Application of Dehydrated Amniotic Membrane Allografts in Advanced Diabetic Foot Ulceration: Case Report and Review of Literature
Abstract
:1. Introduction
2. Case Presentation Section
2.1. Dehydrated Human Amniotic Membrane Allografts
2.2. Patient History
3. Discussion
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Test Kits
- HBcAb: Catalog number: 06P06, Abbott Laboratories, Abbott Park, IL, USA;
- HbsAg: Catalog number: 06P02, Abbott Laboratories, Abbott Park, IL, USA;
- HCV: Catalog number: 06P04, Abbott Laboratories, Abbott Park, IL, USA;
- HIV1, HIV2, plus O: Catalog number: 06P01, Abbott Laboratories, Abbott Park, IL, USA;
- HTLV-I/II: Catalog number: 06P07, Abbott Laboratories, Abbott Park, IL, USA;
- RPR: Catalog number: 900025, Arlington Scientific, Springville, UT, USA;
- HIV1, HCV, HBV, NAT: Catalog number: 303330, 303331, 303719, 303334, 303344;
- WNV: Catalog number: 07001061190, Roche Diagnostics, Indianapolis, IN, USA.
Appendix B
Appendix B.1. Wound Size
- Length × width less than 4 square cm;
- Length × width between 4 and 16 square cm;
- Length × width between 16.1 and 36 square cm;
- Length × width between 36.1 and 80 square cm;
- Length × width greater than 80 square cm.
Appendix B.2. Depth
- Non-blanchable erythema on intact skin;
- Partial-thickness skin loss involving epidermis and/or dermis;
- Full-thickness skin loss involving damage or necrosis of subcutaneous tissue; may extend down to but not through underlying fascia; and/or mixed partial and full-thickness and/or tissue layers obscured by granulation tissue;
- Obscured by necrosis;
- Full-thickness skin loss with extensive destruction, tissue necrosis, or damage to muscle, bone, or supporting structures.
Appendix B.3. Edges
- Instinct, diffuse, none clearly visible;
- Distinct, outline clearly visible, attached, even with wound base;
- Well-defined, not attached to wound base;
- Well-defined, not attached to wound base, rolled under, thickened;
- Well-defined, fibrotic, scarred, or hyperkeratotic.
Appendix B.4. Necrotic Tissue Amount
- None visible;
- <25% of wound bed covered;
- 25% to 50% of wound covered;
- >50% and <75% of wound covered;
- 75% to 100% of wound covered.
Appendix B.5. Epithelialization
- 100% wound covered, surface intact;
- 75% to <100% wound covered and/or epithelial tissue extends >0.5 cm into wound bed;
- 50% to <75% wound covered and/or epithelial tissue extends to <0.5 cm into wound bed;
- 25% to <50% wound covered;
- <25% wound covered.
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2019. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 15 April 2022).
- Centers for Disease Control and Prevention. Incidence of Newly Diagnosed Diabetes. 2020. Available online: https://www.cdc.gov/diabetes/data/statistics-report/newly-diagnosed-diabetes.html (accessed on 15 April 2022).
- Everett, E.; Mathioudakis, N. Update on Management of Diabetic Foot Ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Neville, R.F.; Kayssi, A.; Buescher, T.; Stempel, M.S. The Diabetic Foot. Curr. Probl. Surg. 2016, 53, 408–437. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Coexisting Conditions and Complications. 2021. Available online: https://www.cdc.gov/diabetes/data/statistics-report/coexisting-conditions-complications.html (accessed on 18 April 2022).
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ElHeneidy, H.; Omran, E.; Halwagy, A.; Al-Inany, H.; Al-Ansary, M.; Gad, A. Amniotic Membrane Can Be a Valid Source for Wound Healing. Int. J. Women’s Health 2016, 8, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Snyder, R.J.; Shimozaki, K.; Tallis, A.; Kerzner, M.; Reyzelman, A.; Lintzeris, D.; Bell, D.; Rutan, R.L.; Rosenblum, B. A Prospective, Randomized, Multicenter, Controlled Evaluation of the Use of Dehydrated Amniotic Membrane Allograft Compared to Standard of Care for the Closure of Chronic Diabetic Foot Ulcers. Wounds Compend. Clin. Res. Pract. 2016, 28, 70–77. Available online: https://www.hmpgloballearningnetwork.com/site/wounds/article/prospective-randomizedmulticenter-controlled-evaluation-use-dehydrated-amniotic-membrane (accessed on 14 April 2022).
- Driver, V.R.; Fabbi, M.; Lavery, L.A.; Gibbons, G. The Costs of Diabetic Foot: The Economic Case for the Limb Salvage Team. J. Vasc. Surg. 2010, 52, 17S–22S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblum, B.I. A Retrospective Case Series of a Dehydrated Amniotic Membrane Allograft for Treatment of Unresolved Diabetic Foot Ulcers. J. Am. Podiatr. Med. Assoc. 2016, 106, 328–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporusso, J.; Abdo, R.; Karr, J.; Smith, M.; Anaim, A. Clinical Experience Using a Dehydrated Amnion/Chorion Membrane Construct for the Management of Wounds. Wounds Compend. Clin. Res. Pract. 2019, 31, S19–S27. Available online: https://www.researchgate.net/publication/332624695_Clinical_experience_using_a_dehydrated_amnionchorion_membrane_construct_for_the_management_of_wounds (accessed on 15 April 2022).
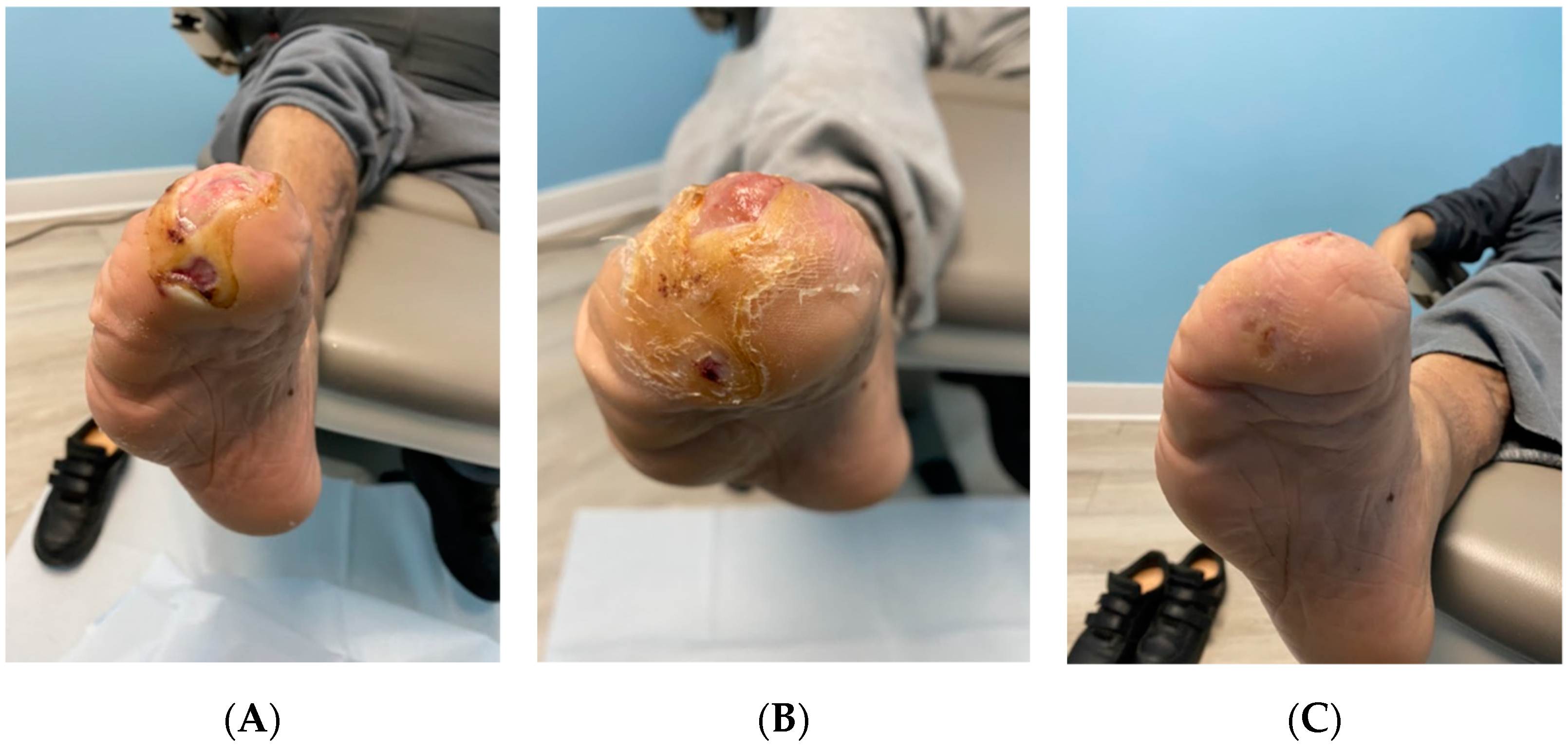
| Date of Examination | DFU Measurements |
|---|---|
| 25 January 2022 | 5.5 cm × 4.5 cm × 0.3 cm |
| 1 February 2022 | 5.3 cm × 4.3 cm × 0.1 cm |
| 8 February 2022 | 5.1 cm × 4.4 cm × 0.1 cm |
| 15 February 2022 | 5.0 cm × 3.9 cm × 0.1 cm |
| 22 February 2022 | 4.2cm × 3.5 cm × 0.1 cm |
| 1 March 2022 | 3.1 cm × 1.8 cm × 0.1 cm |
| 8 March 2022 | 0.9 cm × 0.8 cm × superficial |
| 15 March 2022 | 0.0 cm × 0.0 cm × superficial |
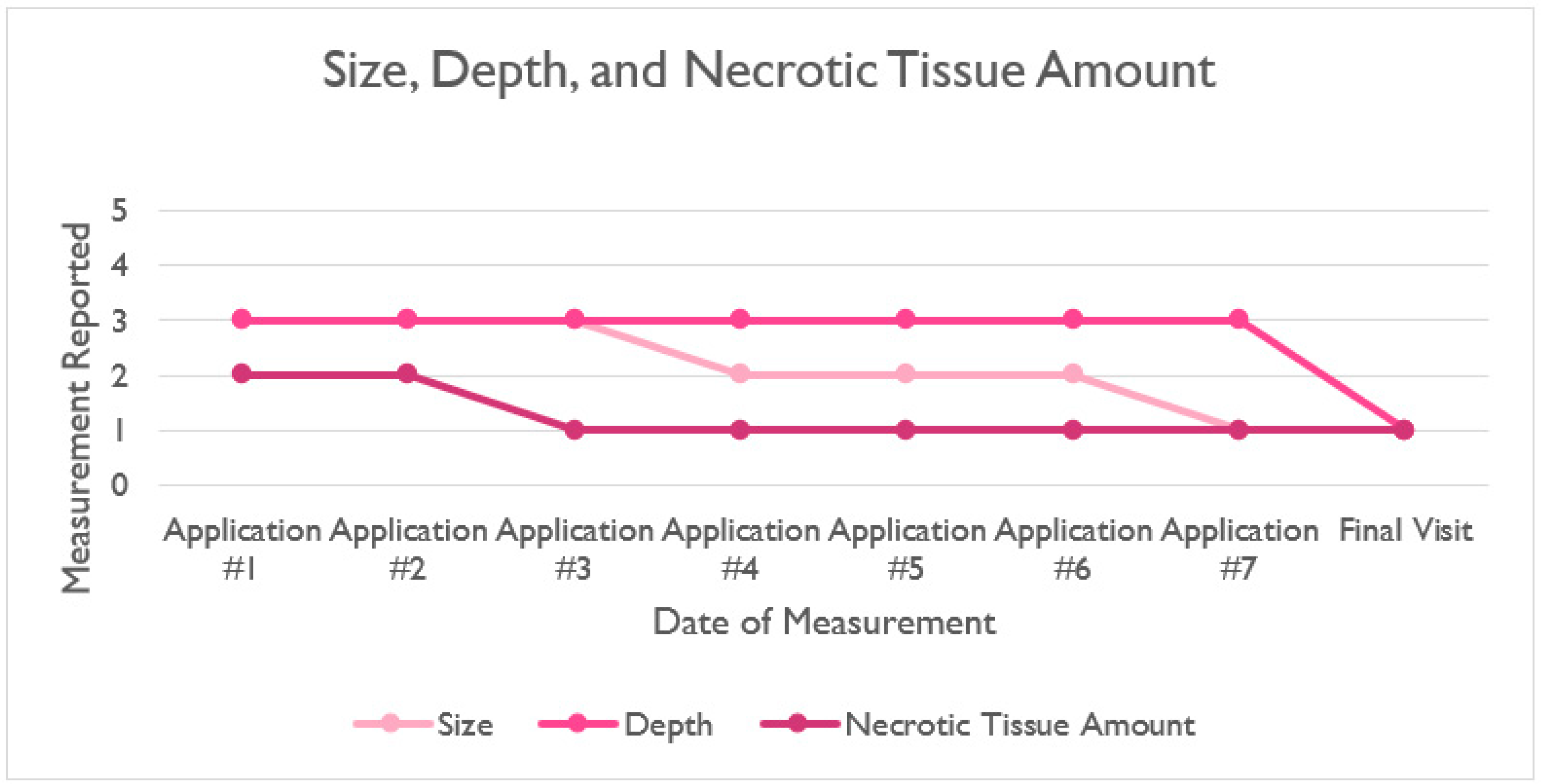
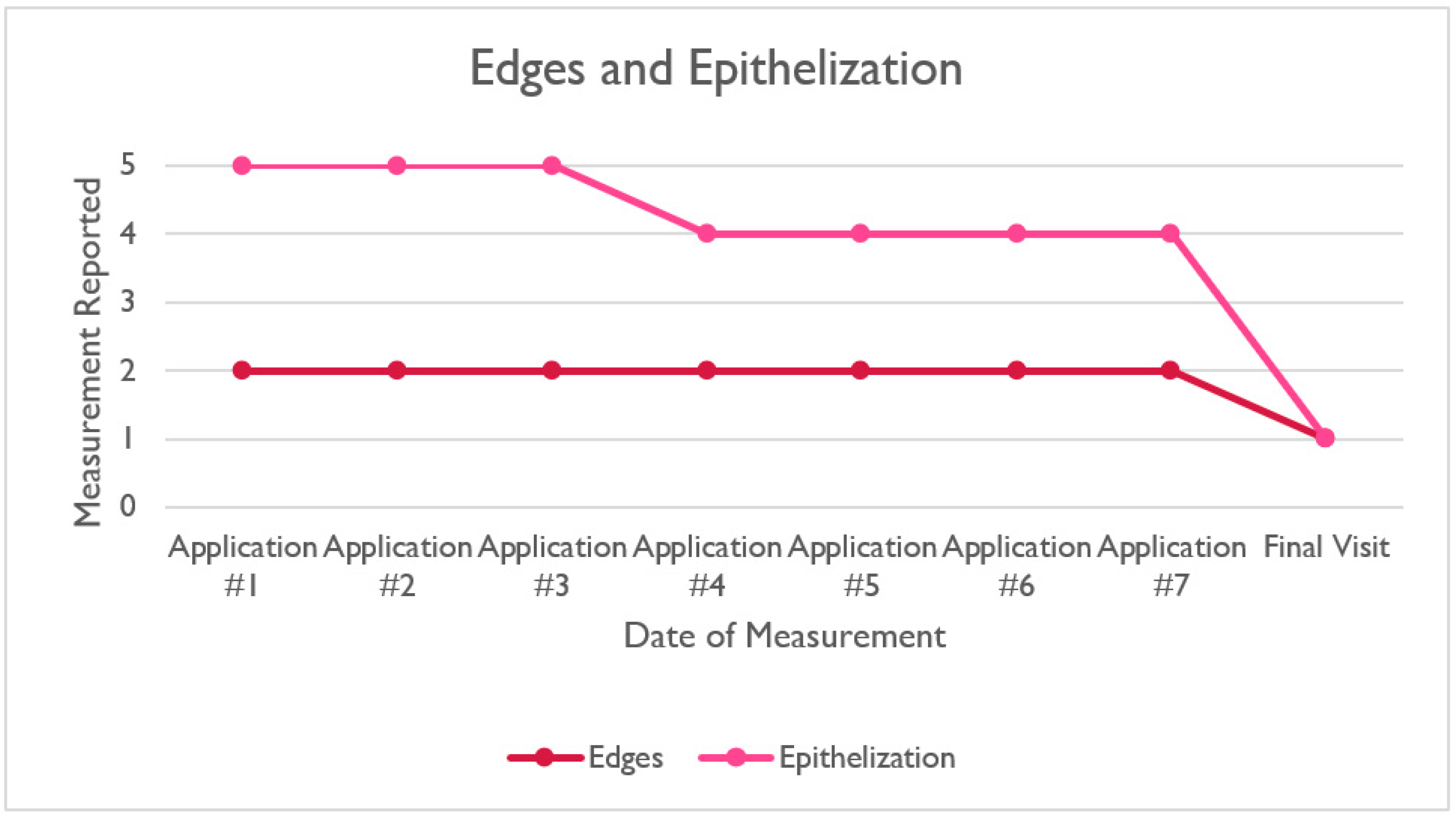
| Application #1 | Application #2 | Application #3 | Application #4 | Application #5 | Application #6 | Application #7 | Final Visit | |
|---|---|---|---|---|---|---|---|---|
| Size | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 |
| Depth | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 |
| Edges | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Necrotic Tissue Amount | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Epithelization | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, C.; Regulski, M.; Martin, S.; Barrett, T. Application of Dehydrated Amniotic Membrane Allografts in Advanced Diabetic Foot Ulceration: Case Report and Review of Literature. Reports 2022, 5, 28. https://doi.org/10.3390/reports5030028
Becker C, Regulski M, Martin S, Barrett T. Application of Dehydrated Amniotic Membrane Allografts in Advanced Diabetic Foot Ulceration: Case Report and Review of Literature. Reports. 2022; 5(3):28. https://doi.org/10.3390/reports5030028
Chicago/Turabian StyleBecker, Catherine, Matthew Regulski, Scott Martin, and Tyler Barrett. 2022. "Application of Dehydrated Amniotic Membrane Allografts in Advanced Diabetic Foot Ulceration: Case Report and Review of Literature" Reports 5, no. 3: 28. https://doi.org/10.3390/reports5030028
APA StyleBecker, C., Regulski, M., Martin, S., & Barrett, T. (2022). Application of Dehydrated Amniotic Membrane Allografts in Advanced Diabetic Foot Ulceration: Case Report and Review of Literature. Reports, 5(3), 28. https://doi.org/10.3390/reports5030028







