., V.P., C.M.M. and R.M. contributed to the data curation, investigation, formal analysis, and supervision; A.R., D.N.M., D.C. and A.O.D. contributed to the validation, formal analysis, supervision, and software; M.-S.P., D.-M.F., V.P., C.M.M., R.M. and D.N.M. contributed to writing—original draft preparation; A.I.D., A.O, A.R., D.C., A.O.D. and P.M. contributed to writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The dataset presented in this study is available from the corresponding author upon reasonable request.
Acknowledgments
This work is part of the special part of PhD thesis of Marian Sorin Popescu.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lemieux, I.; Després, J.P. Metabolic Syndrome: Past, Present and Future. Nutrients 2020, 12, 3507. [Google Scholar] [CrossRef] [PubMed]
- Bence, K.K.; Birnbaum, M.J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 2021, 50, 101143. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Parveen, B.; Zahiruddin, S.; Parveen, R.; Khan, M.A.; Gupta, A.; Ahmad, S. Uplc/Ms Based Phytochemical Screening and Antidiabetic Properties of Picrorhiza Kurroa in Mitigating Glucose-Induced Metabolic Dysregulation and Oxidative Stress. Farmacia 2021, 69, 749–755. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the Paradox of Hepatic Insulin Resistance. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Farshadpour, F.; Taherkhani, R.; Ravanbod, M.R.; Eghbali, S.S. Prevalence and Genotype Distribution of Hepatitis C Virus Infection among Patients with Type 2 Diabetes Mellitus. Med. Princ. Pract. 2018, 27, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Kralj, D.; Virović Jukić, L.; Stojsavljević, S.; Duvnjak, M.; Smolić, M.; Čurčić, I.B. Hepatitis C Virus, Insulin Resistance, and Steatosis. J. Clin. Transl. Hepatol. 2016, 4, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, F.; Orsi, A.; Sticchi, L.; Bruzzone, B.; Icardi, G. Hepatitis C virus in the new era: Perspectives in epidemiology, prevention, diagnostics and predictors of response to therapy. World J. Gastroenterol. 2014, 20, 9633–9652. [Google Scholar] [CrossRef]
- Narne, P. Impaired insulin exocytosis in chronic hepatitis C infection: Contributory role of p38δ MAPK-protein kinase D-golgi complex axis. Clin. Sci. 2020, 134, 1449–1456. [Google Scholar] [CrossRef]
- Himoto, T.; Nomura, T.; Tani, J.; Miyoshi, H.; Morishita, A.; Yoneyama, H.; Haba, R.; Masugata, H.; Masaki, T. Exacerbation of insulin resistance and hepatic steatosis deriving from zinc deficiency in patients with HCV-related chronic liver disease. Biol. Trace Elem. Res. 2015, 163, 81–88. [Google Scholar] [CrossRef]
- Abenavoli, L.; Masarone, M.; Peta, V.; Milic, N.; Kobyliak, N.; Rouabhia, S.; Persico, M. Insulin resistance and liver steatosis in chronic hepatitis C infection genotype 3. World J. Gastroenterol. 2014, 20, 15233–15240. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, K.; Jabłonowska, E.; Omulecka, A.; Piekarska, A. Insulin resistance, adipokine profile and hepatic expression of SOCS-3 gene in chronic hepatitis C. World J. Gastroenterol. 2014, 20, 10449–10456. [Google Scholar] [CrossRef]
- Chien, C.H.; Lin, C.L.; Hu, C.C.; Chang, J.J.; Chien, R.N. Clearance of Hepatitis C Virus Improves Insulin Resistance During and After Peginterferon and Ribavirin Therapy. J. Interferon. Cytokine Res. 2015, 35, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.E.; Nevola, R.; Guerrera, B.; D’Alterio, G.; Marrone, A.; Giordano, M.; Rinaldi, L. Hepatitis C virus clearance by direct-acting antiviral treatments and impact on insulin resistance in chronic hepatitis C patients. J. Gastroenterol. Hepatol. 2018, 33, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Stasi, C.; Sadalla, S.; Carradori, E.; Monti, M.; Petraccia, L.; Madia, F.; Gragnani, L.; Zignego, A.L. Longitudinal evaluation of liver stiffness and outcomes in patients with chronic hepatitis C before and after short- and long-term IFN-free antiviral treatment. Curr. Med. Res. Opin. 2020, 36, 245–249. [Google Scholar] [CrossRef]
- Stasi, C.; Triboli, E.; Arena, U.; Urraro, T.; Petrarca, A.; Gragnani, L.; Laffi, G.; Zignego, A.L. Assessment of liver stiffness in patients with HCV and mixed cryoglobulinemia undergoing rituximab treatment. J. Transl. Med. 2014, 12, 21. [Google Scholar] [CrossRef]
- Adinolfi, L.E.; Petta, S.; Fracanzani, A.L.; Nevola, R.; Coppola, C.; Narciso, V.; Rinaldi, L.; Calvaruso, V.; Pafundi, P.C.; Lombardi, R.; et al. Reduced incidence of type 2 diabetes in patients with chronic hepatitis C virus infection cleared by direct-acting antiviral therapy: A prospective study. Diabetes Obes. Metab. 2020, 22, 2408–2416. [Google Scholar] [CrossRef]
- Cheng, P.N.; Chen, J.Y.; Chiu, Y.C.; Chiu, H.C.; Tsai, L.M. Augmenting central arterial stiffness following eradication of HCV by direct acting antivirals in advanced fibrosis patients. Sci. Rep. 2019, 9, 1426. [Google Scholar] [CrossRef]
- Ichikawa, T.; Miyaaki, H.; Miuma, S.; Motoyoshi, Y.; Narita, S.; Toda, S.; Takahashi, Y.; Honda, T.; Yajima, H.; Uehara, R.; et al. Carotid Intima-media Thickness and Small Dense Low-density Lipoprotein Cholesterol Increase after One Year of Treatment with Direct-acting Antivirals in Patients with Hepatitis C Virus Infection. Intern. Med. 2019, 58, 1209–1215. [Google Scholar] [CrossRef]
- Strauhs-Nitsch, L.; Campiolo, M.F.; Morsoletto, D.B.G.; Pissaia Junior, A.; Ivantes, C.A.P. Curing hepatitis c with the new direct acting antivirals did not improve insulin resistance after one year. Arq. Gastroenterol. 2020, 57, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Elhelbawy, M.; Abdel-Razek, W.; Alsebaey, A.; Hashim, M.; Elshenawy, H.; Waked, I. Insulin resistance does not impair response of chronic hepatitis C virus to direct-acting antivirals, and improves with the treatment. Eur. J. Gastroenterol. Hepatol. 2019, 31, 16–23. [Google Scholar] [CrossRef]
- Özdoğan, O.; Yaraş, S.; Ateş, F.; Üçbilek, E.; Sezgin, O.; Altıntaş, E. The impact of direct-acting antiviral treatment on lipid metabolism and insulin resistance in chronic hepatitis C patients: Temporary? Permanent? Turk. J. Gastroenterol. 2020, 31, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Welzel, T.; Bogdanou, D.; Vermehren, J.; Beckel, A.; Bojunga, J.; Friedrich-Rust, M.; Dietz, J.; Kubesch, A.; Mondorf, A.; et al. Hepatitis C Clearance by Direct-Acting Antivirals Impacts Glucose and Lipid Homeostasis. J. Clin. Med. 2020, 9, 2702. [Google Scholar] [CrossRef]
- Gitto, S.; Cicero, A.F.G.; Loggi, E.; Giovannini, M.; Conti, F.; Grandini, E.; Guarneri, V.; Scuteri, A.; Vitale, G.; Cursaro, C.; et al. Worsening of Serum Lipid Profile after Direct Acting Antiviral Treatment. Ann. Hepatol. 2018, 17, 64–75. [Google Scholar] [CrossRef]
- Doyle, M.-A.; Galanakis, C.; Mulvihill, E.; Crawley, A.; Cooper, C.L. Hepatitis C Direct Acting Antivirals and Ribavirin Modify Lipid but not Glucose Parameters. Cells 2019, 8, 252. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-L.; Hu, J.-H.; Pao, L.-H.; Lin, M.-S.; Kuo, C.-J.; Chen, S.-C.; Fan, C.-M.; Chang, M.-Y.; Chien, R.-N. Critical role of triglycerides for adiponectin levels in hepatitis C: A joint study of human and HCV core transgenic mice. BMC Immunol. 2021, 22, 54. [Google Scholar] [CrossRef]
- Lee, H.; Chien, R.N.; Pao, L.H.; Kuo, C.J.; Huang, P.H.; Chang, M.L. Decoupled Glucose and Lipid Metabolic Recovery after Viral Clearance in Direct-Acting Antiviral-Treated HCV Patients: A 3-Year Prospective Cohort Study. Cells 2021, 10, 2934. [Google Scholar] [CrossRef]
- Alsebaey, A.; Elhelbawy, M.; Abdel-Razek, W.; Hashim, M.; Elshenawy, H.; Waked, I. HCV treatment with direct acting antivirals improves the insulin sensitivity. Expert Rev. Anti. Infect. Ther. 2019, 17, 749–754. [Google Scholar] [CrossRef]
- Abdel Moneim, A.; Suleiman, H.A.; Mahmoud, B.; Mabrouk, D.; Zaky, M.Y.; Mahmoud, B. Viral clearance ameliorates hematological and inflammatory markers among diabetic patients infected with hepatitis C genotype 4. Clin. Exp. Med. 2020, 20, 231–240. [Google Scholar] [CrossRef]
- Cheng, P.-N.; Sun, H.-Y.; Feng, I.C.; Chiu, Y.-C.; Wang, S.-T.; Tan, D.C.; Chiu, H.-C.; Chien, S.-C.; Young, K.-C. Interdependence of glycemic and lipid modulation in cured chronic hepatitis C patients by direct-acting antiviral agents. J. Microbiol. Immunol. Infect. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, A.; Bosio, R.; Bo, S.; Pellegrini, M.; Sacco, M.; Vogliotti, E.; Fassio, G.; Bianco Mauthe Degerfeld, A.G.F.; Gallo, M.; Giordanino, C.; et al. Significant improvement of glycemic control in diabetic patients with HCV infection responding to direct-acting antiviral agents. J. Med. Virol. 2018, 90, 320–327. [Google Scholar] [CrossRef]
- Russo, F.P.; Zanetto, A.; Gambato, M.; Bortoluzzi, I.; Al Zoairy, R.; Franceschet, E.; De Marchi, F.; Marzi, L.; Lynch, E.N.; Floreani, A.; et al. Hepatitis C virus eradication with direct-acting antiviral improves insulin resistance. J. Viral. Hepat. 2020, 27, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, J.J.; Lee, P.L.; Tung, H.D.; Cheng, C.T.; Kao, H.J.; Wu, Y.H.; Pang, M.G.; Chuang, T.W. Lipid profile changes after direct acting antiviral treatment in different genotypes of chronic hepatitis C virus-infected patients. Adv. Dig. Med. 2021, 8, 139–145. [Google Scholar] [CrossRef]
- Alzahaby, A.A.A.E.-H.; Abdel-Halim, M.M.; Hussien, A.A.E.-s. Effect of direct acting anti-viral agents on insulin resistance in chronic HCV patients. Egypt. J. Hosp. Med. 2018, 72, 4413–4419. [Google Scholar] [CrossRef]
- Nevola, R.; Rinaldi, L.; Zeni, L.; Sasso, F.C.; Pafundi, P.C.; Guerrera, B.; Marrone, A.; Giordano, M.; Adinolfi, L.E. Metabolic and renal changes in patients with chronic hepatitis C infection after hepatitis C virus clearance by direct-acting antivirals. JGH Open 2020, 4, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Gualerzi, A.; Bellan, M.; Smirne, C.; Tran Minh, M.; Rigamonti, C.; Burlone, M.E.; Bonometti, R.; Bianco, S.; Re, A.; Favretto, S. Improvement of insulin sensitivity in diabetic and non diabetic patients with chronic hepatitis C treated with direct antiviral agents. PLoS ONE 2018, 13, e0209216. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.E.; Kandeel, H.T.; Hendy, O.M.; El-Mola, K.; El-Raey, F.M.; Attia, M.S. Effect of new direct-acting antiviral drugs on insulin resistance and glycemic control after treatment of chronic hepatitis C virus infection in type 2 diabetic patients. Al-Azhar Assiut Med. J. 2017, 15, 187. [Google Scholar]
- Salomone, F.; Catania, M.; Montineri, A.; Bertino, G.; Godos, J.; Rizzo, L.; Magrì, G.; Li Volti, G. Hepatitis C virus eradication by direct antiviral agents improves glucose tolerance and reduces post-load insulin resistance in nondiabetic patients with genotype 1. Liver Int. 2018, 38, 1206–1211. [Google Scholar] [CrossRef]
- Yosef, T.; Ibrahim, W.A.; El-Ghandour, A.; Attia, S.; El-Nakeep, S. Effect of different direct-acting antiviral regimens for treatment of nondiabetic hepatitis C virus–infected Egyptian patients on insulin resistance and sensitivity. Egypt. J. Intern. Med. 2021, 33, 45. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Guaraldi, G.; Nascimbeni, F.; Romagnoli, D.; Zona, S.; Targher, G. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease - Evidence from three different disease models: NAFLD, HCV and HIV. World J. Gastroenterol. 2016, 22, 9674–9693. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.A.; Nooh, M.Z.; Elgamal, A.A. Factors Associated with Improved Glycemic Control by Direct-Acting Antiviral Agent Treatment in Egyptian Type 2 Diabetes Mellitus Patients with Chronic Hepatitis C Genotype 4. Diabetes Metab. J. 2017, 41, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-L.; Kuo, C.-J.; Pao, L.-H.; Hsu, C.-M.; Chiu, C.-T. The evolving relationship between adiponectin and insulin sensitivity in hepatitis C patients during viral clearance. Virulence 2017, 8, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-L. Metabolic alterations and hepatitis C: From bench to bedside. World J. Gastroenterol. 2016, 22, 1461. [Google Scholar] [CrossRef]
- Knobler, H.; Malnick, S. Hepatitis C and insulin action: An intimate relationship. World J. Hepatol. 2016, 8, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Brenachot, X.; Ramadori, G.; Ioris, R.M.; Veyrat-Durebex, C.; Altirriba, J.; Aras, E.; Ljubicic, S.; Kohno, D.; Fabbiano, S.; Clement, S.; et al. Hepatic protein tyrosine phosphatase receptor gamma links obesity-induced inflammation to insulin resistance. Nat. Commun. 2017, 8, 1820. [Google Scholar] [CrossRef]
- Pasc, P.; Berdea, D.E.; Dobjanschi, L.; Judea-Pusta, C.T.; Popescu, M.I. Descriptive Analysis of Real-World Medication Patterns and One-Year Outcomes of Acute Coronary Syndrome Patients with Metabolic Syndrome in a Tertiary Care Hospital. Farmacia 2021, 69, 498–508. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Simental-Mendía, L.E.; Sahebkar, A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J. Cell Physiol. 2019, 234, 12385–12392. [Google Scholar] [CrossRef]
- He, X.; Wu, D.; Hu, C.; Xu, T.; Liu, Y.; Liu, C.; Xu, B.; Tang, W. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Thyroid 2019, 29, 359–367. [Google Scholar] [CrossRef]
- Kujawska-Luczak, M.; Szulinska, M.; Skrypnik, D.; Musialik, K.; Swora-Cwynar, E.; Kregielska-Narozna, M.; Markuszewski, L.; Grzymislawska, M.; Bogdanski, P. The influence of orlistat, metformin and diet on serum levels of insulin-like growth factor-1 in obeses women with and without insulin resistance. J. Physiol. Pharmacol. 2018, 69, 737–745. [Google Scholar] [CrossRef]
- Gower, B.A.; Fernández, J.R.; Beasley, T.M.; Shriver, M.D.; Goran, M.I. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes 2003, 52, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).