COVID-19 Outcomes in a US Cohort of Persons Living with HIV (PLWH)
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inciarte, A.; Gonzalez-Cordon, A.; Rojas, J.; Torres, B.; de Lazzari, E.; de la Mora, L.; Martinez-Rebollar, M.; Laguno, M.; Callau, P.; Gonzalez-Navarro, A.; et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: A single-center, prospective observational study. AIDS 2020, 34, 1775–1780. [Google Scholar] [CrossRef]
- Huang, J.; Xie, N.; Hu, X.; Yan, H.; Ding, J.; Liu, P.; Ma, H.; Ruan, L.; Li, G.; He, N.; et al. Epidemiological, Virological and Serological Features of Coronavirus Disease 2019 (COVID-19) Cases in People Living with Human Immunodeficiency Virus in Wuhan: A Population-based Cohort Study. Clin. Infect. Dis. 2020, 73, e2086–e2094. [Google Scholar] [CrossRef]
- Gervasoni, C.; Meraviglia, P.; Riva, A.; Giacomelli, A.; Oreni, L.; Minisci, D.; Atzori, C.; Ridolfo, A.; Cattaneo, D. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2276–2278. [Google Scholar] [CrossRef]
- Byrd, K.M.; Beckwith, C.G.; Garland, J.M.; Johnson, J.E.; Aung, S.; Cu-Uvin, S.; Farmakiotis, D.; Flanigan, T.; Gillani, F.S.; Macias-Gil, R.; et al. SARS-CoV-2 and HIV coinfection: Clinical experience from Rhode Island, United States. J. Int. AIDS Soc. 2020, 23, e25573. [Google Scholar] [CrossRef]
- Calza, L.; Bon, I.; Tadolini, M.; Borderi, M.; Colangeli, V.; Badia, L.; Verucchi, G.; Rossini, G.; Vocale, C.; Gaibani, P.; et al. COVID-19 in patients with HIV-1 infection: A single-centre experience in northern Italy. Infection 2021, 49, 333–337. [Google Scholar] [CrossRef]
- Okoh, A.K.; Bishburg, E.; Grinberg, S.; Nagarakanti, S. COVID-19 pneumonia in patients with HIV—A Case Series. J. Acquir. Immune Defic. Syndr. 2020, 85, e4–e5. [Google Scholar] [CrossRef]
- Shalev, N.; Scherer, M.; LaSota, E.D.; Antoniou, P.; Yin, M.T.; Zucker, J.; Sobieszczyk, M.E. Clinical Characteristics and Outcomes in People Living with Human Immunodeficiency Virus Hospitalized for Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 2294–2297. [Google Scholar] [CrossRef] [PubMed]
- Sigel, K.; Swartz, T.; Golden, E.; Paranjpe, I.; Somani, S.; Richter, F.; De Freitas, J.K.; Miotto, R.; Zhao, S.; Polak, P.; et al. Covid-19 and People with HIV Infection: Outcomes for Hospitalized Patients in New York City. Clin. Infect. Dis. 2020, 71, 2933–2938. [Google Scholar] [CrossRef]
- Collins, L.F.; Moran, C.A.; Oliver, N.T.; Moanna, A.; Lahiri, C.D.; Colasanti, J.A.; Kelley, C.F.; Nguyen, M.L.; Marconi, V.C.; Armstrong, W.S.; et al. Clinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS 2020, 34, 1789–1794. [Google Scholar] [CrossRef]
- Ridgway, J.P.; Farley, B.; Benoit, J.-L.; Frohne, C.; Hazra, A.; Pettit, N.; Pho, M.; Pursell, K.; Saltzman, J.; Schmitt, J.; et al. A Case Series of Five People Living with HIV Hospitalized with COVID-19 in Chicago, Illinois. AIDS Patient Care STDS 2020, 34, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ming, F.; Feng, Y.; Zhang, Q.; Mo, P.; Liu, L.; Gao, M.; Tang, W.; Liang, K. Patterns of HIV and SARS-CoV-2 co-infection in Wuhan, China. J. Int. AIDS Soc. 2020, 23, e25568. [Google Scholar] [CrossRef]
- Boulle, A.; Davies, M.-A.; Hussey, H.; Ismail, M.; Morden, E.; Vundle, Z.; Zweigenthal, V.; Mahomed, H.; Paleker, M.; Pienaar, D.; et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2020, 73, e2005–e2015. [Google Scholar] [CrossRef]
- Miyashita, H.; Kuno, T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV Med. 2021, 22, e1–e2. [Google Scholar] [CrossRef]
- Tesoriero, J.M.; Swain, C.-A.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living with or without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef]
- Geretti, A.M.; Stockdale, A.J.; Kelly, S.H.; Cevik, M.; Collins, S.; Waters, L.; Villa, G.; Docherty, A.; Harrison, E.M.; Turtle, L.; et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization among People with Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin. Infect. Dis. 2021, 73, e2095–e2106. [Google Scholar] [CrossRef]
- Vizcarra, P.; Pérez-Elías, M.J.; Quereda, C.; Moreno, A.; Vivancos, M.J.; Dronda, F.; Casado, J.L.; COVID-19 ID Team. Description of COVID-19 in HIV-infected individuals: A single-centre, prospective cohort. Lancet HIV 2020, 7, e554–e564. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Sun, K.; Ma, Y.; Rodriguez, F.; Secemsky, E.A.; Parikh, R.V.; Hsue, P.Y. Association of HIV infection with outcomes among adults hospitalized with COVID-19. AIDS 2022, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Rentsch, C.T.; MacKenna, B.; Schultze, A.; Mehrkar, A.; Bates, C.J.; Eggo, R.M.; Morton, C.E.; Bacon, S.C.J.; Inglesby, P.; et al. HIV infection and COVID-19 death: A population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021, 8, e24–e32. [Google Scholar] [CrossRef]
- Dandachi, D.; Geiger, G.; Montgomery, M.W.; Karmen-Tuohy, S.; Golzy, M.; Antar, A.A.R.; Llibre, J.M.; Camazine, M.; Díaz-De Santiago, A.; Carlucci, P.M.; et al. Characteristics, Comorbidities, and Outcomes in a Multicenter Registry of Patients with HIV and Coronavirus Disease-19. Clin. Infect. Dis. 2021, 73, e1964–e1972. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Blanco, J.L.; Reyes-Urueña, J.M.; Davies, M.-A.; Sued, O.; Marcos, M.A.; Martínez, E.; Bertagnolio, S.; Alcamí, J.; Miro, J.M.; et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV 2021, 8, e294–e305. [Google Scholar] [CrossRef]
- Nugent, J.; Aklilu, A.; Yamamoto, Y.; Simonov, M.; Li, F.; Biswas, A.; Ghazi, L.; Greenberg, J.; Mansour, S.; Moledina, D.; et al. Assessment of Acute Kidney Injury and Longitudinal Kidney Function after Hospital Discharge Among Patients with and without COVID-19. JAMA Netw. Open 2021, 4, e211095. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.; Neugarten, J.; Bellin, E.; Yunes, M.; Stahl, L.; Johns, T.S.; Abramowitz, M.K.; Levy, R.; Kumar, N.; Mokrzycki, M.H.; et al. AKI in Hospitalized Patients with and without COVID-19: A Comparison Study. J. Am. Soc. Nephrol. 2020, 31, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; Northwell COVID-19 Research Consortium; et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Ojha, V.; Verma, M.; Pandey, N.N.; Mani, A.; Malhi, A.S.; Kumar, S.; Jagia, P.; Roy, A.; Sharma, S. Cardiac Magnetic Resonance Imaging in Coronavirus Disease 2019 (COVID-19): A Systematic Review of Cardiac Magnetic Resonance Imaging Findings in 199 Patients. J. Thorac. Imaging 2021, 36, 73–83. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Hanff, T.C.; Mohareb, A.M.; Giri, J.; Cohen, J.B.; Chirinos, J.A. Thrombosis in COVID-19. Am. J. Hematol. 2020, 95, 1578–1589. [Google Scholar] [CrossRef]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29, 100639. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute Ischemic Stroke and COVID-19: An Analysis of 27 676 Patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Merkler, A.E.; Parikh, N.S.; Mir, S.; Gupta, A.; Kamel, H.; Lin, E.; Lantos, J.; Schenck, E.J.; Goyal, P.; Bruce, S.S.; et al. Risk of Ischemic Stroke in Patients with Coronavirus Disease 2019 (COVID-19) vs Patients with Influenza. JAMA Neurol. 2020, 77, 1366. [Google Scholar] [CrossRef]
- Palella, F.J.; Hart, R.; Armon, C.; Tedaldi, E.; Yangco, B.; Novak, R.; Battalora, L.; Ward, D.; Li, J.; Buchacz, K.; et al. Non-AIDS comorbidity burden differs by sex, race, and insurance type in aging adults in HIV care. AIDS 2019, 33, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.F.; Sheth, A.N.; Mehta, C.C.; Naggie, S.; Golub, E.T.; Anastos, K.; French, A.L.; Kassaye, S.; Taylor, T.; Fischl, M.A.; et al. The Prevalence and Burden of Non-AIDS Comorbidities Among Women Living with or at Risk for Human Immunodeficiency Virus Infection in the United States. Clin. Infect. Dis. 2021, 72, 1301–1311. [Google Scholar] [CrossRef]
- Schouten, J.; Wit, F.W.; Stolte, I.G.; Kootstra, N.A.; van der Valk, M.; Geerlings, S.E.; Prins, M.; Reiss, P.; AGEhIV Cohort Study Group. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV cohort study. Clin. Infect. Dis. 2014, 59, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Ronit, A.; Gerstoft, J.; Nielsen, L.; Mohey, R.; Wiese, L.; Kvinesdal, B.; Obel, N.; Ahlströhm, M.G. Non-AIDS Comorbid Conditions in Persons Living with Human Immunodeficiency Virus (HIV) Compared With Uninfected Individuals 10 Years Before HIV Diagnosis. Clin. Infect. Dis. 2018, 67, 1291–1293. [Google Scholar] [CrossRef]
- Facts and Figures. Available online: https://www.medstarhealth.org/mhs/about-medstar/facts-and-figures/ (accessed on 12 July 2021).
- Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2018. HIV Surveill. Suppl. Rep. 2020, 25. Available online: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-25-2.pdf (accessed on 12 July 2021).
- Navickas, R.; Petric, V.-K.; Feigl, A.B.; Seychell, M. Multimorbidity: What do we know? What should we do? J. Comorb. 2016, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Burns, E.M.; Rigby, E.; Mamidanna, R.; Bottle, A.; Aylin, P.; Ziprin, P.; Faiz, O.D. Systematic review of discharge coding accuracy. J. Public Health 2012, 34, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Peck, R.; Olsen, C.; Devore, J.L. Introduction to Statistics and Data Analysis; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Schriger, D.L. Book and media review. Ann. Emerg. Med. 2008, 52, 480. [Google Scholar] [CrossRef]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients with COVID-19 in a New York City Health System. JAMA 2020, 324, 799–801. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients with COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Naicker, S.; Rahmanian, S.; Kopp, J.B. HIV and chronic kidney disease. Clin. Nephrol. 2015, 83, 32–38. [Google Scholar] [CrossRef]
- So-Armah, K.; Benjamin, L.A.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. Lancet HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef]
- Losina, E.; Hyle, E.P.; Borre, E.D.; Linas, B.P.; Sax, P.E.; Weinstein, M.C.; Rusu, C.; Ciaranello, A.L.; Walensky, R.P.; Freedberg, K.A. Projecting 10-year, 20-year, and Lifetime Risks of Cardiovascular Disease in Persons Living With Human Immunodeficiency Virus in the United States. Clin. Infect. Dis. 2017, 65, 1266–1271. [Google Scholar] [CrossRef]
- Bibas, M.; Biava, G.; Antinori, A. HIV-Associated Venous Thromboembolism. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011030. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Bangash, H.I.; Habiba, M.; Lei, Y.; Xie, T.; Sun, J.; Wei, Z.; Hong, Z.; Shao, L.; Zhang, Q. Immune dysregulation and system pathology in COVID-19. Virulence 2021, 12, 918–936. [Google Scholar] [CrossRef]
- Braunstein, S.L.; Lazar, R.; Wahnich, A.; Daskalakis, D.C.; Blackstock, O.J. COVID-19 Infection Among People with HIV in New York City: A Population-Level Analysis of Matched Surveillance Data. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Yendewa, G.A.; Perez, J.A.; Schlick, K.; Tribout, H.; McComsey, G.A. Clinical Features and Outcomes of Coronavirus Disease 2019 Among People with Human Immunodeficiency Virus in the United States: A Multicenter Study from a Large Global Health Research Network (TriNetX). Open Forum Infect. Dis. 2021, 8, ofab272. [Google Scholar] [CrossRef] [PubMed]
- Hadi, Y.B.; Naqvi SF, Z.; Kupec, J.T.; Sarwari, A.R. Characteristics and outcomes of COVID-19 in patients with HIV: A multicentre research network study. AIDS 2020, 34, F3–F8. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, J.; Polo, R.; Moreno, S.; Díaz, A.; Martínez, E.; Arribas, J.R.; Jarrín, I.; Hernán, M.A.; The Spanish HIV/COVID-19 Collaboration. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy: A Cohort Study. Ann. Intern. Med. 2020, 173, 536–541. [Google Scholar] [CrossRef]
- Jassat, W.; Cohen, C.; Masha, M.; Goldstein, S.; Kufa-Chakezha, T.; Savulescu, D.; Walaza, S.; Bam, J.-L.; Davies, M.-A.; Prozesky, H.W.; et al. A national cohort study of COVID-19 in-Hospital Mortality in South Africa: The Intersection of Communicable and Non-Communicable Chronic Diseases in a High HIV Prevalence Setting. medRxiv 2020. [Google Scholar] [CrossRef]
- Rosenthal, E.M.; Rosenberg, E.S.; Patterson, W.; Ferguson, W.P.; Gonzalez, C.; DeHovitz, J.; Udo, T.; Rajulu, D.T.; Hart-Malloy, R.; Tesoriero, J. Factors associated with SARS-CoV-2-related hospital outcomes among and between persons living with and without diagnosed HIV infection in New York State. PLoS ONE 2022, 17, e0268978. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Eastment, M.C.; Dominitz, J.A.; Fan, V.S. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10 131 US Veterans with SARS-CoV-2 Infection. JAMA Netw. Open 2020, 3, e2022310. [Google Scholar] [CrossRef]
- Panagiotou, O.A.; Kosar, C.M.; White, E.M.; Bantis, L.E.; Yang, X.; Santostefano, C.M.; Feifer, R.A.; Blackman, C.; Rudolph, J.L.; Gravenstein, S.; et al. Risk Factors Associated With All-Cause 30-Day Mortality in Nursing Home Residents with COVID-19. JAMA Intern. Med. 2021, 181, 439–448. [Google Scholar] [CrossRef]
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, J.; Patel, R.C.; Zhang, J.; Guo, S.; Zheng, Q.; Olex, A.L.; Olatosi, B.; Weissman, S.B.; Islam, J.Y.; et al. Associations between HIV infection and clinical spectrum of COVID-19: A population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021, 8, e690–e700. [Google Scholar] [CrossRef]
- Brown, L.B.; Spinelli, M.A.; Gandhi, M. The interplay between HIV and COVID-19: Summary of the data and responses to date. Curr. Opin. HIV AIDS 2021, 16, 63. [Google Scholar] [CrossRef]
- Nomah, D.K.; Reyes-Urueña, J.; Díaz, Y.; Moreno, S.; Aceiton, J.; Bruguera, A.; Vivanco-Hidalgo, R.M.; Llibre, J.M.; Domingo, P.; Falcó, V.; et al. Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: A retrospective cohort study. Lancet HIV 2021, 8, e701–e710. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Kim, A.Y.; Ard, K.L.; Basgoz, N.; Chu, J.T.; Hurtado, R.M.; Lee, C.K.; He, W.; Minukas, T.; Nelson, S.; et al. Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS 2020, 34, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, A.; Lee, D.; Gordon, S.H.; Allen, H.; Sommers, B.D. Comparison of Income Eligibility for Medicaid vs. Marketplace Coverage for Insurance Enrollment Among Low-Income US Adults. JAMA Health Forum 2021, 2, e210771. [Google Scholar] [CrossRef]
- Shafer, P.R.; Anderson, D.M.; Whitaker, R.; Wong, C.A.; Wright, B. Association of Unemployment with Medicaid Enrollment By Social Vulnerability In North Carolina During COVID-19. Health Aff. 2021, 40, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, N.E.; Purcell, L.N.; Karam, B.S.; Dudley, R.A.; Usher, M.G.; Warlick, C.A.; Allen, M.L.; Melton, G.B.; Charles, A.; Tignanelli, C.J. Racial and Ethnic Disparities in Hospital Admissions from COVID-19: Determining the Impact of Neighborhood Deprivation and Primary Language. J. Gen. Intern. Med. 2021, 36, 3462–3470. [Google Scholar] [CrossRef]
- Dalsania, A.K.; Fastiggi, M.J.; Kahlam, A.; Shah, R.; Patel, K.; Shiau, S.; Rokicki, S.; DallaPiazza, M. The Relationship Between Social Determinants of Health and Racial Disparities in COVID-19 Mortality. J. Racial Ethn. Health Disparities 2021, 9, 288–295. [Google Scholar] [CrossRef]
- Liebovitz, D.M.; Fahrenbach, J. COUNTERPOINT: Is ICD-10 Diagnosis Coding Important in the Era of Big Data? No. Chest 2018, 153, 1095–1098. [Google Scholar] [CrossRef]
- Weiner, M.G. POINT: Is ICD-10 Diagnosis Coding Important in the Era of Big Data? Yes. Chest 2018, 153, 1093–1095. [Google Scholar] [CrossRef]
- ICD-ICD-10-CM-International Classification of Diseases, (ICD-10-CM/PCS) Transition. 2019. Available online: https://www.cdc.gov/nchs/icd/icd10cm_pcs_background.htm (accessed on 14 July 2021).
- Wiley, Z.; Kubes, J.N.; Cobb, J.; Jacob, J.T.; Franks, N.; Plantinga, L.; Lea, J. Age, Comorbid Conditions, and Racial Disparities in COVID-19 Outcomes. J. Racial Ethn. Health Disparities 2022, 9, 117–123. [Google Scholar] [CrossRef]
- Fu, J.; Reid, S.A.; French, B.; Hennessy, C.; Hwang, C.; Gatson, N.T.; Duma, N.; Mishra, S.; Nguyen, R.; Hawley, J.E.; et al. Racial Disparities in COVID-19 Outcomes Among Black and White Patients with Cancer. JAMA Netw. Open 2022, 5, e224304. [Google Scholar] [CrossRef]
- Shortreed, S.M.; Gray, R.; Akosile, M.A.; Walker, R.L.; Fuller, S.; Temposky, L.; Fortmann, S.P.; Albertson-Junkans, L.; Floyd, J.S.; Bayliss, E.A.; et al. Increased COVID-19 Infection Risk Drives Racial and Ethnic Disparities in Severe COVID-19 Outcomes. J. Racial Ethn. Health Disparities 2022. [Google Scholar] [CrossRef]
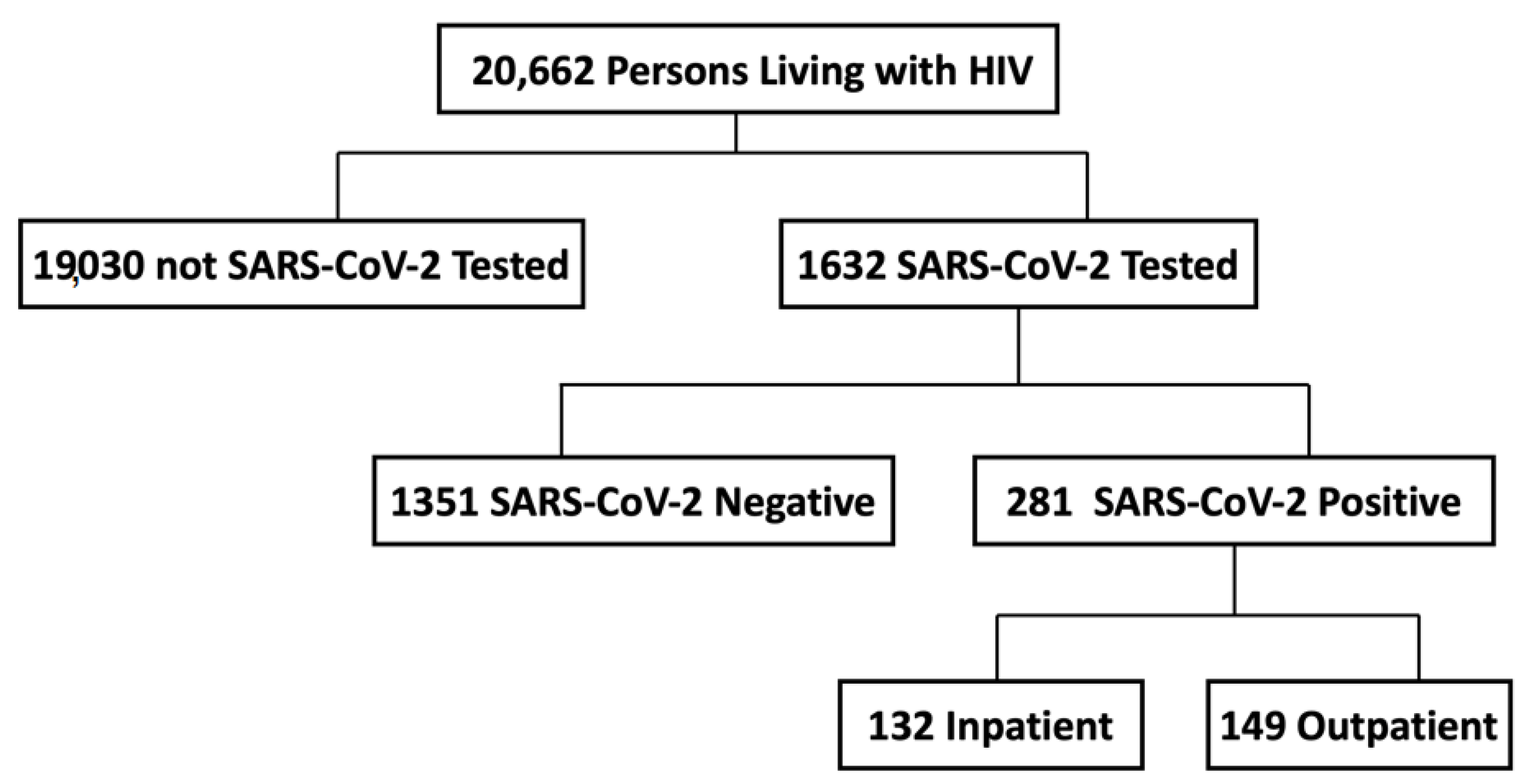
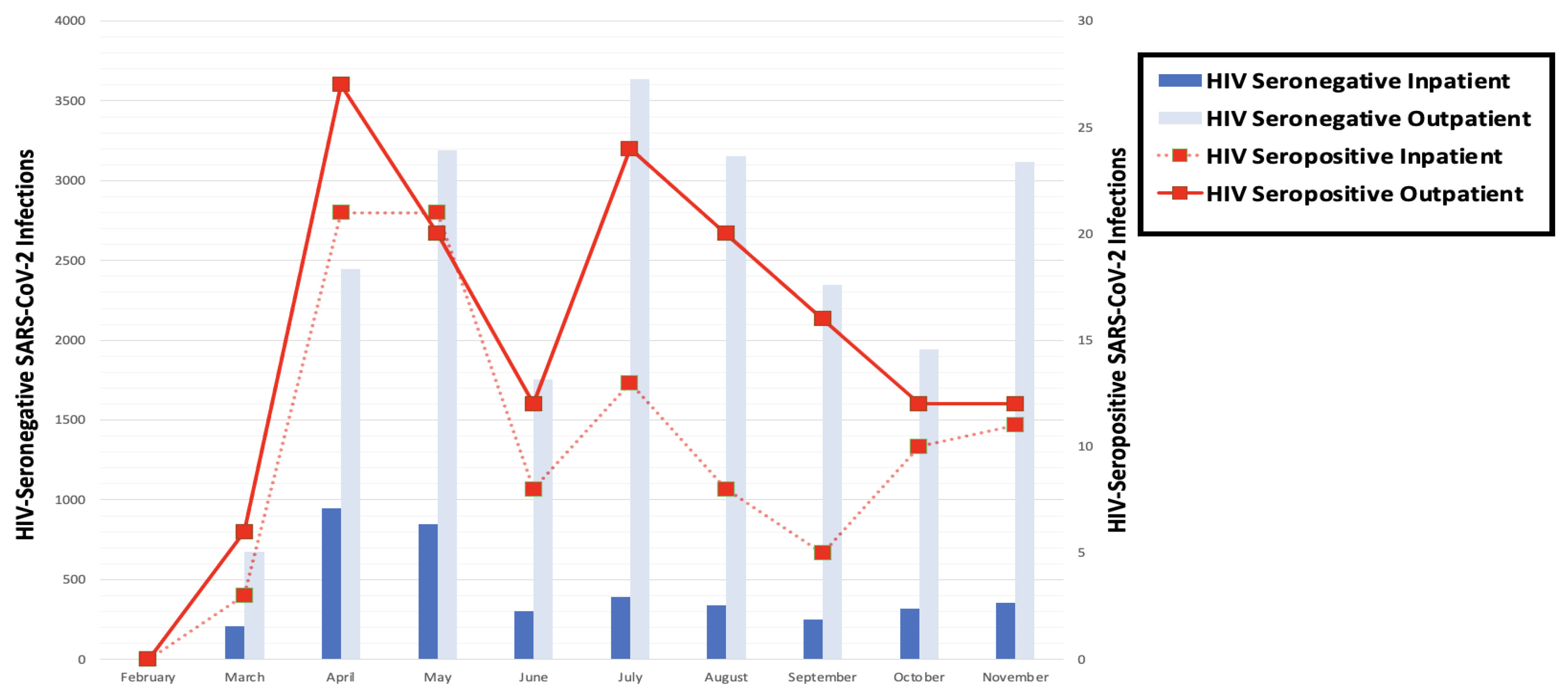
| Characteristic | HIV-Seronegative (n = 1124) | HIV-Seropositive (n = 281) | p-Value |
|---|---|---|---|
| Age mean years (SD) | 51.2 (13.7) | 51.5 (12.7) | 1 |
| Sex at Birth, n (%) | |||
| Male | 708 (63) | 177 (63) | 1 |
| Female | 416 (37) | 104 (37) | |
| Race, n (%) | |||
| African American/Black | 388 (44) | 237 (86) | <0.001 |
| White | 341 (39) | 20 (7) | |
| Other | 157 (18) | 18 (7) | |
| Ethnicity | |||
| Non-Hispanic | 813 (92) | 258 (98) | 0.001 |
| Hispanic | 69 (8) | 6 (2) | |
| Insurance, n (%) | |||
| Private | 818 (75) | 161 (58) | <0.001 |
| Medicaid | 79 (7) | 67 (24) | |
| Medicare | 146 (13) | 45 (16) | |
| Non-Insured | 46 (4) | 6 (2) | |
| Co-Morbid Conditions, n (%) | |||
| Cardiovascular Disease | 423 (38) | 174 (62) | <0.001 |
| Hypertension | 403 (36) | 165 (59) | <0.001 |
| Obesity | 280 (25) | 111 (40) | <0.001 |
| Diabetes Mellitus | 226 (20) | 92 (33) | <0.001 |
| Chronic Renal Disease | 117 (10) | 31 (11) | <0.001 |
| Chronic Liver Disease | 62 (6) | 70 (25) | <0.001 |
| Malignancy | 19 (2) | 28 (10) | <0.001 |
| Transplant | 14 (1) | 8 (3) | 0.062 |
| Post-Infection Events, n (%) | |||
| Thrombotic | 2 (1) | 1 (0.4) | 0.489 |
| Infections | 14 (1) | 7 (2) | 0.206 |
| Cardiovascular | 22 (2) | 9 (3) | 0.253 |
| Acute Kidney Injury | 6 (1) | 4 (1) | 0.120 |
| INPATIENT | N = 269 | N = 132 | |
| Median Length of Stay, days (IQR) | 6 (3, 11) | 5.5 (3, 11) | 0.889 |
| ICU Median Length of Stay, days (IQR) | 7 (3, 15) | 3 (1, 7.25) | 0.008 |
| Deceased, n (%) | 33 (13) | 18 (14) | 0.750 |
| Comorbid Conditions, n (%) | |||
| Diabetes Mellitus | 110 (41) | 50 (38) | 0.589 |
| Cardiovascular Disease | 67 (35) | 35 (27) | 0.716 |
| Chronic Renal Disease | 30 (11) | 22 (17) | 0.154 |
| Chronic Liver Disease | 10 (4) | 21 (16) | <0.001 |
| Malignancy | 19 (7) | 25 (18) | <0.001 |
| Post-Infection Events, n (%) | |||
| Thrombotic | 1 (0.4) | 1 (0.4) | 0.551 |
| Infections | 12 (5) | 3 (2) | 0.421 |
| Cardiovascular | 20 (7) | 5 (4) | 0.190 |
| Acute Kidney Injury | 4 (2) | 1 (1) | 1 |
| COVID Treatments a, n (%) | |||
| Remdesivir | 39 (15) | 18 (14) | 0.880 |
| Dexamethasone | 65 (24) | 32 (24) | 1 |
| Azithromycin | 120 (45) | 58 (44) | 0.915 |
| Hydroxychloroquine | 49 (18) | 22 (17) | 0.781 |
| Tocilizumab | 18 (7) | 6 (5) | 0.504 |
| Supplemental Oxygen, n (%) | |||
| Room Air | 38 (18) | 27 (25) | |
| Nasal Cannula | 82 (39) | 33 (30) | 0.097 |
| Non-Rebreather/HFNC | 29 (14) | 25 (23) | |
| Ventilator | 53 (25) | 21 (19) | |
| Laboratory Data (Admission b), (IQR) | |||
| Median WBC (×103 cells/µL) | 7 (5.3, 9.6) | 6.80 (4.60, 9.20) | 0.060 |
| Median Absolute Lymphocyte count (×103 cells/µL) | 1.05 (0.80, 1.50) | 1.30 (0.80, 1.80) | 0.032 |
| Mean Hemoglobin (gm/dL) | 12.98 (12.98) | 12.23 (2.40) | 0.006 |
| Mean Platelets (×103 cells/µL) | 233 (95.52) | 211.07 (91.78) | 0.036 |
| Median Creatinine (mg/dL) | 1.11 (0.83, 1.71) | 1.17 (0.89, 2.48) | 0.175 |
| Mean eGFR (mL/min/1.73 m2) | 50.07 (17.96) | 46.10 (20.67) | 0.062 |
| Median ALT (IU/L) | 37.00 (23, 58.50) | 33.00 (22, 49.50) | 0.224 |
| Median CPK (units/L) | 148.50 (78.25, 326) | 142 (75, 394) | 0.834 |
| Median Troponin (ng/mL) | 0.02 (0.01, 0.03) | 0.02 (0.01, 0.04) | 0.248 |
| Median Procalcitonin (ng/mL) | 0.19 (0.10, 0.73) | 0.34 (0.10, 0.86) | 0.398 |
| Median Ferritin (ng/mL) | 592 (300, 1330.40) | 565.15 (262.40, 1367.22) | 0.573 |
| Median Lactate Dehydrogenase (units/L) | 343.50 (264.50, 460.50) | 312.50 (238.5, 161.5) | 0.477 |
| Median D-Dimer (mcg/mL FEU) | 1.66 (0.78, 3.06) | 1.44 (0.78, 3.46) | 0.902 |
| Median C-Reactive Protein (mg/L) | 82.80 (35.05, 127.50) | 93.70 (53.50, 161.50) | 0.285 |
| Laboratory Data (Peak), (IQR) | |||
| Median WBC (×103 cells/µL) | 10.20 (6.93, 14.97) | 8.90 (6, 12.70) | 0.010 |
| Median Platelets (×103 cells/µL) | 307 (236.75, 417.75) | 263 (194, 371) | 0.008 |
| Median Procalcitonin (ng/mL) | 0.22 (0.10, 2.30) | 0.39 (0.10, 1.70) | 0.498 |
| Median Ferritin (ng/mL) | 896.1 (373.45, 1817.15) | 707.40 (345, 1798.20) | 0.420 |
| Median Lactate Dehydrogenase (units/L) | 400.50 (284.50, 574) | 355.5 (271, 576.75) | 0.615 |
| Median D-Dimer (mcg/mL FEU) | 1.88 (1.09, 5.07) | 1.94 (0.93, 3.83) | 0.655 |
| Mean C-Reactive Protein (mg/L) | 109 (47.40) | 121.50 (61.70, 172.25) | 0.573 |
| Median Interleukin-6 (pg/mL) | 12.10 (5, 43.50) | 9 (5, 18.60) | 0.412 |
| Characteristic | OR | p-Value | aOR * | p-Value |
|---|---|---|---|---|
| Age, years | 1.05 (1.03, 1.07) | <0.001 | 1.04 (1.01, 1.06) | 0.002 |
| Sex at Birth | ||||
| Female (reference) | − | − | − | − |
| Male | 1.12 (0.69, 1.83) | 0.646 | 1.5 (0.87, 2.62) | 0.152 |
| Race | ||||
| White (reference) | − | − | − | − |
| African American/Black | 0.6 (0.23, 1.5) | 0.278 | − | − |
| Other | 0.26 (0.06, 0.96) | 0.051 | − | − |
| Ethnicity | ||||
| Non-Hispanic (reference) | − | − | − | − |
| Hispanic | 1.13 (0.21, 6.22) | 0.88 | − | − |
| HIV Viral Load | ||||
| <200 (reference) | − | − | − | − |
| >200 | 1 (1, 1) | 0.34 | ||
| CD4 + T Lymphocyte | ||||
| >200 (reference) | − | − | − | − |
| <200 | 0.98 (0.91, 1.06) | 0.624 | − | − |
| Insurance | ||||
| Private (reference) | − | − | − | − |
| Medicaid | 2.22 (1.25, 4) | 0.007 | 2.61 (1.39, 4.97) | 0.003 |
| Medicare | 2.4 (1.23, 4.77) | 0.011 | 1.41 (0.67, 3.01) | 0.362 |
| Uninsured/Self-Pay | 0.8 (0.11, 4.22) | 0.798 | 0.78 (0.09, 5.39) | 0.803 |
| Multimorbidity ** | 3.74 (2.29, 6.21) | <0.001 | 2.98 (1.72, 5.23) | <0.001 |
| Characteristic | OR (CI 95%) | p-Value | aOR * | p-Value |
|---|---|---|---|---|
| Age, years | 1.06 (1.03, 1.1) | <0.0001 | 1.06 (1.01, 1.11) | 0.013 |
| Sex at Birth | ||||
| Female (reference) | − | − | − | − |
| Male | 0.87 (0.4, 1.93) | 0.72 | 0.97 (0.42, 2.34) | 0.94 |
| Race | ||||
| White (reference) | − | − | − | − |
| African American/Black | 2.34 (0.46, 42.92) | 0.416 | − | − |
| Other | 0 (0, 2.44 × 1015) | 0.987 | − | − |
| Ethnicity | ||||
| Non-Hispanic (reference) | − | − | − | − |
| Hispanic | 1.95 (0.1, 12.76) | 0.55 | − | − |
| HIV Viral Load | ||||
| <200 (reference) | − | − | − | − |
| >200 | 0.97 (0.84, 1) | 0.592 | − | − |
| CD4 + T Lymphocyte | ||||
| >200 (reference) | − | − | − | − |
| <200 | 1.05 (0.86, 1.31) | 0.64 | − | − |
| Insurance | ||||
| Private (reference) | − | − | − | − |
| Medicaid | 2.97 (1.19, 7.49) | 0.019 | 3.6 (1.36, 9.74) | 0.01 |
| Medicare | 2.78 (0.95, 7.73) | 0.051 | 1.33 (0.42, 3.99) | 0.614 |
| Uninsured/Self-Pay | 7.55 (0.97, 43.98) | 0.029 | 12.09 (1.17, 1.26 × 102) | 0.027 |
| Multimorbidity ** | 6.55 (2.47, 22.68) | <0.001 | 4.4 (1.55, 15.9) | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spence, A.B.; Desale, S.; Lee, J.; Kumar, P.; Huang, X.; Cooper, S.E.; Fernandez, S.; Kassaye, S.G. COVID-19 Outcomes in a US Cohort of Persons Living with HIV (PLWH). Reports 2022, 5, 41. https://doi.org/10.3390/reports5040041
Spence AB, Desale S, Lee J, Kumar P, Huang X, Cooper SE, Fernandez S, Kassaye SG. COVID-19 Outcomes in a US Cohort of Persons Living with HIV (PLWH). Reports. 2022; 5(4):41. https://doi.org/10.3390/reports5040041
Chicago/Turabian StyleSpence, Amanda Blair, Sameer Desale, Jennifer Lee, Princy Kumar, Xu Huang, Stanley Evan Cooper, Stephen Fernandez, and Seble G. Kassaye. 2022. "COVID-19 Outcomes in a US Cohort of Persons Living with HIV (PLWH)" Reports 5, no. 4: 41. https://doi.org/10.3390/reports5040041
APA StyleSpence, A. B., Desale, S., Lee, J., Kumar, P., Huang, X., Cooper, S. E., Fernandez, S., & Kassaye, S. G. (2022). COVID-19 Outcomes in a US Cohort of Persons Living with HIV (PLWH). Reports, 5(4), 41. https://doi.org/10.3390/reports5040041







