Abstract
A 39-year-old male had a stomachache for 10 days before abnormal liver function tests were detected by a local doctor. Then, he was referred to us and admitted to our hospital for examination and treatment of elevated transaminases. He had taken benzbromarone to treat his hyperuricemia for seven months, and we diagnosed him with benzbromarone-induced liver injury. After the termination of benzbromarone, he finally recovered from his illness. There are several reports about benzbromarone-induced liver injury. In conclusion, as periodic liver function tests seem not to be completely performed, clinicians should regularly monitor liver function tests in patients taking benzbromarone.
1. Introduction
Underexcretion of urate is the major cause of hyperuricemia in people with gout and another cause is the overproduction of urate through the purine degradation pathway [1]. Hyperuricemia is caused by the excess production of uric acid or the reduction of uric acid excretion [2]. Benzbromarone inhibits urate transporter 1 (URAT1), which is a major urate transporter involved in renal uric acid reabsorption and excretion [2]. Additionally, benzbromarone has uricosuric action and normalizes serum uric acid levels [2]. Benzbromarone has been compared with allopurinol, which inhibits overproduction of urate, in chronic gout patients with renal impairment [3]. Benzbromarone demonstrated a significantly greater reduction in serum uric acid levels compared with the allopurinol regimen [3]. In Japan, benzbromarone has been used to treat hyperuricemia since 1979 and is still widely used [4].
There have been several reports that hepatotoxicity is associated with benzbromarone [5,6,7,8,9,10,11]. Acute liver injuries including acute liver failure (ALF) have occasionally been observed in patients treated with benzbromarone [5,6,7,8,9,11]. Benzbromarone and benzarone are used for the treatment of peripheral venous disorders in Europe, and they induce drug-induced autoimmune hepatitis and chronic active hepatitis, respectively [10,12]. Benzbromarone and benzarone have similar structures to amiodarone, which causes mitochondrial toxicity and liver injury [10]. Liver injury induced by amiodarone causes hepatic steatosis and benzbromarone also aggravates hepatic steatosis in obese patients [13].
Recently, we had a medical opportunity to observe a patient with benzbromarone-induced liver injury. The patient had taken this drug for more than 6 months before his abnormal liver function tests were detected. Periodic liver function tests should be performed more frequently in patients with benzbromarone administration. Herein, we discuss the hepatotoxicity of benzbromarone and its future measures.
2. Case Report
A 39-year-old male had been diagnosed with hyperuricemia and allopurinol was changed to benzbromarone (Torii Pharmaceutical, Tokyo Japan) 50 mg daily seven months prior. He was also administered 200 mg of bilastine (Taiho, Tokyo, Japan) daily for his allergic rhinitis six months prior. He also took one tablet of Hyakusosan (unknown company), Japanese traditional medicine, several times for his epigastric distress three months ago. Benzbromarone was the only drug he had taken when he came to our hospital. He had no history of liver diseases but a history of asthma and a history of surgery for his left inguinal hernia. He was not a drinker of alcohol and had no history of transfusion, tattoo, drug abuse, or drug allergy. He had no family history of liver diseases.
After he had a stomachache for 10 days, he initially visited a local doctor. There, abnormal liver function tests (aspartate aminotransferase (AST), 1077 IU/L; alanine transaminase (ALT), 1297 IU/L; γ-glutamyl transpeptidase (γ-GTP), 231 IU/L; total bilirubin, 9.4 mg/dL; and direct bilirubin, 6.3 mg/dL) were detected. He was immediately referred to us and admitted to our hospital for examination and treatment of his elevated transaminases.
On admission, his chief complaint was stomachache. His height, body weight, body mass index, and body temperature were 1.72 m, 71.6 kg, 24.2 kg/m2, and 37.3 °C, respectively. His consciousness was alert. His bulbar conjunctiva and skin were icteric. His liver and spleen were not palpable. Edema of the legs was not detected.
The laboratory data on admission are listed in Table 1. Liver dysfunction was observed in addition to severe transaminase levels. Hepatitis viral markers and autoantibodies were negative. Thyroid function was within normal limits: thyroid stimulating hormone, 1.66 μIU/mL; free tri-iodothyronine, 3.25 pg/mL; and free thyroxine, 1.49 ng/dL. We also performed a drug lymphocyte stimulation test (DLST). DLST was positive for benzbromarone (stimulation index (SI), 561%), Hyakusosan (SI, 406%), and bilastine (SI, 403%). These drugs were provided by this patient. On the DLST, the drug at 0.1, 1 or 10-fold of maximum plasma concentration was added to the lymphocytes separated from our patient’s plasma. Lymphocytes were cultured for 72 h, and 3H-thymidine was then added [14]. After culturing the lymphocytes for 16–18 h, radioactivity resulting from the uptake of 3H-thymidine was measured by the cells during deoxyribonucleic acid (DNA) synthesis in counts per minutes (cpm). The SI was calculated as the ratio of proliferation (cpm) with the drug/proliferation (cpm) without the drug [14]. Abdominal ultrasound, computed tomography and magnetic resonance imaging results indicated acute liver injury (Figure 1). We did not observe any signatures of chronic hepatitis or cirrhosis.
After admission, benzbromarone was immediately stopped. As his liver function tests had improved (AST, 68 IU/L; ALT, 86 IU/L; γ-GTP, 81 IU/L; and total bilirubin, 3.23 mg/dL), he was discharged on day 27 after admission. After one month and three months after discharge, his liver function tests were further improved (AST, 28 IU/L and 24 IU/L; ALT, 25 IU/L and 25 IU/L; γ-GTP, (not available) and 22 IU/L; and total bilirubin, 1.98 mg/dL and 0.93 mg/dL). We ruled out acute viral hepatitis, autoimmune hepatitis, and others. Although DLST were positive for three drugs, he took only benzbromarone on admission. The score based on the Japanese criteria on drug-induced liver injury was 8, which strongly suggests drug-induced liver injury by benzbromarone [12]. Finally, we diagnosed the cause of his liver injury to be benzbromarone.

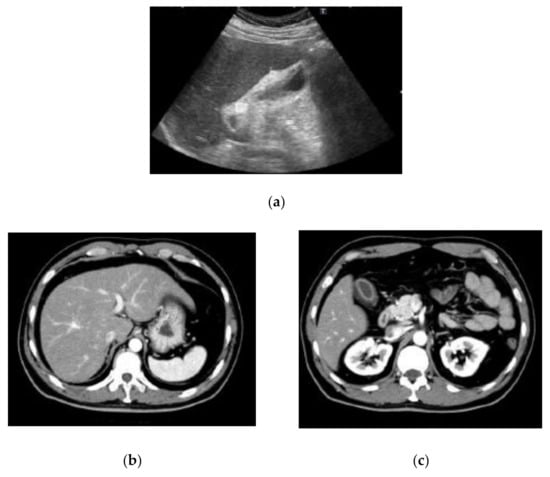
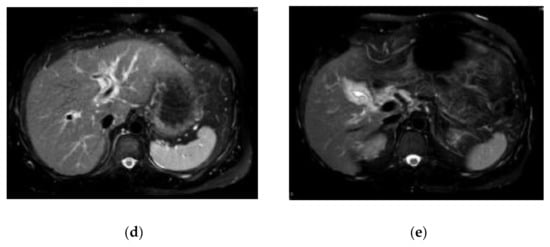

Table 1.
The laboratory data on admission in the present case.
Table 1.
The laboratory data on admission in the present case.
| Item | Values | Item | Values | Item | Values |
|---|---|---|---|---|---|
| WBC | 5300/μL | TP | 7.4 g/dL | HBsAg | negative |
| RBC | 5,260,000/μL | Albumin | 3.8 g/dL | Anti-HBs | negative |
| Hemoglobin | 15.9 g/dL | T. CHO | 159 mg/dL | Anti-HBc | negative |
| Hematocrit | 47.7% | TG | 211 mg/dL | IgM anti-HBc | negative |
| Platelets | 252,000/μL | BUN | 7.1 mg/dL | Anti-HCV | negative |
| PT | 78% | Creatinine | 0.85 mg/dL | Anti-HIV | negative |
| INR | 1.15 | CK | 72 IU/L | IgM anti-HAV | negative |
| AST | 1008 IU/L | Amylase | 47 IU/L | IgA anti-HEV | negative |
| ALT | 1241 IU/L | BS | 112 mg/dL | IgG | 1955 mg/dL |
| LDH | 369 IU/L | HbA1c | 5.8% | IgA | 463 mg/dL |
| γ-GTP | 186 IU/L | NH3 | 52 μg/dL | IgM | 36 mg/dL |
| ALP | 1241 IU/L | CRP | 0.47 mg/dL | IgE | 680 IU/mL |
| T. Bil | 9.71 mg/dL | AFP | 18.0 ng/mL | ANA | negative |
| D. Bil | 7.22 mg/dL | sIL2R | 2530 U/mL | AMAM2 | negative |
WBC: white blood cells, RBC: red blood cells, PT: prothrombin time, INR: international normalized ratio, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, T. Bil: total bilirubin, D. Bil: direct bilirubin, TP: total protein, T.CHO: total cholesterol, TG: triglyceride, BUN: blood urea nitrogen, Cre: creatinine, CK: creatine kinase, BS: blood sugar, HbA1c: hemoglobin A1c, NH3: ammonia, CRP: C-reactive protein, AFP: alpha fetoprotein, sIL-2R: soluble interleukin-2 receptor, HBsAg: hepatitis B surface antigen, anti-HBs: anti-hepatitis B surface antibody, anti-HBc: anti-hepatitis B core antibody, anti-HCV: anti-hepatitis C virus antibody, anti-HIV: anti-human immunodeficiency virus antibody, Ig: immunoglobulin, ANA: anti-nuclear antibody, AMAM2: anti-mitochondrial M2 antibody.


Figure 1.
Findings of abdominal ultrasound (US) (a), computed tomography (CT) (b,c) and magnetic resonance imaging (MRI) (d,e) examinations. US indicated acute liver injury with thickening of the gall bladder wall and minimal ascites on the underside of the liver (a). The contrast-enhanced CT scan also indicated acute liver injury with thickening of the gall bladder wall (b,c). MRI indicated acute liver injury with periportal abnormal intensity (d) and thickening of the gall bladder wall (e).
3. Discussion
A male patient with liver injury induced by benzbromarone is presented. He took benzbromarone for seven months, but after the stoppage of benzbromarone, his abnormal liver function tests were improved. Other liver diseases, such as viral hepatitis and autoimmune liver diseases, were ruled out. Zhang et al. reported nine cases of benzbromarone-induced hepatotoxicity with liver failure associated with benzbromarone [11]. In only one of these nine cases, the course of benzbromarone was more than 6 months [15]. Of note, even though more than 6 months had passed since the commencement of benzbromarone, it is possible that benzbromarone could induce liver injury, including ALF [9].
For more than 30 years, benzbromarone has been used in Japan. Imai et al. reported that a periodic liver function test (1–90 days of initial benzbromarone administration) was implemented in only 28.7% of patients [16]. As benzbromarone occasionally causes severe hepatotoxicity, periodic liver function tests should be performed for the first six months and thereafter following benzbromarone administration.
Outside of Asian countries, hepatotoxicity associated with benzbromarone may be a relatively rare event [17]. Benzbromarone has not been approved for use in the United States because of concerns over reports of acute liver injury and associated deaths [18]. Poor cytochrome P450 (CYP)2C9 metabolizers may be at a heightened risk of benzbromarone-induced hepatotoxicity [19]. Modification of the P450 enzyme by the reactive metabolite could be a common trait of drugs that induce idiosyncratic hepatotoxicity [20]. The metabolic activation of benzbromarone can induce the mitochondrial membrane permeability transition [21]. If users of benzbromarone have important nucleotide polymorphisms associated with adverse events, the hepatotoxicity associated with benzbromarone may decrease. This is the case for the Nudix hydrolase 15 (NUDT15) polymorphism in users of azathioprine [22]. Further investigation is needed.
Rana et al. determined the number of reports for hepatotoxicity at a group level and an individual drug level using the reporting odds ratio (ROR) [23]. Benzbromarone, troglitazone, isoniazid, and rifampin had ROR values higher than 18, and were associated with mitochondrial mechanisms of toxicity [23]. They also reported that an older mean patient age was associated with cases of drug-induced liver injury that act through a mitochondrial mechanism, as mitochondrial function declines with age. However, our case is a 39-year-old male.
Uric acid metabolism-related inflammation is involved in the pathogenesis of metabolic syndrome components, such as atherosclerosis and nonalcoholic steatohepatitis [24]. The incidence of metabolic dysfunction-associated liver disease is increasing in cases of ACLF [25,26]. Drug-induced liver injury presenting as ACLF is an important entity that is not often addressed as an acute disease in literature [27]. A Japanese nationwide survey demonstrated that drug-induced liver injury was present in 2% of ACLF cases as an acute disease [28]. Careful attention should be given to hyperuricemia and its drugs in patients with liver diseases.
In the present case, we did not find any abnormality of urine simple test, such as hematuria, proteinuria, or glycosuria on his admission. Benzbromarone shows protection for nephrotoxicity with lowering uric acid levels in rat experimental models [29]. There have been rare reports on benzbromarone-induced nephrotoxicity [30].
The present case took one tablet of Hyakusosan, Japanese traditional medicine, several times for his epigastric distress three months ago. It is unknown whether the previous use of a Japanese traditional medicine based on natural products, via a drug–drug interaction with benzbromarone, can cause drug-induced liver injury. There are many examples of hepatotoxicity induced by herbal traditional medicine [31,32,33]. Thus, drug-induced liver injury is an unpredictable event [34].
Although DLST are positive for three drugs in the present case, the patient took only benzbromarone at the time of the liver injury. Regarding drug-induced liver injury, both DLST-positive and DLST-negative cases exist [10,35]. Another limitation of this study is not performing a liver biopsy, following the patient’s wishes.
4. Conclusions
We report here a case of recent liver injury induced by benzbromarone. Unfortunately, in Japan, benzbromarone-induced hepatotoxicity is occasionally observed. In patients treated with benzbromarone, periodic liver function tests should be performed for the first six months and thereafter following benzbromarone administration, although, drug-induced liver injury is an unpredictable event.
Author Contributions
Conceptualization, T.I., K.H. and T.K.; formal analysis, T.I.; investigation, T.K.; writing—original draft preparation, T.K.; writing—review and editing, T.I., K.H., M.H., Y.Y., R.S.-T., M.K., S.K., T.M., N.M., R.M., K.N., H.Y., M.M. and T.K.; supervision, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, but ethical review and approval were waived for this study due to the case report’s nature.
Informed Consent Statement
Written informed consent for publication was obtained from the patient involved in the study.
Data Availability Statement
All data underlying this article are available in this article.
Acknowledgments
The authors thank all staff members seeing and taking care of patients at Nihon University School of Medicine Itabashi Hospital.
Conflicts of Interest
Tatsuo Kanda would like to report research grants received from AbbVie Inc. and Towa Pharmaceutical Co., Ltd., and lecture fees received from Gilead Sciences, Inc., AbbVie Inc., and MSD K.K. outside the submitted work. Kazushige Nirei would like to report lecture fees received from Gilead Sciences, Inc., outside the submitted work. Mitsuhiko Moriyama would like to report research grants received from Towa Pharmaceutical Co., Ltd., AbbVie Inc., and MSD K.K. outside the submitted work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Borghi, C. The management of hyperuricemia: Back to the pathophysiology of uric acid. Curr. Med. Res. Opin. 2017, 33 (Suppl. 3), 1–4. [Google Scholar] [CrossRef]
- Li, X.; Yan, Z.; Tian, J.; Zhang, X.; Han, H.; Ye, F. Urate Transporter URAT1 in Hyperuricemia: New Insights from Hyperuricemic Models. Ann. Clin. Lab. Sci. 2019, 49, 756–762. [Google Scholar]
- Perez-Ruiz, F.; Calabozo, M.; Fernandez-Lopez, M.J.; Herrero-Beites, A.; Ruiz-Lucea, E.; Garcia-Erauskin, G.; Duruelo, J.; Alonso-Ruiz, A. Treatment of chronic gout in patients with renal function impairment: An open, randomized, actively controlled study. J. Clin. Rheumatol. 1999, 5, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K. Drug-induced hepatic injury, the challenge for cause investigation. Rinsho Byori 2011, 59, 1117–1122, (In Japanese with English Abstract). [Google Scholar] [PubMed]
- Van der Klauw, M.M.; Houtman, P.M.; Stricker, B.H.; Spoelstra, P. Hepatic injury caused by benzbromarone. J. Hepatol. 1994, 20, 376–379. [Google Scholar] [CrossRef]
- Wagayama, H.; Shiraki, K.; Sugimoto, K.; Fujikawa, K.; Shimizu, A.; Takase, K.; Nakano, T.; Tameda, Y. Fatal fulminant hepatic failure associated with benzbromarone. J. Hepatol. 2000, 32, 874. [Google Scholar] [CrossRef]
- Arai, M.; Yokosuka, O.; Fujiwara, K.; Kojima, H.; Kanda, T.; Hirasawa, H.; Saisho, H. Fulminant hepatic failure associated with benzbromarone treatment: A case report. J. Gastroenterol. Hepatol. 2002, 17, 625–626. [Google Scholar] [CrossRef]
- Haring, B.; Kudlich, T.; Rauthe, S.; Melcher, R.; Geier, A. Benzbromarone: A double-edged sword that cuts the liver? Eur. J. Gastroenterol. Hepatol. 2013, 25, 119–121. [Google Scholar] [CrossRef]
- Haga, Y.; Yasui, S.; Kanda, T.; Hattori, N.; Wakamatsu, T.; Nakamura, M.; Sasaki, R.; Wu, S.; Nakamoto, S.; Arai, M.; et al. Successful Management of Acute Liver Failure Patients Waiting for Liver Transplantation by On-Line Hemodiafiltration with an Arteriovenous Fistula. Case Rep. Gastroenterol. 2016, 10, 139–145. [Google Scholar] [CrossRef]
- Kumagai, J.; Kanda, T.; Yasui, S.; Haga, Y.; Sasaki, R.; Nakamura, M.; Wu, S.; Nakamoto, S.; Arai, M.; Iino, Y.; et al. Autoimmune hepatitis following drug-induced liver injury in an elderly patient. Clin. J. Gastroenterol. 2016, 9, 156–159. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Niu, J.Q.; Wen, X.Y.; Jin, Q.L. Liver failure associated with benzbromarone: A case report and review of the literature. World J. Clin. Cases 2019, 7, 1717–1725. [Google Scholar] [CrossRef]
- Takikawa, H.; Murata, Y.; Horiike, N.; Fukui, H.; Onji, M. Drug-induced liver injury in Japan: An analysis of 1676 cases between 1997 and 2006. Hepatol. Res. 2009, 39, 427–431. [Google Scholar] [CrossRef]
- Babany, G.; Larrey, D.; Pessayre, D.; Degott, C.; Rueff, B.; Benhamou, J.P. Chronic active hepatitis caused by benzarone. J. Hepatol. 1987, 5, 332–335. [Google Scholar] [CrossRef]
- Katoh, Y.; Natsume, O.; Matsunaga, M.; Takayanagi, F.; Uchida, H.; Yasuoka, R. Diagnosis of non-immediate hypersensitivity to amoxicillin in children by skin test and drug provocation tests: A retrospective case-series study. Allergol. Int. 2022, 71, 131–136. [Google Scholar] [CrossRef]
- Sun, P.; Zhu, J.J.; Wang, T.; Huang, Q.; Zhou, Y.R.; Yu, B.W.; Jiang, H.L.; Wang, H.Y. Benzbromarone aggravates hepatic steatosis in obese individuals. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864 Pt A, 2067–2077. [Google Scholar] [CrossRef]
- Imai, S.; Nasuhara, Y.; Momo, K.; Oki, H.; Kashiwagi, H.; Sato, Y.; Miyai, T.; Sugawara, M.; Takekuma, Y. Implementation Status of Liver Function Tests for Monitoring Benzbromarone-Induced Hepatotoxicity: An Epidemiological Survey Using the Japanese Claims Database. Biol. Pharm. Bull. 2021, 44, 1499–1505. [Google Scholar] [CrossRef]
- Azevedo, V.F.; Kos, I.A.; Vargas-Santos, A.B.; da Rocha Castelar Pinheiro, G.; Dos Santos Paiva, E. Benzbromarone in the treatment of gout. Adv. Rheumatol. 2019, 59, 37. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Benzbromarone. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548732 (accessed on 21 January 2022).
- Roberts, R.L.; Wallace, M.C.; Wright, D.F.; Cadzow, M.; Dalbeth, N.; Jones, P.B.; Stamp, L.K.; Harrison, A.A.; Black, M.A.; Merriman, T.R. Frequency of CYP2C9 polymorphisms in Polynesian people and potential relevance to management of gout with benzbromarone. Jt. Bone Spine 2014, 81, 160–163. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Kondo, S. Inactivation of CYP3A4 by Benzbromarone in Human Liver Microsomes. Drug Metab. Lett. 2016, 10, 16–21. [Google Scholar] [CrossRef]
- Shirakawa, M.; Sekine, S.; Tanaka, A.; Horie, T.; Ito, K. Metabolic activation of hepatotoxic drug (benzbromarone) induced mitochondrial membrane permeability transition. Toxicol. Appl. Pharmacol. 2015, 288, 12–18. [Google Scholar] [CrossRef]
- Lee, K.S.; Song, I.S.; Kim, E.S.; Kim, H.I.; Ahn, K.H. Association of preterm birth with medications: Machine learning analysis using national health insurance data. Arch. Gynecol. Obstet. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Aleo, M.D.; Wen, X.; Kogut, S. Hepatotoxicity reports in the FDA adverse event reporting system database: A comparison of drugs that cause injury via mitochondrial or other mechanisms. Acta. Pharm. Sin. B 2011, 11, 3857–3868. [Google Scholar] [CrossRef] [PubMed]
- Kushiyama, A.; Nakatsu, Y.; Matsunaga, Y.; Yamamotoya, T.; Mori, K.; Ueda, K.; Inoue, Y.; Sakoda, H.; Fujishiro, M.; Ono, H.; et al. Role of Uric Acid Metabolism-Related Inflammation in the Pathogenesis of Metabolic Syndrome Components Such as Atherosclerosis and Nonalcoholic Steatohepatitis. Mediat. Inflamm. 2016, 2016, 8603164. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Gallardo, J.; Keaveny, A.P.; Qi, X.; Méndez-Sánchez, N. Metabolic associated fatty liver disease and acute-on-chronic liver failure: Common themes for common problems. Eur. J. Gastroenterol. Hepatol. 2021, 33 (Suppl. 1), e84–e93. [Google Scholar] [CrossRef]
- Zheng, K.I.; Zheng, M.H. The uprising of metabolic dysfunction-associated fatty liver disease (MAFLD) in acute-on-chronic liver failure (ACLF). Hepatobiliary Surg. Nutr. 2021, 10, 857–859. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, N.; Uemura, H.; Uchida, Y.; Imai, Y.; Tomiya, T.; Terai, S.; Yoshiji, H.; Genda, T.; Ido, A.; Inoue, K.; et al. Nationwide survey for patients with acute-on-chronic liver failure occurring between 2017 and 2019 and diagnosed according to proposed Japanese criteria. J. Gastroenterol. 2021, 56, 1092–1106. [Google Scholar] [CrossRef]
- Mazali, F.C.; Johnson, R.J.; Mazzali, M. Use of uric acid-lowering agents limits experimental cyclosporine nephropathy. Nephron Exp. Nephrol. 2012, 120, e12–e19. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Razek, E.A.; Abo-Youssef, A.M.; Azouz, A.A. Benzbromarone mitigates cisplatin nephrotoxicity involving enhanced peroxisome proliferator-activated receptor-alpha (PPAR-α) expression. Life Sci. 2020, 243, 117272. [Google Scholar] [CrossRef]
- Kanda, T.; Yokosuka, O.; Okada, O.; Suzuki, Y.; Saisho, H. Severe hepatotoxicity associated with Chinese diet product ‘Onshidou-Genbi-Kounou’. J. Gastroenterol. Hepatol. 2003, 18, 354–355. [Google Scholar] [CrossRef]
- Kanda, T.; Yokosuka, O.; Tada, M.; Kurihara, T.; Yoshida, S.; Suzuki, Y.; Nagao, K.; Saisho, H. N-nitroso-fenfluramine hepatotoxicity resembling chronic hepatitis. J. Gastroenterol. Hepatol. 2003, 18, 999–1000. [Google Scholar] [CrossRef]
- Tarantino, G.; Pezzullo, M.G.; di Minno, M.N.; Milone, F.; Pezzullo, L.S.; Milone, M.; Capone, D. Drug-induced liver injury due to “natural products” used for weight loss: A case report. World J. Gastroenterol. 2009, 15, 2414–2417. [Google Scholar] [CrossRef]
- Leise, M.D.; Poterucha, J.J.; Talwalkar, J.A. Drug-induced liver injury. Mayo Clin. Proc. 2014, 89, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Yokosuka, O.; Fujiwara, K.; Saisho, H.; Shiga, H.; Oda, S.; Okuda, K.; Sugawara, Y.; Makuuchi, M.; Hirasawa, H. Fulminant hepatic failure associated with triazolam. Dig. Dis. Sci. 2002, 47, 1111–1114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).