Abstract
Background: This study aims to describe COVID-19–related clinical outcomes after immunotherapies (ICIs) for cancer patients. Methods: In this meta-analysis, we searched databases to collect data that addressed outcomes after immunotherapies (ICIs) during the COVID-19 pandemic. The primary endpoint was COVID-19–related mortality. Secondary endpoints included COVID-related hospital readmission, emergency room (ER) visits, opportunistic infections, respiratory complications, need for ventilation, and thrombo-embolic events. Pooled event rates (PERs) were calculated and a meta-regression analysis was performed. Results: A total of 262 studies were identified. Twenty-two studies with a total of forty-four patients were eligible. The PER of COVID-19–related mortality was 39.73%, while PERs of COVID-19–related ER visits, COVID-19–related pulmonary complications, and COVID-19–related ventilator needs were 40.75%, 40.41%, and 34.92%, respectively. The PER of opportunistic infections was 34.92%. The PERs of the use of antivirals, antibiotics, steroids, prophylactic anticoagulants, and convalescent plasma were 62.12%, 57.12%, 51.36%, 41.90%, and 26.48%, respectively. There was a trend toward an association between previous respiratory diseases and COVID-19–related mortality. Conclusion: The rates of COVID-19–related mortality, ER visits, pulmonary complications, need for a ventilator, and opportunistic infections are still high after ICIs during the COVID-19 pandemic. There was a trend toward an association between previous respiratory diseases and COVID-19–related mortality.
1. Introduction
Cancer patients could be more susceptible to COVID-19 infection because of their vulnerable immunity status due to the cancer itself, as well as the cancer treatment [1]. Administering immune checkpoint inhibitors (ICIs) during the COVID-19 era comes with challenges [2,3]. However, the data addressing the impact of ICIs on COVID-19–related outcomes are unclear [4,5], considering the known fact that ICIs restore immune competency [6]. Some data showed that receipt of ICIs does not negatively impact the outcomes after COVID-19 infection [5]. Thus, such challenges, debatable outcomes, and limited existing data necessitate a systematic review.
The challenges of administering ICIs during the COVID-19 era include the potential overlap between COVID-19–related interstitial pneumonia and possible ICI-induced lung injury [2,3,7]. The overall incidence rate of ICI-induced pneumonitis ranges from 2.5% to 10%; yet, it could be fatal, accounting for 35% of ICI-related mortality [2,8]. This challenge is greater in lung cancer patients receiving ICIs with or without local radiotherapy who are at risk for COVID-19 infection [9]. The immune hyperactivation induced by ICIs initiates cytokine release syndrome (CRS) (elevated interleukins and cytokines with subsequent organ failure and death). Similar cytokine storms have been observed after COVID-19 infection with similarly fatal outcomes of organ failure and death [10,11]. Given the similarity of the presentations of underlying COVID-19–induced and ICI-induced lung injury, diagnostic difficulty or delay and the synergistic effect of ICI- and COVID-19–induced lung injury could add to the fatality of the outcomes [12]. Fortunately, ICI-induced CRS is quite rare, and a COVID-19–induced cytokine storm is not an early event in the COVID-19 trajectory [7]. Such observations leave space for early intervention and careful patient screening/selection and monitoring to allow cancer patients in need of ICIs to receive their treatment safely and effectively during the COVID-19 era.
Given that the duration of the pandemic and the trajectory of COVID-19 infections are still unknown and unpredictable, we undertook a systematic review to obtain solid data showing patient characteristics and COVID-19–related outcomes after ICIs during the COVID-19 era. Care providers need these data to create effective, tolerable ICI treatment plans without compromising safety or outcomes. The objective of this systematic review was to address the clinical outcomes after ICIs for cancer patients during the COVID-19 era. The primary endpoint was COVID-19–related mortality and the secondary endpoints included COVID-19–related therapy, readmission to the hospital, ER visits, opportunistic infections, respiratory complications, need for ventilation, need for tracheostomy, and thrombo-embolic events.
2. Methodology
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The Newcastle–Ottawa Quality Assessment Scale for cohort studies was used [13].
2.1. Literature Search
We searched the Ovid MEDLINE, Ovid Embase, Clarivate Analytics Web of Science, PubMed, and Wiley-Blackwell Cochrane Library databases for publications in the English language from 1 December 2019 to 15 October 2020. The following concepts were searched for using subject headings and keywords as needed: “COVID-19”, “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “coronavirus infections”, “novel coronavirus”, “cancer”, “neoplasms”, “tumor”, “leukemia”, “lymphoma”, “melanoma”, “carcinoma”, “sarcoma”, “oncology”, “checkpoint inhibitors”, “programmed cell death 1”, “programmed death ligand 1”, “PD-1”, “PD-L1”, “cytotoxic T lymphocyte associated antigen 4”, “CTLA 4”, “ipilimumab”, “pembrolizumab”, “nivolumab”, “atezolizumab”, “durvalumab”, “avelumab”, “cemiplimab”, “chimeric antigen receptor t-cell therapy”, “adoptive immunotherapy”, etc. The search terms were combined by “or” if they represented similar concepts and combined by “and” if they represented different concepts. The complete search strategies are detailed in Tables S1–S4.
2.2. Study Selection
Eligible studies were required to evaluate measurable outcomes related to COVID-19 infection in cancer patients on ICIs during the COVID-19 pandemic. Owing to limited publications in this unique cohort, we included case presentations and case studies. To ensure inclusion of all available data, all bibliographies were searched for potential eligible studies (i.e., backward snowballing). Nevertheless, abstracts, reviews, and expert opinions were excluded, as were studies that were not exclusively of ICI-treated patients and studies with insufficient information about the characteristics or outcomes (listed below).
2.3. Data Extraction and Endpoints
Two reviewers (M.K. and A.Q.) independently assessed the eligibility. Then M.K., A.Q., and J.J. extracted the data from the eligible studies and tabulated the data using Excel software (Microsoft Corporation, Redmond, WA, USA).
Data on study period, study center, country, type of cancer, type of study, and sample size were retrieved. We abstracted age, gender, presence of hypertension, diabetes mellitus, renal insufficiency, smoking history, pre-existing chronic obstructive pulmonary disease, cerebrovascular accident, and dyslipidemia.
We also collected information about previous and current cancer treatments, type of cancer and ICI(s), cancer status, in-hospital COVID-19 infection, onset of COVID-19 infection in relation to receipt of ICIs, and laboratory and pulmonary findings at diagnosis of COVID-19 infection and their follow-up data if presented. To assess COVID-19–related therapy use, we recorded use of steroids (yes/no, dosage, and duration), use of antivirals, antibiotics, convalescent plasma, prophylactic coagulations, and antibodies. Finally, we assessed the following outcomes when they occurred because of COVID-19 infection: rates of readmission, emergency room (ER) visits, intensive care unit (ICU) admission, need for tracheostomy, need for ventilation, mortality, and complications, for instance pulmonary problems, thrombo-embolic events, and fungal and other opportunistic infections.
The primary endpoint of the analysis was COVID-19–related mortality. Secondary endpoints included COVID-19–related therapy, readmission to the hospital, ER visits, opportunistic infections, respiratory complications, need for ventilation, need for tracheostomy, and thrombo-embolic events.
2.4. Statistical Analysis
Pooled event rates (PERs) with 95% confidence intervals (CIs) were calculated for the study outcomes. Meta-regression was performed to explore the relationship between COVID-19–related mortality and clinical characteristics. These results were reported as a regression coefficient (i.e., beta). In all analyses, studies were weighted by the inverse of the variance of the estimate for that study, and between-study variance was estimated with the DerSimonian–Laird method with a random-effects model. Studies with zeros were included in the meta-analysis, and treatment arm continuity correction was applied in studies with zero cell frequencies.
Heterogeneity was based on the Cochran Q test, with I2 values. In the case of heterogeneity I2 > 50%, individual study inference analysis was performed through a “leave-one-out” sensitivity analysis. Funnel plots by graphical inspection and Egger regression test were used for assessment of publication bias. In the case of asymmetry positivity, visual assessment and Duval and Tweedie’s “trim and fill” method were used for further assessment.
Hypothesis testing for equivalence was set at the two-tailed 0.05 level. All analyses were performed using R version 4.1.0 (R Project for Statistical Computing) and RStudio version 1.4.1717, using the “meta” and “metafor” packages.
3. Results
A total of 262 studies were identified in the databases. After exclusion of duplicates, 162 studies were screened. Then, we excluded 122 non-eligible studies. Forty full-text articles were assessed for eligibility. Finally, 22 studies with a total of 44 patients met the eligibility criteria. Supplementary Figure S1 shows the PRISMA flow diagram. Table 1 shows the studies’ characteristics and patient demographics. Supplementary Table S5 shows the overall baseline patient demographics. Patients’ average age was 57.2 ± 17.4 years. A total of 66% were men, and 53% were current/former smokers. Totals of 61%, 36%, 30%, and 15% had hypertension, pre-existing chronic obstructive pulmonary disease, diabetes mellitus, and cerebrovascular accident, respectively. A total of 58% of patients had previous cancer therapy before receipt of ICIs. The top presenting COVID-19 symptoms were fever (74%), cough (57%), and dyspnea (52%), while ground glass opacity (64%), infiltrate (27%), and consolidation (27%) were the top radiologic findings. The Newcastle–Ottawa Quality Assessment Scale for cohort studies is shown in Supplementary Table S6 [13].

Table 1.
Characteristics of the eligible studies and demographics of the patients in the included studies.
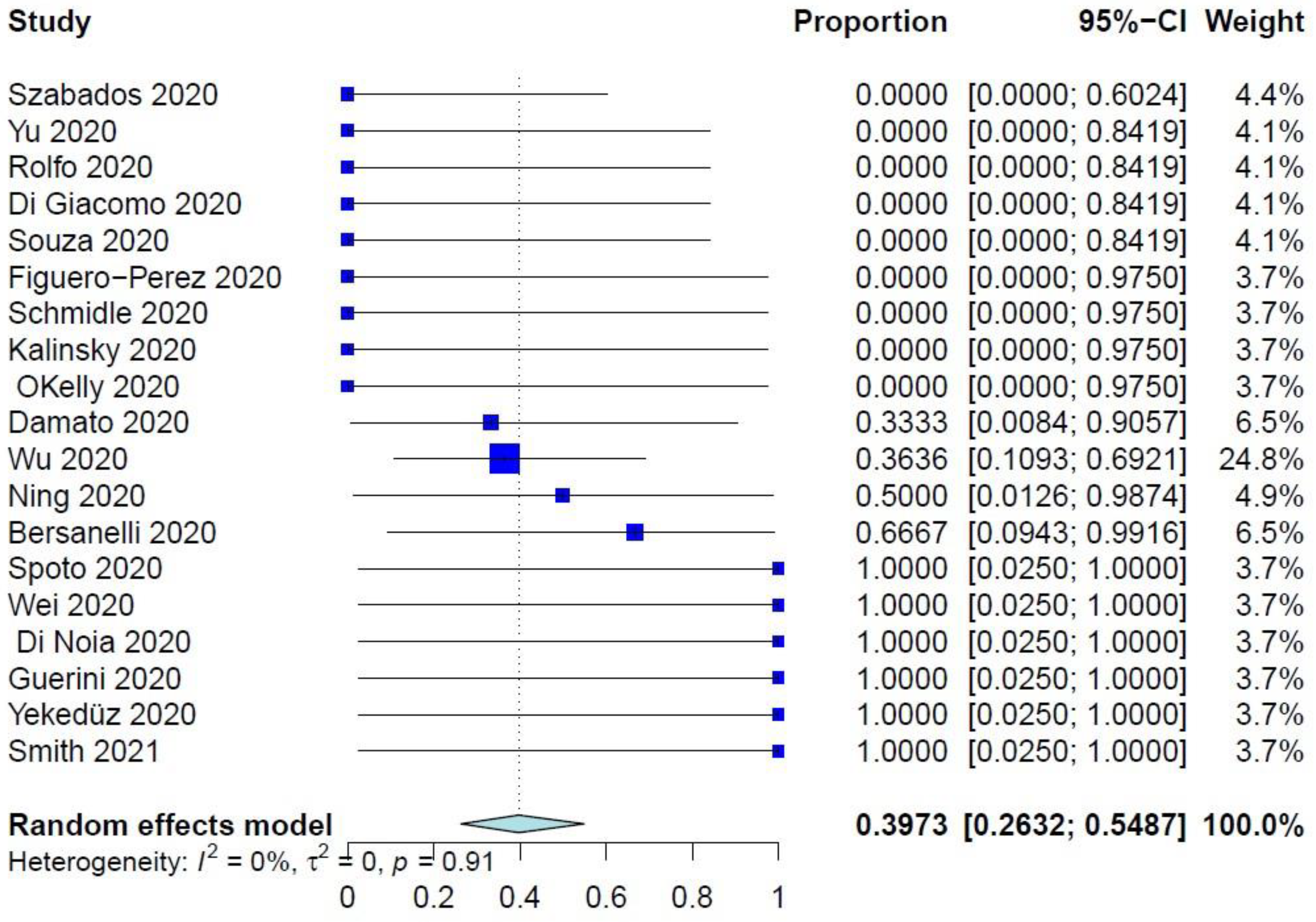
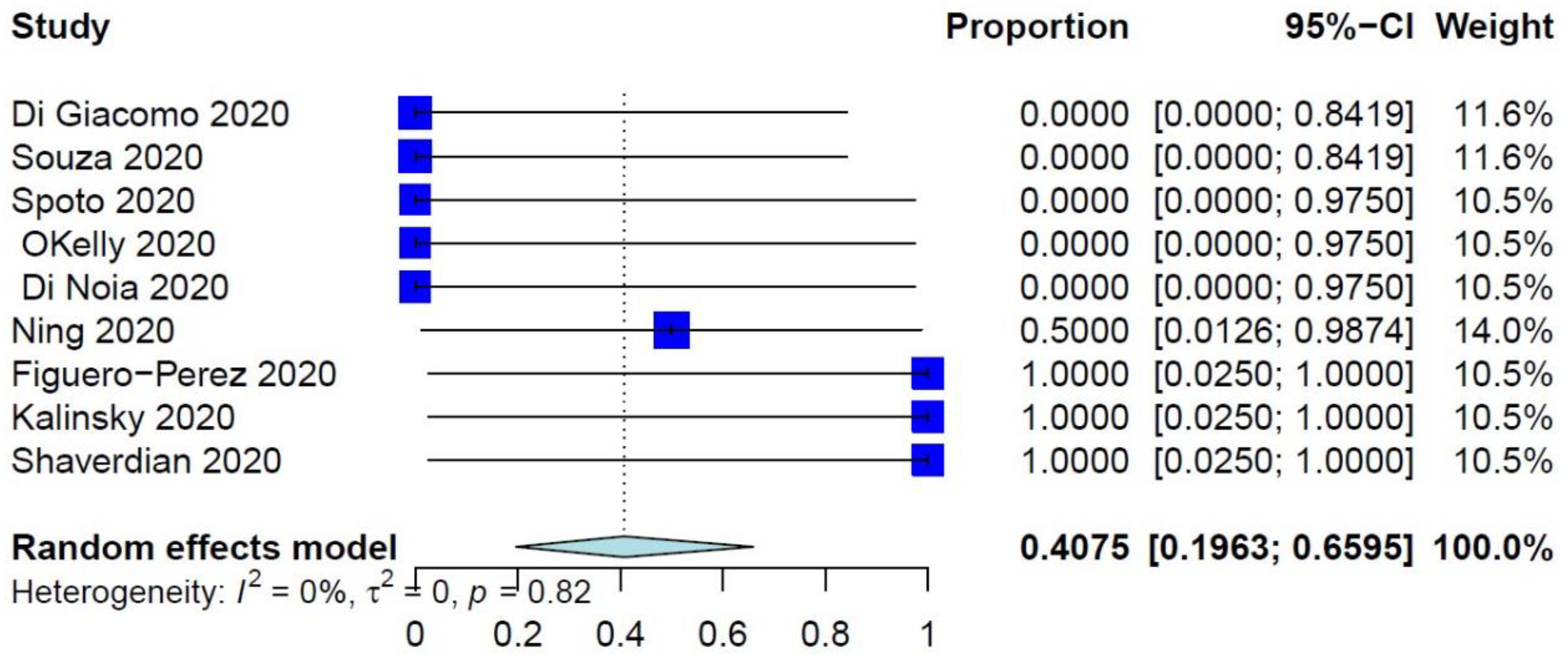
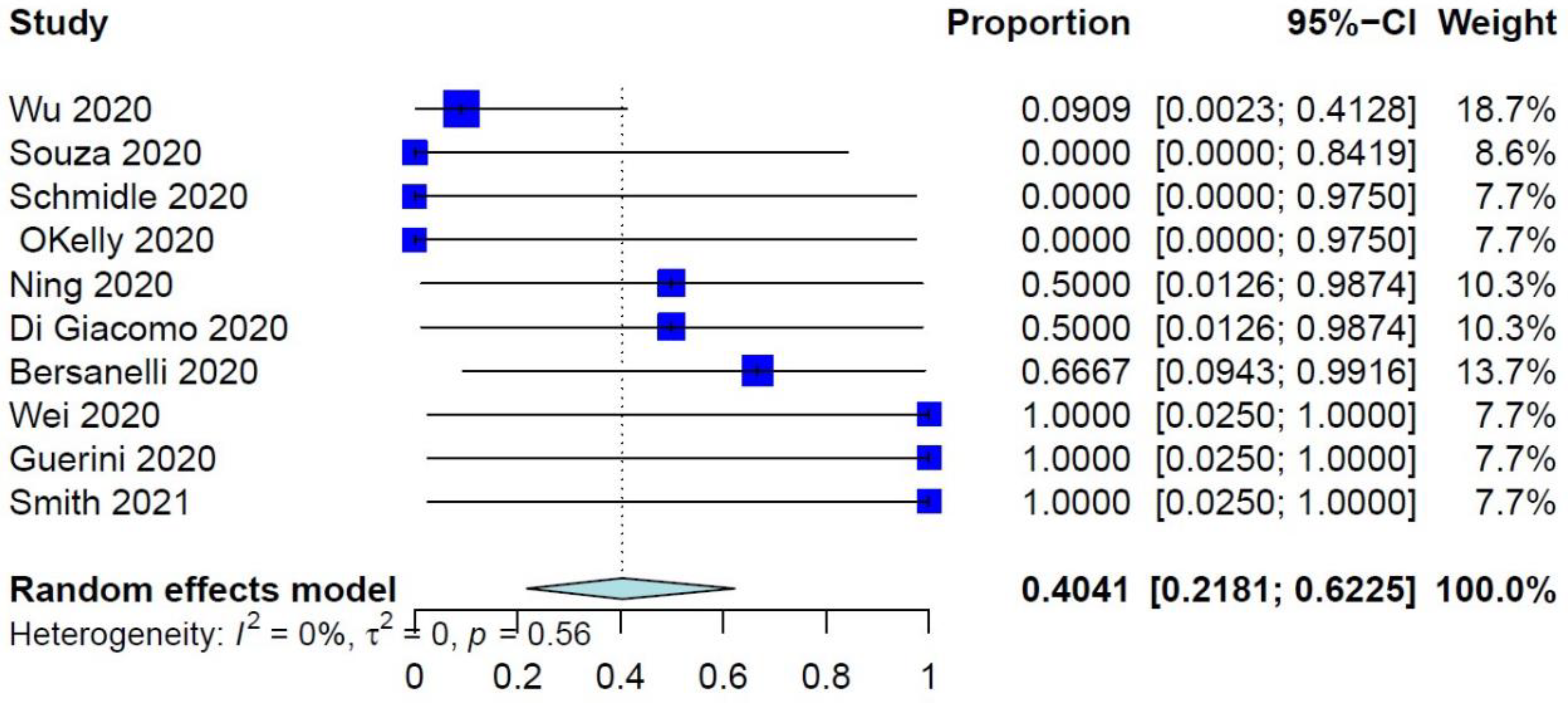
The PER of COVID-19–related mortality was 39.73% (95% CI: 26.32–54.87%) (Figure 1), while the PER of COVID-19–related ER visits, pulmonary complications, and need for ventilation were 40.75% (95% CI: 19.63–65.95%), 40.41% (95% CI: 21.81–62.25%), and 34.92% (95% CI: 17.34–57.86%), respectively (Figure 2 and Figure 3, Supplementary Figure S2). The PER of opportunistic infections was 34.92% (95% CI: 17.34–57.86%) (Supplementary Figure S3). Table 2 and Supplementary Figures S4–S8 show the PERs of the use of antivirals (62.12%), antibiotics (57.12%), steroids (51.36%), prophylactic anticoagulants (41.90%), and convalescent plasma (26.48%). As shown in Table 2, none of the patients in the included studies received antibodies, needed readmission, needed tracheostomy, or developed thrombo-embolic events due to COVID-19 infection. Nevertheless, 27% of patients had airway problems after COVID-19 infection in the nine included studies that assessed this outcome.
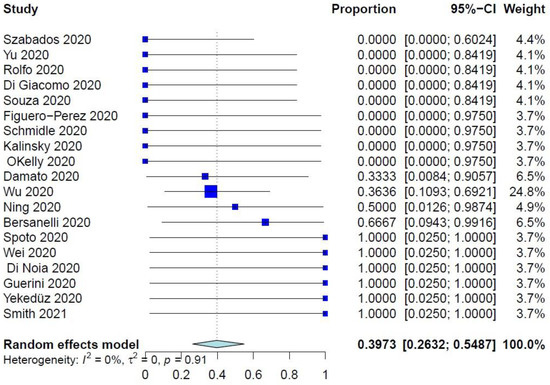
Figure 1.
Forest plot of the primary endpoint of COVID-19–related mortality.
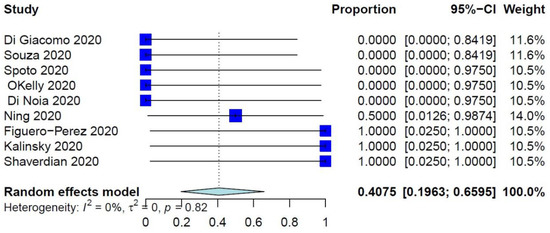
Figure 2.
Forest plot of COVID-19–related ER visits.
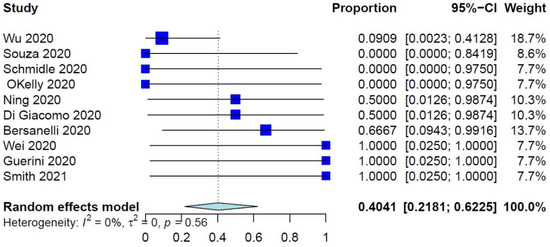
Figure 3.
Forest plots of pulmonary complications due to COVID-19 infection.

Table 2.
Outcomes summary.
The meta-regression (Table 3) indicated a trend toward association between previous respiratory diseases and COVID-19–related mortality (p = 0.0861). No other characteristic showed a significant association with COVID-19–related mortality in the meta-regression analysis.

Table 3.
Meta-regression of COVID-related mortality.
4. Discussion
Our systematic review of COVID-19–related outcomes after ICIs reported the rates of COVID-19–related mortality, ER visits, pulmonary complications, need for a ventilator, and opportunistic infections in cancer patients on ICIs during the COVID-19 pandemic. While there was a trend toward association between previous respiratory diseases and COVID-19-related mortality, no other characteristic was associated with COVID-19-related mortality in the meta-regression analysis.
Immunotherapies have revolutionized cancer care. Nevertheless, immunotherapies modulate the immune system, induce unique adverse events, and are usually administered for long durations. Further, managing the resultant, potentially fatal morbidities after immunotherapies is a clinical challenge, especially during the pandemic [1,14]. However, the exact impact of COVID-19 infection on the risk of mortality and morbidities after immunotherapies is still uncertain. Our data showed that the PER of COVID-19–related mortality was 39.73% in cancer patients treated with ICIs during the pandemic. Similarly high COVID-19–related mortality rates in patients on ICI therapy during the pandemic were reported by Dai et al. (33%) [1] and Robilotti et al. (36%). Yet, Robilotti et al. [15] highlighted that receiving ICIs did not impact the death rate during the COVID-19 era.
While patients on ICIs have a certain level of risk for developing infectious diseases [16], the risk of COVID-19 infection after ICIs increased only after the use of corticosteroids and/or TNF-α inhibitors [17]. However, other studies reported that COVID-19 infection rates are low after ICIs and that receipt of ICIs did not increase the risk of COVID-19 infection [18]. These low rates have been attributed in part to the high compliance with social distancing and mask-wearing in cancer-setting care. Additionally, the immunosuppressive effect of ICIs modulates the cytokine release syndrome associated with severe COVID-19 infection [19,20,21,22]. For these reasons, some ICI-treated patients with COVID-19 infection are asymptomatic and subsequently do not seek to be tested for COVID-19. Further, at certain stages of the treatment course, ICIs restore cellular immunocompetence, which makes patients on ICIs less prone to infection [6,23]. However, close monitoring is still needed.
Based on the data from this meta-analysis, the top presenting COVID-19 symptoms were fever (74%), cough (57%), and dyspnea (52%), while ground glass opacity (64%), infiltrate (27%), and consolidation (27%) were the most common imaging findings. Considering the high rate of pulmonary complications and need for ventilators (40% and 35%), close and cautious monitoring is warranted [24], with particular focus on excluding bacterial co-infection, which has been found to increase the risk of poor outcomes. The similarities in presentation, response to steroids/antibodies, chest imaging findings, and pathological characterization between the lung injury induced by COVID-19 and ICIs are clinical challenges in the management of cases treated with ICIs during the COVID-19 era [2,11,12]. The massive amount of activated immune cells after ICI therapy may delay the diagnosis of COVID-19 infection, as these cells are very hypermetabolic on fluorodeoxyglucose positron emission tomography [25]. Further, steroids could relieve both COVID-19– and ICI-induced lung injury. On the basis of pathological findings after COVID-19 infection (hyaline membrane formation and pulmonary edema), steroids could resolve COVID-19–induced lung injury. However, steroid use should be timely optimized to treat severe respiratory stress after COVID-19 infection [11]. Additionally, monoclonal antibodies showed improvement in levels of organ toxicity induced by either ICIs or COVID-19 [26,27]. Yet, the efficacy of monoclonal antibodies in treating COVID-19–induced injury is still under investigation. Further, the granulocyte colony-stimulating factor and erythropoietin play important roles whenever indicated [28,29].
Managing COVID-19–related complications in patients on ICIs is another challenge. We found that the PER of opportunistic infections was 34.92%. Nevertheless, none of the patients in the included studies needed readmission, needed tracheostomy, or developed thrombo-embolic events due to COVID-19 infection. However, 27% of the patients in nine included studies had airway problems after COVID-19 infection. We also presented PERs of the use of antivirals (62.12%), antibiotics (57.12%), steroids (51.36%), prophylactic anticoagulants (41.90%), and convalescent plasma (26.48%) after COVID-19 infection. Most cancer care centers agree on continuing ICIs after COVID-19 infection [4,30], and Amin et al. advised continuing the standard management of immunotherapy-induced adverse events in these patients as long as protective measures are closely adhered to [21]. Nevertheless, timing is key; since most patients experience immunotherapy-induced adverse events within the first 6 months of treatment [7], patients who are going to start ICIs during the pandemic must be carefully selected and monitored. Furthermore, pathological activation of immune response usually occurs during the late stage of COVID-19 infection [11].
Some authors have explored the effect of treatment frequency and time elapsed after ICIs on COVID-19 infection severity. Robilotti et al. [15] mentioned that ICIs were one of the predictors of the need for hospitalization and developing severe COVID-19 infection, while others did not observe any statistically significant association between receipt of ICIs and the severity of COVID-19 infection [18,31]. We may better explain these findings when we have a better understanding of the crosstalk between the respective immune activation pathways that are secondary to ICI treatment and COVID-19–induced cytokine release syndrome. Nevertheless, modulating the dosage and schedule of ICIs may benefit individual patients [32]. On the other hand, the severity of COVID-19 infection has been observed to be high in patients with lung cancer [33,34], especially after ICIs, as reported by Robilotti et al. [15]. However, Robilotti et al. [15] mentioned that the severity of COVID-19 infection was similarly high in non-lung-cancer patients who had ICIs. Nevertheless, other studies did not find an association between receipt of ICIs and poor outcomes of COVID-19 infection [4,18,33]. Of note, Robilotti et al. attributed the difference between their findings and other studies to their inclusion of more patients and their assessment of infection severity in terms of significant oxygen need rather than death, which was the outcome evaluated by studies that did not show any association between severity and outcomes.
We found a trend toward the association between previous respiratory diseases and COVID-19–related mortality. No other characteristic showed a significant association with COVID-19–related mortality in the meta-regression analysis. Our systematic review provides essential information to guide the care after ICIs during the COVID-19 era. Yet, we acknowledge that the existing data are still limited. Global, harmonized data collection is exceptionally needed to support solid guidelines. We believe that further understanding of the COVID-19- and ICI-induced lung injury will improve our management of patients during the COVID-19 era.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/reports5030031/s1, Figure S1: Preferred reporting items for systematic reviews and meta-analyses diagram of the included studies; Figure S2: Forest plots of the need for ventilation due to COVID-19 infection; Figure S3: Forest plot of opportunistic infections; Figure S4: Forest plot of the use of antivirals for COVID-19 infection; Figure S5: Forest plot of the use of antibiotics for COVID-19 infection; Figure S6: Forest plot of the use of steroids due to COVID-19 infection; Figure S7: Forest plot of the use of prophylactic anticoagulants due to COVID-19 infection; Figure S8: Forest plot of the use of prophylactic convalescent plasma due to COVID-19 infection; Table S1: Ovid MEDLINE search strategy; Table S2: Ovid Embase search strategy; Table S3: Web of Science search strategy; Table S4: Cochrane Library search strategy; Table S5: Overall baseline patient demographics; Table S6: Newcastle–Ottawa Scale of included studies.
Author Contributions
Conceptualization, M.K. and Y.G.; methodology, M.K., Y.G. and M.B.; software, M.B.; validation, M.K., Y.G., M.B., A.Q. and J.J.; formal analysis, M.B.; investigation, M.K., Y.G., M.B., A.Q. and J.J.; data curation, M.K., Y.G., M.B., A.Q. and J.J.; writing—original draft preparation, M.K., Y.G. and M.B.; writing—review and editing, M.K., Y.G., M.B., A.Q. and J.J.; visualization, M.B. and M.K.; supervision, M.K.; project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the support from the Research Medical Library at The University of Texas MD Anderson Cancer Center, especially the great help from Sarah Bronson for editing the draft.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, K.R.; Ramaiya, N.H.; Laukamp, K.R.; Gupta, A. Immune Checkpoint Inhibitor Therapy–related Pneumonitis: Patterns and Management. RadioGraphics 2019, 39, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020, 12, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.Y.; Cazier, J.B.; Angelis, V.; Arnold, R.; Bisht, V.; Campton, N.A.; Chackathayil, J.; Cheng, V.W.; Curley, H.M.; Fittall, M.W.; et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Szabados, B.; Abu-Ghanem, Y.; Grant, M.; Choy, J.; Bex, A.; Powles, T. Clinical Characteristics and Outcome for Four SARS-CoV-2-infected Cancer Patients Treated with Immune Checkpoint Inhibitors. Eur. Urol. 2020, 78, 276–280. [Google Scholar] [CrossRef]
- Bersanelli, M.; Scala, S.; Affanni, P.; Veronesi, L.; Colucci, M.E.; Banna, G.L.; Cortellini, A.; Liotta, F. Immunological insights on influenza infection and vaccination during immune checkpoint blockade in cancer patients. Immunotherapy 2020, 12, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.R.; Ju, Z.Y.; He, W.F. Advances in the research of mechanism and related immunotherapy on the cytokine storm induced by coronavirus disease 2019. Zhonghua Shao Shang Za Zhi 2020, 36, 471–475. [Google Scholar]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Artigas, C.; Lemort, M.; Mestrez, F.; Gil, T.; Flamen, P. COVID-19 Pneumonia Mimicking Immunotherapy-Induced Pneumonitis on 18F-FDG PET/CT in a Patient Under Treatment with Nivolumab. Clin. Nucl. Med. 2020, 45, e381–e382. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 March 2022).
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.V.; Babady, N.E.; Mead, P.A.; Rolling, T.; Perez-Johnston, R.; Bernardes, M.; Bogler, Y.; Caldararo, M.; Figueroa, C.J.; Glickman, M.S.; et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020, 26, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kim, Y.H.; Kanai, O.; Yoshida, H.; Mio, T.; Hirai, T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir. Med. 2019, 146, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, M.; Romero, F.A.; Argüello, E.; Kyi, C.; Postow, M.A.; Redelman-Sidi, G. The Spectrum of Serious Infections Among Patients Receiving Immune Checkpoint Blockade for the Treatment of Melanoma. Clin. Infect. Dis. 2016, 63, 1490–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Rizvi, H.; Egger, J.V.; Preeshagul, I.R.; Wolchok, J.D.; Hellmann, M.D. Impact of PD-1 Blockade on Severity of COVID-19 in Patients with Lung Cancers. Cancer Discov. 2020, 10, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, L.; Leung, T. Evaluating the effectiveness of social distancing interventions to delay or flatten the epidemic curve of coronavirus disease. Emerg. Infect. Dis. 2020, 26, 1740. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Yang, L.; Tonelli, R.; Barbagallo, M.; Lopalco, P.; Lagolio, E.; Celotto, S.; Pizzol, D.; Zou, L.; et al. Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature. Front. Med. 2020, 7, 170. [Google Scholar] [CrossRef]
- Amin, R.; Thomas, A.S.; Khurana, S.; Panneerselvam, K.; Zou, F.; Ma, W.; Chari, S.T.; Wang, Y. Management of Immune-Related Colitis During the COVID-19 Pandemic. Inflamm. Bowel Dis. 2020, 26, e110–e111. [Google Scholar] [CrossRef]
- Rotz, S.J.; Leino, D.; Szabo, S.; Mangino, J.L.; Turpin, B.K.; Pressey, J.G. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr. Blood Cancer 2017, 64, e26642. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Giannarelli, D.; Castrignanò, P.; Fornarini, G.; Panni, S.; Mazzoni, F.; Tiseo, M.; Rossetti, S.; Gambale, E.; Rossi, E.; et al. Influenza vaccine indication during therapy with immune checkpoint inhibitors: A transversal challenge. The INVIDIa Study. Immunotherapy 2018, 10, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Souza, I.L.; Fernandes, Í.; Taranto, P.; Buzaid, A.C.; Schvartsman, G. Immune-related pneumonitis with nivolumab and ipilimumab during the coronavirus disease 2019 (COVID-19) pandemic. Eur. J. Cancer 2020, 135, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, H.; Shen, H.; Li, Z.; Geng, J.; Han, H.; Cai, J.; Li, X.; Kang, W.; Weng, D.; et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 200, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Stroud, C.R.; Hegde, A.; Cherry, C.; Naqash, A.R.; Sharma, N.; Addepalli, S.; Cherukuri, S.; Parent, T.; Hardin, J.; Walker, P. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J. Oncol. Pharm. Pract. 2019, 25, 551–557. [Google Scholar] [CrossRef]
- Horisberger, A.; La Rosa, S.; Zurcher, J.P.; Zimmermann, S.; Spertini, F.; Coukos, G.; Obeid, M. A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J. Immunother. Cancer 2018, 6, 156. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, A.; Mortezazadeh, M.; Kolahdouzan, K.; Alavian, G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J. Med. Virol. 2020, 92, 915–918. [Google Scholar] [CrossRef] [Green Version]
- Sereno, M.; Gutiérrez-Gutiérrez, G.; Sandoval, C.; Falagan, S.; Jimenez-Gordo, A.M.; Merino, M.; López-Menchaca, R.; Martínez-Martin, P.; Roa, S.; Casado, E.; et al. A favorable outcome of pneumonia COVID 19 in an advanced lung cancer patient with severe neutropenia: Is immunosuppression a risk factor for SARS-CoV2 infection? Lung Cancer 2020, 145, 213–215. [Google Scholar] [CrossRef]
- Vivarelli, S.; Falzone, L.; Grillo, C.M.; Scandurra, G.; Torino, F.; Libra, M. Cancer Management during COVID-19 Pandemic: Is Immune Checkpoint Inhibitors-Based Immunotherapy Harmful or Beneficial? Cancers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Wu, Q.; Chu, Q.; Zhang, H.; Yang, B.; He, X.; Zhong, Y.; Yuan, X.; Chua, M.L.; Xie, C. Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors. Cancer Commun. 2020, 40, 374–379. [Google Scholar] [CrossRef]
- Goldstein, D.A.; Ratain, M.J.; Saltz, L.B. Weight-Based Dosing of Pembrolizumab Every 6 Weeks in the Time of COVID-19. JAMA Oncol. 2020, 6, 1694–1695. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Rizvi, H.; Preeshagul, I.R.; Egger, J.V.; Hoyos, D.; Bandlamudi, C.; McCarthy, C.G.; Falcon, C.J.; Schoenfeld, A.J.; Arbour, K.C.; et al. COVID-19 in patients with lung cancer. Ann. Oncol. 2020, 31, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).