Abstract
Background: The COVID-19 pandemic exploits existing inequalities in the social determinants of health (SDOH) that influence disease burden and access to healthcare. The role of health behaviours and socioeconomic status in genitourinary (GU) malignancy has also been highlighted. Our aim was to evaluate predictors of patient-level and neighbourhood-level factors contributing to disparities in COVID-19 outcomes in GU cancer patients. Methods: Demographic information and co-morbidities for patients screened for COVID-19 across the Mount Sinai Health System (MSHS) up to 10 June 2020 were included. Descriptive analyses and ensemble feature selection were performed to describe the relationships between these predictors and the outcomes of positive SARS-CoV-2 RT-PCR test, COVID-19-related hospitalisation, intubation and death. Results: Out of 47,379 tested individuals, 1094 had a history of GU cancer diagnosis; of these, 192 tested positive for SARS-CoV-2. Ensemble feature selection identified social determinants including zip code, race/ethnicity, age, smoking status and English as the preferred first language—being the majority of significant predictors for each of this study’s four COVID-19-related outcomes: a positive test, hospitalisation, intubation and death. Patient and neighbourhood level SDOH including zip code/ NYC borough, age, race/ethnicity, smoking status, and English as preferred language are amongst the most significant predictors of these clinically relevant outcomes for COVID-19 patients. Conclusion: Our results highlight the importance of these SDOH and the need to integrate SDOH in patient electronic medical records (EMR) with the goal to identify at-risk groups. This study’s results have implications for COVID-19 research priorities, public health goals, and policy implementations.
1. Introduction
The current COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), represents the third occurrence of widespread disease caused by a coronavirus in 20 years [1]. First identified in Wuhan, China, in December 2019, the rapid spread of SARS-CoV-2 has produced over 7 million cases and over 350,000 deaths worldwide as of June 2020 [2]. Emerging global data indicates older age, male sex, and several underlying conditions/diseases are predisposing factors to higher severity COVID-19 disease [3,4]. Furthermore, immunocompromised patients with cancer appear to be more susceptible to infection, have a higher risk of severe events, and ultimately poorer outcomes [5]. Pathogenesis of SARS-CoV-2 infection is mediated, in part, by angiotensin-converting enzyme 2 (ACE-2) and transmembrane protease serine 2 (TMPRSS2). SARS-CoV-2 host cell entry is facilitated by viral spike proteins, primed by TMPRSS2-mediated cleavage, which bind to ACE-2 and gain access [6]. TMPRSS2 is highly expressed in prostate epithelial cells; a minor percentage of the prostate club and hillock cells express both ACE-@ and TMPRSS2 [7]. Additionally, prostate adenocarcinoma cells may have the highest TMPRSS2 expression of all cancers, highlighting the need to further examine the relationship between genitourinary (GU) cancer and COVID-19 [8].
First described over 100 years ago by sociologists such as W.E.B. DuBois, social determinants of health (SDOH) are conditions in which people are born, raised, and currently live in, and the greater socioecological systems creating the economic policies, political systems, and social norms that shape the conditions of daily life. SDOH are primarily responsible for the severe health inequities seen today [9,10]. Factors such as race and socioeconomic status have been repeatedly linked to differences in overall health and survival of communities in all medical literature, including in the field of urology [9,11]. Mortality rates for GU malignancies also vary in rural and urban dwellings [12,13,14]. Disparities related to prostate cancer, bladder cancer, and kidney cancer—three of the most commonly diagnosed malignancies in the United States—are heavily linked to patient and community (i.e., neighbourhood) levels SDOH. As examples, low SES communities have high prostate cancer mortality rates (e.g., Appalachian, Kentucky residents) and the mortality rate of African American men with prostate cancer is 2.4 times higher than White men; men with less than high school education have a 20% increased risk of bladder cancer compared to those with postgraduate education; and kidney cancer mortality directly correlates to lower-ranked healthcare systems and lower healthcare expenditures [15,16,17,18].
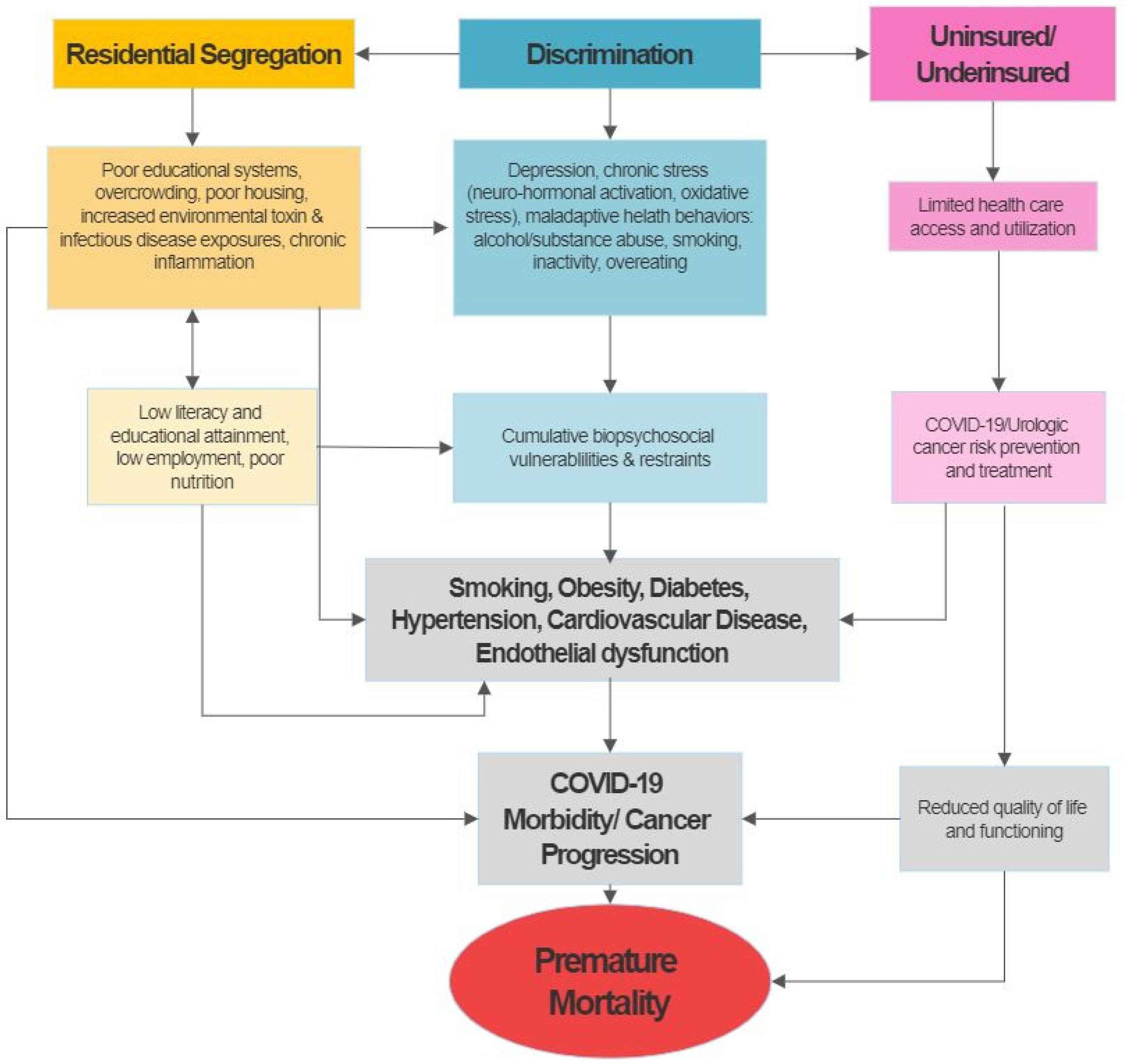
To explore the potential impact of patient and neighbourhood level SDOH on COVID-19 outcomes, we propose to adapt Nicholas and colleagues’ Socioeconomic Deprivation and Chronic Kidney Disease model to examine potential clinical (e.g., comorbidities), behavioural (e.g., smoking, obesity), and neighbourhood predictors (zip code), of COVID-19 outcomes. This conceptual framework emphasises the importance of socioeconomic factors as a mediator of key disease prevention and treatment pathways and highlights its vast impact on urologic disease outcomes (Figure 1). The figure shows that many of the determinants of disparities, such as comorbidities including diabetes and hypertension, may have their foundation in socioeconomic deprivation and its consequences. These include, but are not limited to, discrimination and segregation, substandard living conditions, limited access to quality healthcare among the uninsured or underinsured, limited health literacy, and chronic stress resulting in measurable and quantifiable pathologic factors that contribute to and enhance the development of urologic disease and eventually premature mortality [9,11,15]. Increasing evidence from the COVID-19 reports and emerging data points to potential overlaps in drivers of health disparities in both urologic cancer and COVID-19, suggested that factors fuelling cancer disparities also rendered this patient population more vulnerable to worse COVID-19 outcomes (i.e., morbidity and mortality).
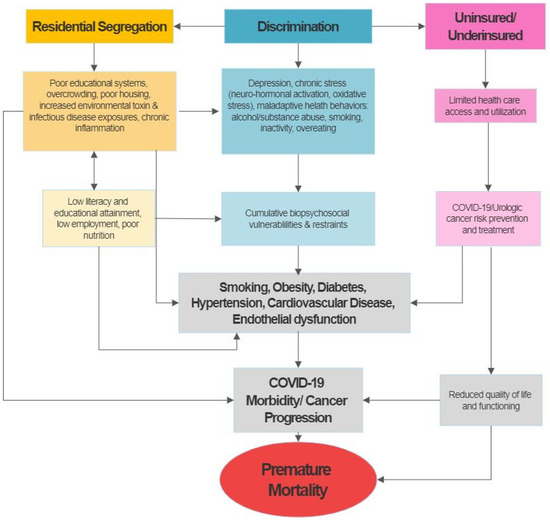
Figure 1.
Theoretical framework for the potential of socioeconomic, behavioral, psychosocial and medical co-morbidities as drivers of GU cancers and COVID-19 disparities in the United States (adapted from Nicholas et al.) [11].
This current analysis of a multi-ethnic cohort of GU cancer patients aims to delineate the patient- and neighbourhood-level factors contributing to disparities in COVID-19 test positivity, morbidity and mortality.
2. Materials and Methods
2.1. Patient Data
Symptomatic patients presenting to the Mount Sinai Healthcare System (MSHS), a network of 10 institutes and facilities across New York City, were tested and included in this dataset (n = 47,379). SARS-CoV-2 testing was performed by reverse transcriptase PCR assay following a nasopharyngeal swab. The MSHS Ethics Committee approved a waiver of documentation of informed consent.
De-identified patient data was obtained from the MSHS Data Warehouse (https://msdw.mountsinai.org/). Demographic and social determinants available for analysis included age, sex, and first language preference being English, as well as race/ethnicity and smoking status. City borough of residence, hereafter referred to as “zip -code”, was derived from the first three digits of a patient’s zip code and included in models.
The MSHS Ethics Committee approved a waiver of documentation of informed consent; de-identified patient data was obtained from the MSHS Data Warehouse (https://msdw.mountsinai.org/).
2.2. Statistical Analysis
Continuous data were presented as medians (interquartile range [IQR]) and categorical data were presented as numbers (percentage). The χ2 test was used to compare differences in clinical outcomes between COVID test-Positive and COVID test-Negative groups. No single feature selection methodology seems capable of ensuring optimal results in predictive performance and stability in medical datasets, therefore, ensemble feature selection (EFS) was utilised to overcome these limitations. EFS reduces data dimensionality, removing irrelevant, redundant, or confounding features, leaving only those most relevant to the outcome [16,17].
Briefly; six feature selection methods for binary classifications were utilised; namely median, Pearson, Spearman-correlation, logistic regression, and two variable importance measures embedded in the random forest algorithm. The median method compares positive samples with negative samples by a Mann–Whitney U Test; the smaller the p-value, the higher the importance. Spearman-correlation was used to select features that are highly correlated with the dependent variable, but showed low correlation with other features and avoids multi-collinearity. Logistic regression involves a pre-processing step (Z-transformation) to ensure comparability between the different ranges of feature values and the β-coefficients of the resulting regression equation represent the importance measure. The random forest are themselves ensembles of multiple decision trees, which gain their randomness from the randomly chosen starting feature for each tree [19]. The random forest approach provides an importance measure based on the Gini-index (Gini_RF), which measures the node impurity in the trees and the error rate-based method (ER_RF) measure the difference before and after permuting the class variable. Each feature selection method was normalised to a common scale—an interval from 0 to 1/n—where n is the number of conducted feature selection methods (Figure 2).
A simple social determinants risk scale was computed by assigning equal weight to each of the five identified features per respective COVID-19 outcome: testing positive, hospitalisation, intubation and death. Calculated scores for each patient were determined and charted against the proportion of patients who experienced each outcome (Figure 3). All analyses were performed using R software [20].
3. Results
Of 47,379 tested patients, 10,444 (22%) tested positive for SARS-CoV-2. There were 1094 GU cancer patients in this cohort with 192 (17.6%) who tested positive. This cohort includes 659 prostate cancer patients with 134 (20.3%) who tested positive; bladder cancer patients (n = 283) with 37 (13.1%) confirmed positive; kidney cancer patients (n = 194) with 10.3%, 16% (n = 31) tested positive, and testis (n = 29) cancer patients of whom 10.3% (n = 3) tested positive for SARS-CoV-2. Of all 192 GU cancer patients who tested positive for SARS-CoV-2, 128 were hospitalised, 19 were intubated, and 39 died of the disease (Table 1).

Table 1.
Urologic cancer patient social and clinical demographics. * indicates p < 0.05; † indicates p < 0.001. Total percentages for prostate, bladder, kidney and testis cancer do not sum to 100% as patients may have had a diagnosis of more than one malignancy.
Of the 1094 GU cancer patients that presented to MSHS for testing, 997 (91.1%) were male. Regarding their race/ethnicity, 459 (42%) were White, 273 (25.5%) were of African ancestry, 204 (18.6%) were Hispanic/Latinx, 41 (37.5%) were Asian, and 117 (10.7%) were Other/Unknown. There was a significant difference in the rate of positive tests between these groups (p < 0.001), with the highest proportion of positive tests occurring in Hispanic/Latinx patients with GU cancer (31.3%). There were no significant statistical differences in the rates of hospitalisation, intubation, or death between these groups.
Older age was associated with a significantly higher risk of testing positive (p = 0.002), being hospitalised (p=0.01) and death due to COVID-19 in this cohort (p = 0.01) but not intubation (p = 0.09) (Table 1).
The majority of patients (933; 85%) in this cohort spoke English as their preferred first language. While there were no statistical differences between those who did and did not speak English as their first language for each of the outcomes on univariate analysis, English was the preferred first language of 81% of patients who tested positive and 74.4% who expired due to COVID-19 in this cohort.
The borough of residence was significantly associated with testing positive for COVID-19 and intubation (p = 0.009), but not hospitalisation or death. Manhattan had the highest rate of positive tests (108/513; 21.1%) and Queens had the highest mortality rate (12/28; 42.9%). There was a significant difference in ever smokers vs. never smokers receiving COVID-19 diagnosis (p = 0.004). Additionally, while the rates of hospitalisation (68% vs. 65.3%), intubation (11.3% vs. 8.4%) and death (23.7% vs. 16.8%) were higher amongst ever smokers, these were not significant.
Figure 2 outlines the results of EFS analysis, where age, race/ethnicity, diabetes, current/former smoker and prostate cancer diagnosis were the top five features selected as the most parsimonious, and biologically reasonable model to describe the relationship between those testing positive, and the features from social, demographic and medical co-morbidities. In a similar manner, the identified features in this cohort predicting the risk of hospitalisation were: older age, prostate and bladder cancer diagnosis, hypertension, and zip code. For risk of intubation, the identified factors were zip code, age, obesity, English as the preferred 1st language, and hypertension. For risk of death, the identified factors were: age, zip code, race/ethnicity, prostate cancer, and smoking status.
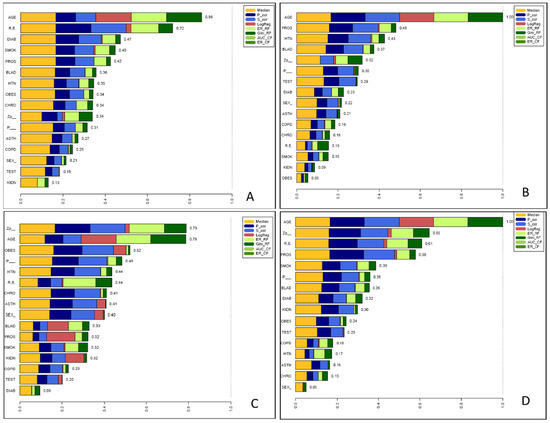
Figure 2.
Ensemble feature analysis (EFS) of social, demographic and medical co-morbidities of GU cancer patients for the risk of (A) Positive COVID-19 test, (B) hospitalisation, (C) intubation and (D) death from COVID-19. EFS combined six feature selection methods—namely median, Pearson (P_cor), Spearman-correlation (S_cor), logistic regression (LogReg) and two two variable importance measures embedded in the random forest algorithm; the Gini index (Gini_RF) and error rate based detection (ER_RF). Each selection method was normalised to a common scale; a score of 1 indicates this parameter is the most prominent parameter selected by every method. R.E = race/ethnicity; DIAB = diabetes mellitus; SMOK= current/former smoker; PROS = prostate cancer diagnosis; BLAD = bladder cancer diagnosis; HTN = hypertension; OBES = obese; CHRO = chronic HIV infection; Zp_= zip code; P_ = English as the preferred 1st language; ASTH = asthma; COPD = chronic obstructive pulmonary disease; KIDN = chronic kidney disease.
Figure 2.
Ensemble feature analysis (EFS) of social, demographic and medical co-morbidities of GU cancer patients for the risk of (A) Positive COVID-19 test, (B) hospitalisation, (C) intubation and (D) death from COVID-19. EFS combined six feature selection methods—namely median, Pearson (P_cor), Spearman-correlation (S_cor), logistic regression (LogReg) and two two variable importance measures embedded in the random forest algorithm; the Gini index (Gini_RF) and error rate based detection (ER_RF). Each selection method was normalised to a common scale; a score of 1 indicates this parameter is the most prominent parameter selected by every method. R.E = race/ethnicity; DIAB = diabetes mellitus; SMOK= current/former smoker; PROS = prostate cancer diagnosis; BLAD = bladder cancer diagnosis; HTN = hypertension; OBES = obese; CHRO = chronic HIV infection; Zp_= zip code; P_ = English as the preferred 1st language; ASTH = asthma; COPD = chronic obstructive pulmonary disease; KIDN = chronic kidney disease.
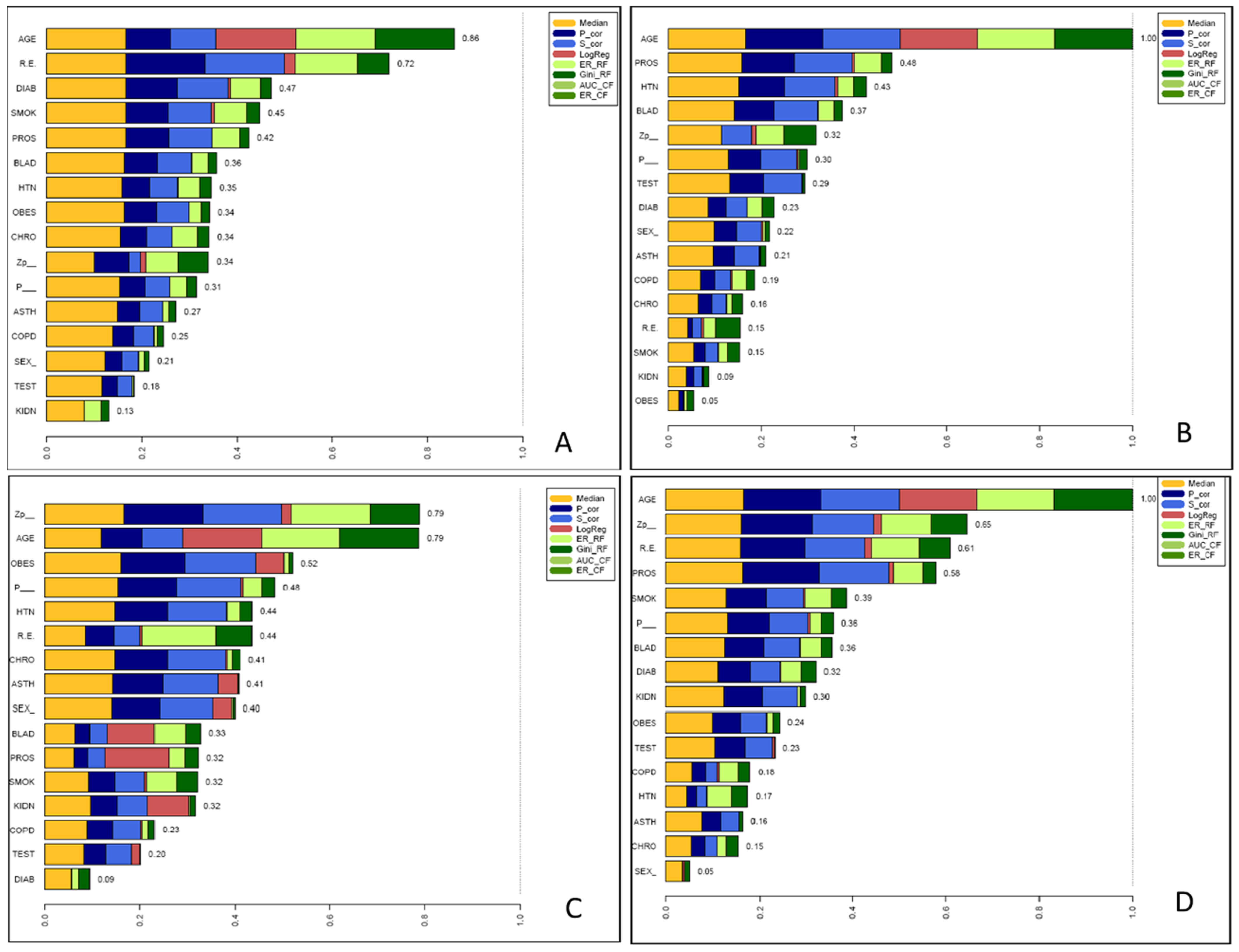
Figure 3 demonstrates progressive increases in the risk for each outcome across the scale from 0 through to ≥4 for the risk of a positive test, risk of intubation, and risk of death. Factors comprising each scale are also displayed in Table 2. For GU cancer patients who scored ≥4 points or more on these scales, more than 30% tested positive for COVID-19, 10% were hospitalised, more than 80% were intubated, and over 50% died. (Figure 3).

Table 2.
The most parsimonious parameters as identified EFS analysis for each of this study’s outcomes: risk of testing positive for SARS-CoV-2 infection and COVID-19-related hospitalisation, intubation and death in a cohort of patients with genitourinary cancers.
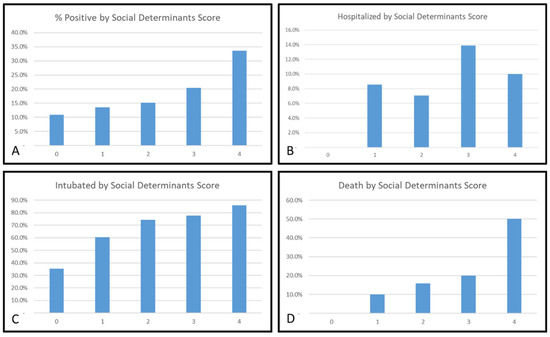
Figure 3.
Relationship between social and medical determinants and COVID-19 outcomes: (A) positive COVID-19 test, (B) hospitalisation, (C) intubation and (D) mortality for patients with urologic cancers. A unique social determinant scale of (A) age, race/ethnicity, diabetes, current/former smoker, prostate cancer; (B) age, prostate cancer, hypertension, bladder cancer, zip code, (C) zip code, age, obesity, English as preferred 1st language, hypertension and (D) age, zip code, race/ethnicity, prostate cancer, current/former smoker was created—in which each determinant was awarded 1 point. Of those urologic cancer patients who scored 4 points on these scales, >30% tested positive for COVID-19; 10% were hospitalised, >80% were intubated and >50% died.
Figure 3.
Relationship between social and medical determinants and COVID-19 outcomes: (A) positive COVID-19 test, (B) hospitalisation, (C) intubation and (D) mortality for patients with urologic cancers. A unique social determinant scale of (A) age, race/ethnicity, diabetes, current/former smoker, prostate cancer; (B) age, prostate cancer, hypertension, bladder cancer, zip code, (C) zip code, age, obesity, English as preferred 1st language, hypertension and (D) age, zip code, race/ethnicity, prostate cancer, current/former smoker was created—in which each determinant was awarded 1 point. Of those urologic cancer patients who scored 4 points on these scales, >30% tested positive for COVID-19; 10% were hospitalised, >80% were intubated and >50% died.
4. Discussion
This is one of the first studies to report on the impact of SDOH on COVID-19 outcomes for patients with urologic cancers. Our study findings provide evidence for the effects of patient and neighbourhood levels of SDOH on COVID-19 test positivity, morbidity, and mortality. The diverse cohort and geographic variation within New York City allowed for novel identification of underlying SDOH patient and neighbourhood-specific factors influencing COVID-19 outcomes.
Our results show that patient and neighbourhood-SDOH-specific factors (e.g., age, race, zip Code) play decisive roles in GU cancer morbidity and mortality from COVID-19. As this data reveals, zip code and race/ethnicity, established proxies for socioeconomic class, are strongly associated with urologic cancer patient outcomes following COVID-19 diagnosis. These findings highlight the importance of SDOH and the need to comprehensively address individual patient’s risk factors among GU-cancer patients.
4.1. Urological Cancer Outcomes and Social Determinants
The relationship between urologic cancer outcomes and socioeconomic class has been well described in the literature. In regards to bladder cancer, more patients present with advanced-stage disease in countries with the highest poverty levels; white-collar workers with bladder cancer have a longer length of survival than blue-collar workers; and even when adjusted for smoking status, people with less than high school education have a 20% higher risk of bladder cancer compared to those with postgraduate education [21,22,23]. Similar data have been published regarding prostate and kidney cancer, ultimately demonstrating that lower income is a predictor of more advanced-stage cancer and worse postoperative outcomes [24,25,26].
Not only is socioeconomic class itself largely impactful on urologic cancer outcomes, it is also uniquely linked to behavioural factors that also correlate to more severe outcomes. Behavioural factors such as diet, physical activity, and smoking all contribute to the development of co-morbidities such as hypertension and obesity, which are associated with worse outcomes in this analysis. In this cohort, smoking was also associated with testing positive and death after COVID-19 diagnosis. As one of the largest risk factors for bladder and kidney cancer diagnosis, smoking is two times more prevalent in those who are below the poverty level versus those above it [27]. Additionally, poor diet and nutritional deficiency at the time of cystectomy or nephrectomy predicts perioperative mortality and 90-day mortality [28]. These health behaviours develop from far more than just individual decision-making; they are influenced by many systemic, socioeconomic and cultural factors critical for urologists to recognise.
Large disparities also exist regarding physical and built environments in relation to urologic cancers. As shown in this analysis as well, location and zip code significantly impact health outcomes for urologic cancer patients. The role of occupational environment on urologic cancer diagnosis has been extensively studied. Blue-collar workers such as car mechanics, construction workers, painters, and factory workers have much higher exposure to bladder and renal carcinogens [29]. Furthermore, environmental pollutants such as low levels of arsenic in drinking water have been associated with increased bladder, prostate, and kidney cancer risk [30,31].
A communities’ built environments are also one of the major factors contributing to differing access to healthcare. Non-insured patients and those with Medicaid have 60% greater odds of presenting with more locally-advanced cancer and 50–70% greater mortality, compared to privately insured patients. This may be explained in part by Schrag and colleagues’ analysis of Medicare/Medicaid data that found that only 40% of non-privately insured patients received adequate follow-up after bladder cancer diagnosis [32]. Even if patients are insured, factors such as distrust in the medical community, inadequate transportation, and provider density contribute to treatment delays. Additionally, higher hospital volume, specifically in New York State, is tied to better outcomes, including decreased operative mortality and decreased length of stay, after major cancer surgeries such as prostatectomy and cystectomy [33]. As reflected in this study, where a person with urologic cancer lives, it significantly impacts their mortality through multiple avenues. Especially when considering the well-documented differences in geographic access and availability of care in the COVID-19 era, this analysis further supports that where one lives directly impacts one’s health.
4.2. Social Disparities in COVID-19
Understanding COVID-19-related racial/ethnic disparities can be challenging, as they are often rooted in historic, socio-structural inequalities. Minorities are often subjected to living in segregated, suboptimal neighbourhoods with poor housing and environmental conditions, as well as limited economic mobility and access to healthcare—a complex interplay of factors that may contribute to increased susceptibility and vulnerability to COVID-19. Racial/ethnic disparities in COVID-19 may also stem from labour inequalities, lack of workplace protections, and large household size, which decrease the ability to adhere to social distancing. Additionally, racial/ethnic minorities are more likely to have respiratory and cardio-metabolic comorbidities due to suboptimal built environments that reduce opportunities for engaging in health promoting activities and may be in close proximity to petrochemical and manufacturing plants or superfund sites.
This study also demonstrated that non-modifiable determinants such as age and race/ethnicity were among the top predictors of worse outcomes following COVID-19 diagnosis. This finding, specifically for urologic cancer patients, echoes numerous recent reports of racial disparities in COVID-19 outcomes in the general population. In the United States, African Americans and Hispanic people have experienced significantly higher COVID-19 mortality than White people [34]. Along with race, increasing age was another non-modifiable factor found to significantly impact COVID-19 outcomes for urologic cancer patients. While these older adults are more likely to be diagnosed with a urologic malignancy, there are other social determinants closely associated with ageing, which negatively impacts health outcomes for older adults [18]. Differences in employment, caretaker roles, language barriers, and transportation access are just a few of the many SDOH that can be attributed to worse health outcomes among older patients. The evident influence that these non-modifiable individual factors have on health outcomes highlights the need for the medical field to address these disparities and actively work towards reducing them.
Our results confirm shared demographic and clinical characteristics between COVID-19 risk and documented urologic cancer health disparities in the U.S. (e.g., race, older age, comorbidities). In order to reduce the effect of COVID-19 on existing urologic cancer disparities and to improve the health of vulnerable patients, healthcare systems must invest in optimising clinical cancer care and reducing the risk of infection and worse outcomes of COVID-19. A recent COVID-19 paper argued that parameters for the prediction of the need for admission to ICUs are urgently needed for patients with nephritis to enable timely management and appropriate resource allocation [35]. Routine data collection of differential clinical (morbidity, mortality) and SDOH (socioeconomic factors, healthcare access, physical environment, individual and collective health behaviours) within electronic medical records and health equity surveillance systems are necessary to optimise understanding of the cancer–COVID-19 double burden [36,37,38,39,40]. The surveillance system would benefit from local knowledge and active involvement of clinical supportive care staff (e.g., oncology and medical social workers) to facilitate understanding of broader contextual factors that can drive mortality and morbidity associated with outbreaks, including COVID-19 [35,36,37,41].
4.3. Strengths and Limitations
Our study presents important findings. With the largest sample size to date, of over 47,000 patients from the epicentre of the pandemic in the U.S., our study is reflective of a broad patient demographic and outcomes in New York City. However, our study does have limitations. The specific cancer clinical information (e.g., time since diagnosis and treatment received) is not available in the dataset, therefore, the severity of the cancer’s stage cannot be determined. Nor do we have data on the long-term outcomes for these patients to assess for post-intensive care syndrome [42]. Another limitation is that our study does not account for cases and deaths outside of the MSHS system, such as patients who were homebound or in nursing homes and other care facilities.
5. Conclusions
This large population-based cohort of patients tested for COVID-19 was taken from the epicentre of the pandemic in the U.S. Our results show that SDOH, including zip code/ NYC borough, age, race/ethnicity, smoking status, and English as the preferred language are significant predictors of COVID-19 outcomes in patients with GU cancers. Our results highlight the importance of taking SDOH into consideration when addressing each individual patient’s risk factors in patients with GU cancers.
We found that various medical and social determinants, when used together in a point scoring system, can risk stratify those GU cancer patients susceptible to COVID-19 diagnosis, hospitalisation, intubation, and death. Urologists, oncologists and others involved in the care of GU cancer patients should consider and account for the importance of social determinants when managing patients.
Author Contributions
R.A.M.: manuscript writing and editing; J.S.O.: manuscript writing and editing; B.D.K.: project development, data management, data analysis, manuscript writing; D.S.: manuscript writing; D.M.B.: project development, manuscript writing and editing; N.K.: project development, manuscript writing and editing; P.W.: project development, manuscript writing and editing; A.L.: project development, manuscript writing and editing; N.M.: project development, manuscript writing and editing; H.H.G.: project development, manuscript writing and editing; D.J.L.: project development, data management, data analysis, manuscript writing; A.T.: project development, manuscript writing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Nihal E. Mohamed is supported by scholar grants from the Department of Defense (W81XWH-17-1-0590 Log#PC160194), and the National Institute of Nursing Research (1R21 NR016518-01A1).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the MSHS Ethics Committee.
Informed Consent Statement
The MSHS Ethics Committee approved a waiver of documentation of informed consent as de-identified patient data was used.
Data Availability Statement
Data managed by msdw.mountsinai.org.
Acknowledgments
This work was supported in part through the computational and data resources and staff expertise provided by Scientific Computing at the Icahn School of Medicine at Mount Sinai.
Conflicts of Interest
A.K. Tewari: CONFLICTS OF INTEREST. COMPANY RELATIONSHIP TYPE FINANCIAL. Uretheral Catheterless Radical Prostatectomy: Patent No. DNA-Based Bicistronic Vectors with Inducible and Constitutive Promoters—ID#: 160608: Patent No. High-Intensity Focus Ultrasound and CPG-BrachyurysiRNA for Treatment of Prostate Cancer—ID# 160403: Patent No. Patent for a Catheterless Device and Approach Patent No. *Promaxo Leadership Position Yes. *Promaxo Equity Ownership YES. Global Prostate Cancer Research Foundation Leadership Position No. Kalyani Prostate Cancer Institute Leadership Position No. Prostate Cancer Foundation: Leadership Position No. Roivant Consultant No. Blank Family Foundation Grant Yes. Intuitive Surgical Scientific Study or Trial Yes. Department of Defense (DOD) Scientific Study or Trial Yes. AxoGen, Inc. Scientific Study or Trial Yes. Oncovir, Inc—Poly ICLC Scientific Study or Trial Yes. National Institute of Health (NIH/DHHS) Scientific Study or Trial Yes. National Cancer Institute Scientific Study or Trial Yes. National Institute on Drug Abuse Scientific Study or Trial Yes. Kite Pharma Scientific Study or Trial Yes. Lumicell, Inc. Scientific Study or Trial Yes. Dendreon Scientific Study or Trial Yes.
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, X.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Kannan, S.; Ali, P.S.S.; Sheeza, A.; Hemalatha, K. COVID-19 (Novel Coronavirus 2019)—Recent trends. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2006–2011. [Google Scholar] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Song, H.; Seddighzadeh, B.; Cooperberg, M.R.; Huang, F.W. Expression of ACE2, the SARS-CoV-2 Receptor, and TMPRSS2 in Prostate Epithelial Cells. Eur. Urol. 2020, 78, 296–298. [Google Scholar] [CrossRef]
- Katopodis, P.; Anikin, V.; Randeva, H.S.; Spandidos, D.A.; Chatha, K.; Kyrou, I.; Karteris, E. Pancancer analysis of transmembrane protease serine 2 and cathepsin L that mediate cellular SARSCoV2 infection leading to COVID-19. Int. J. Oncol. 2020, 57, 533–539. [Google Scholar] [CrossRef]
- Norris, K.; Nissenson, A.R. Race, gender, and socioeconomic disparities in CKD in the United States. J. Am. Soc. Nephrol. 2008, 19, 1261–1270. [Google Scholar] [CrossRef]
- Lundon, D.J.; Mohamed, N.; Lantz, A.; Goltz, H.H.; Kelly, B.D.; Tewari, A.K. Social Determinants Predict Outcomes in Data From a Multi-Ethnic Cohort of 20,899 Patients Investigated for COVID-19. Front. Public Health 2020, 8, 571364. [Google Scholar] [CrossRef]
- Nicholas, S.B.; Kalantar-Zadeh, K.; Norris, K.C. Socioeconomic disparities in chronic kidney disease. Adv. Chronic Kidney Dis. 2015, 22, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla Ruvolo, C.; Stolzenbach, L.F.; Nocera, L.; Deuker, M.; Wenzel, M.; Tian, Z.; La Rocca, R.; Creta, M.; Capece, M.; Saad, F.; et al. Higher Cancer Mortality in Rural Upper Urinary Tract Urothelial Carcinoma Patients. Urol. Int. 2021, 105, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Deuker, M.; Stolzenbach, L.F.; Ruvolo, C.C.; Nocera, L.; Tian, Z.; Roos, F.C.; Becker, A.; Kluth, L.A.; Tilki, D.; Shariat, S.F.; et al. Bladder cancer stage and mortality: Urban vs. rural residency. Cancer Causes Control 2021, 32, 139–145. [Google Scholar] [CrossRef]
- Stolzenbach, L.F.; Deuker, M.; Collà-Ruvolo, C.; Nocera, L.; Tian, Z.; Maurer, T.; Tilki, D.; Briganti, A.; Saad, F.; Mirone, V.; et al. Differences between rural and urban prostate cancer patients. World J. Urol. 2021, 39, 2507–2514. [Google Scholar] [CrossRef]
- Calderon, J.L.; Zadshir, A.; Norris, K. A survey of kidney disease and risk-factor information on the World Wide Web. MedGenMed 2004, 6, 3. [Google Scholar] [PubMed]
- Neumann, U.; Genze, N.; Heider, D. EFS: An ensemble feature selection tool implemented as R-package and web-application. BioData Min. 2017, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Polikar, R. Ensemble based systems in decision making. IEEE Circ. Syst. Mag. 2006, 6, 21–45. [Google Scholar] [CrossRef]
- Buac, N.P.; Khusid, J.A.; Sturgis, M.R.; Gupta, M.; Lundon, D.J.; Chow, A.K.; Becerra, A.Z. Disparities in patient and system factors explain racial/ethnic disparities in delayed time to treatment in muscle invasive bladder cancer. Urol. Oncol. 2022, 40, 343.e15–343.e20. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Mouw, T.; Koster, A.; Wright, M.; Blank, M.M.; Moore, S.C.; Hollenbeck, A.; Schatzkin, A. Education and risk of cancer in a large cohort of men and women in the United States. PLoS ONE 2008, 3, e3639. [Google Scholar] [CrossRef]
- Greenlee, R.T.; Howe, H.L. County-level poverty and distant stage cancer in the United States. Cancer Causes Control 2009, 20, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Vagero, D.; Persson, G. Cancer survival and social class in Sweden. J. Epidemiol. Commun. Health 1987, 41, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weprin, S.A.; Parker, D.C.; Jones, J.D.; Kaplan, J.R.; Giusto, L.L.; Mydlo, J.H.; Yu, S.-J.S.; Lee, D.I.; Eun, D.D.; Reese, A.C. Association of Low Socioeconomic Status With Adverse Prostate Cancer Pathology Among African American Men Who Underwent Radical Prostatectomy. Clin. Genitourin. Cancer 2019, 17, e1054–e1059. [Google Scholar] [CrossRef]
- Maurice, M.J.; Zhu, H.; Kiechle, J.E.; Kim, S.P.; Abouassaly, R. Nonclinical Factors Predict Selection of Initial Observation for Renal Cell Carcinoma. Urology 2015, 86, 892–899. [Google Scholar] [CrossRef]
- Izadmehr, S.; Lundon, D.J.; Mohamed, N.; Katims, A.; Patel, V.; Eilender, B.; Mehrazin, R.; Badani, K.K.; Sfakianos, J.P.; Tsao, C.-K.; et al. The Evolving Clinical Management of Genitourinary Cancers Amid the COVID-19 Pandemic. Front. Oncol. 2021, 11, 734963. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Jemal, A.; Cokkinides, V.; Singh, G.K.; Cardinez, C.; Ghafoor, A.; Thun, M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J. Clin. 2004, 54, 78–93. [Google Scholar] [CrossRef]
- Ko, K.; Park, Y.H.; Lee, J.W.; Ku, J.H.; Kwak, C.; Kim, H.H. Influence of nutritional deficiency on prognosis of renal cell carcinoma (RCC). BJU Int. 2013, 112, 775–780. [Google Scholar] [CrossRef]
- Michalek, I.M.; Martinsen, J.I.; Weiderpass, E.; Kjaerheim, K.; Lynge, E.; Sparen, P.; Tryggvadottir, L.; Pukkala, E. Occupation and risk of cancer of the renal pelvis in Nordic countries. BJU Int. 2019, 123, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, J.S.; Alexander, D.D.; Perez, V.; Mink, P.J. Arsenic exposure and bladder cancer: Quantitative assessment of studies in human populations to detect risks at low doses. Toxicology 2014, 317, 17–30. [Google Scholar] [CrossRef]
- Bulka, C.M.; Jones, R.M.; Turyk, M.E.; Stayner, L.T.; Argos, M. Arsenic in drinking water and prostate cancer in Illinois counties: An ecologic study. Environ. Res. 2016, 148, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Schrag, D.; Hsieh, L.J.; Rabbani, F.; Bach, P.B.; Herr, H.; Begg, C.B. Adherence to surveillance among patients with superficial bladder cancer. J. Natl. Cancer Inst. 2003, 95, 588–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omidele, O.O.; Finkelstein, M.; Omorogbe, A.; Palese, M. Radical Prostatectomy Sociodemographic Disparities Based on Hospital and Physician Volume. Clin. Genitourin. Cancer 2019, 17, e1011–e1019. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W. COVID-19 and African Americans. JAMA 2020, 323, 1891–1892. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Liu, W.; Liu, K.; Fang, Y.-Y.; Shang, J.; Zhou, L.; Wang, K.; Leng, F.; Wei, S.; Chen, L.; et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: A retrospective study. Chin. Med. J. 2020, 133, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H. Racial and Ethnic Disparities in Health Insurance Coverage: Dynamics of Gaining and Losing Coverage over the Life-Course. Popul. Res. Policy Rev. 2017, 36, 181–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Lundon, D.J.; Kelly, B.D.; Nair, S.; Bolton, D.M.; Kyprianou, N.; Wiklund, P.; Tewari, A. Early mortality risk stratification after SARS-CoV-2 infection. Med. Intensiva 2020, 45, e40–e42. [Google Scholar] [CrossRef]
- Lundon, D.J.; Kelly, B.D.; Nair, S.; Bolton, D.M.; Patel, G.; Reich, D.; Tewari, A. A COVID-19 Test Triage Tool, Predicting Negative Results and Reducing the Testing Burden on Healthcare Systems During a Pandemic. Front. Med. 2021, 8, 563465. [Google Scholar] [CrossRef]
- Lundon, D.J.; Kelly, B.D.; Shukla, D.; Bolton, D.M.; Wiklund, P.; Tewari, A. A Decision Aide for the Risk Stratification of GU Cancer Patients at Risk of SARS-CoV-2 Infection, COVID-19 Related Hospitalization, Intubation, and Mortality. J. Clin. Med. 2020, 9, 2799. [Google Scholar] [CrossRef]
- Gross, O.; Moerer, O.; Weber, M.; Huber, T.B.; Scheithauer, S. COVID-19-associated nephritis: Early warning for disease severity and complications? Lancet 2020, 395, e87–e88. [Google Scholar] [CrossRef]
- Nakanishi, N.; Liu, K.; Kawakami, D.; Kawai, Y.; Morisawa, T.; Nishida, T.; Sumita, H.; Unoki, T.; Hifumi, T.; Iida, Y.; et al. Post-Intensive Care Syndrome and Its New Challenges in Coronavirus Disease 2019 (COVID-19) Pandemic: A Review of Recent Advances and Perspectives. J. Clin. Med. 2021, 10, 3870. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).