Abstract
Primary hepatic leiomyoma (PHL) is a rare entity, with very few cases reported in the literature. Even more rarely, until now practically undescribed, is the transformation of a hepatic leiomyoma into leiomyosarcoma with pancreatic metastases. Here, we report a single case of the progression of PHL in primary hepatic leiomyosarcoma, with clinical–surgical and histopathological features, and we conducted a review of the literature of related cases that can be found.
1. Introduction
Primary hepatic leiomyoma (PHL) is a rare entity, of which a limited number of cases are described in the literature [1]. In the latest World Health Organization (WHO) “Digestive Tumours” of 2019, leiomyomas of the gastrointestinal (GI) tract are listed as “relatively rare”, more commonly found in the esophagus, stomach and colon [2]. Although rare, cases of primary liver leiomyosarcoma have also been reported in the literature, but cases of the malignant transformation of a primary hepatic leiomyoma into its malignant counterpart have practically never been reported. In this paper, we report a case of the malignant transformation of a PHL, provide clinical–radiological and histo-morphological information, and conduct a literature review of similar cases.
2. Material and Methods
This case report was presented after approval from informed patient consent; the samples were taken in the operating room and fixed in 10% buffered formaldehyde, sampled according to national guidelines, processed, dipped in paraffin and cut with a microtome. Sections 5 μm thick were obtained, and stained with hematoxylin–eosin and with antibodies for the immunostaining for smooth muscle actin (1A4, Dako-Agilent, Santa Clara, CA, USA 1:250) Desmin (PA5-16705, ThermoFisher, Waltham, MA, USA 1:200), Ki67 (MIB-1, Dako-Agilent, 1:500).
In addition, we conducted a review of the literature using PubMed and Web of Science (WoS) as search engines, typing the following keywords: “primitive hepatic leiomyoma” OR “hepatic leiomyoma” OR “primitive hepatic leiomyosarcoma” OR “primitive mesenchimal hepatic tumours” in combination with “case report” OR “review” OR “case presentation”. Only articles in English were selected. The last search was performed on 28 August 2021. Eligible articles were assessed according to the Oxford Centre for Evidence-Based Medicine 2011 guidelines and rated as level 3 or 4 evidence for clinical research. Case reports, review articles, meta-analyses, observational studies, letters to the editor, and comments to the letters were all included. Other potentially relevant articles were identified by manually checking the references in the included literature.
An independent extraction of the articles was performed by two investigators according to the inclusion criteria. Disagreements were resolved by discussions between the two review authors. We focused on the histopathological diagnosis of leiomyoma or leiomyosarcoma, on the size and topography of the lesion, and on any symptoms mentioned by the patient. The articles and data obtained are presented in Table 1, and the review was performed according to the PRISMA guidelines (Figure 1).

Table 1.
Summary of the reported cases of primary leiomyoma and leiomyosarcoma of the liver in literature.
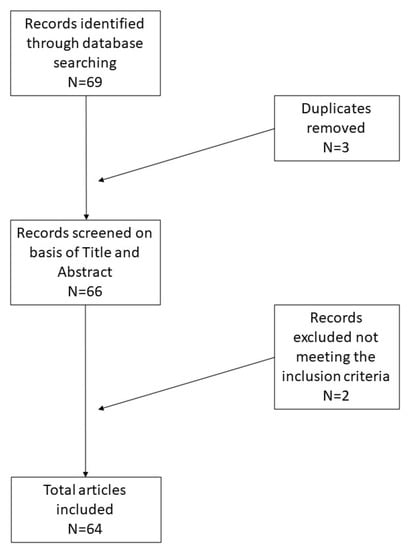
Figure 1.
Selection of articles following PRISMA guidelines.
3. Results
3.1. Case Presentation
In 2014, a 53-year-old woman had been suffering from the onset of non-specific gastrointestinal symptoms for some months, including difficulty in digestion, slowed digestion, vomiting and changes in evacuation habits. Following a consultation with the general practitioner, it was decided to refer the patient for a visit to the general surgeon who, on the CT scan, identified the presence of a voluminous neoformation of the cecum, with peri-cecal fluid collection and a suspected metastatic lesion of about 15 mm, between segments 2 and 4 of the liver. Hematological and serum biochemical profiles were within normal ranges. Tumor markers including alpha-fetoprotein (AFP), carcinoembryonic (CEA), carbohydrate antigens 199 (CA199), and carbohydrate antigens 125 (CA125) were also normal.
3.2. Histological Features
A hepatic segmentectomy was performed, and at the post-operative histological examination, a moderately differentiated adenocarcinoma of the large intestine was diagnosed, involving the ileocecal valve, with an expansive growth pattern, which infiltrated the wall up to the proper muscular tunic. There was no vascular invasion in the examined sections, and it was concluded for a staging (pTNM): pT2N0M0. At the hepatic level, on the other hand, a nodular formation was found, well demarcated, consisting of medium–large cellular elements, mainly arranged in an intertwined bundle architecture, with a single focus of central necrosis. There were no cellular elements of the adenocarcinomatous type. Immunohistochemical investigations were positive for smooth muscle actin (Act-mL) and desmin. On the other hand, the reactions for CD117 (c-kit), DOG-1, S-100 protein, CD34 and vimentin were negative. The evaluation of the mitotic index, carried out by measuring the individual mitotic figures on 50 high-magnification fields (HPF, Original Magnification: 40×), was 1 mitosis/50 HPF, a figure confirmed by evaluation of the neoplastic proliferation index (evaluated by Ki67+) which was 1%. On the basis of these data, the diagnosis of primary leiomyoma of the liver was made (Figure 2A,B). The lesion reached close to the Glissoniana, without ever infiltrating it; the surgical resection margins were free from neoplasia. The patient was considered disease-free and continued to have six-monthly follow-ups. In 2019, about 5 years later, at a checkup, a new liver lesion was found on the computed tomography scan, in correspondence with the S3 segment. In agreement with the patient, a hepatic needle biopsy was performed, which revealed a proliferation of leiomuscular elements with mild cytological atypia and low mitotic index (5 mitosis/50 HPF). Additionally, in this case, the immunohistochemical reactions were positive for smooth muscle actin and desmin. The fraction of neoplastic proliferation (again evaluated by Ki67) was, this time, about 10%. A diagnosis of a recurrent smooth muscle tumor of the liver was therefore made. Following this needle biopsy, the patient was again subjected to hepatic segmentectomy (S3.5-8) which revealed a nodular neoformation of 7.3 × 6.3 × 4 cm, whitish in color, and a collated appearance when cut. On this occasion, a consensual cholecystectomy was also performed. The histological diagnosis concluded with mesenchymal malignant neoplasm, with spindle elements with pleomorphic nuclei, arranged in variously intertwined bundles; the mitotic index was equal to 6 mitosis/50 HPF and the proliferative index (Ki67+) was about 20%. Furthermore, multiple and sometimes confluent foci of necrosis were described (Figure 3A–C). However, there were no clear signs of ease of invasion. Additionally, in this case, the immunohistochemical investigations were strongly positive for smooth muscle actin (Figure 3D) and desmin. In consideration of these new data, the diagnosis of primary hepatic leiomyosarcoma, grade 1 (total score: 3), according to the grading system of the French Federation of Cancer Centers Sarcoma Group (FNCLCC), was made. In May 2021, about 2 years after the last diagnosis, the patient was again operated on, focusing on the thyroid gland for the echo-tomography (ETG) detection of a 29 × 16 mm, hypoechoic nodule, located in the middle/lower third of the left lobe. In addition, she underwent pancreatic caudectomy for an expansive formation in the distal third of the pancreas. The histological diagnosis concluded with an oncocytic adenoma of the thyroid gland and a localization of leiomyosarcoma in the pancreas (Figure 4A,B).
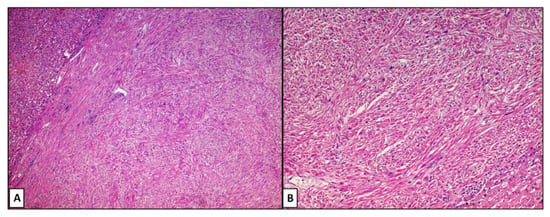
Figure 2.
(A,B). Photomicrograph comprising a proliferation with mesenchymal habitus, consisting of bundles of fibers intersecting with each other, often at right angles, without evident atypia and/or mitotic figures. Note the well-defined and sharp edges of the lesion (hematoxylin–eosin, original magnification: 4× (A) and 20× (B).
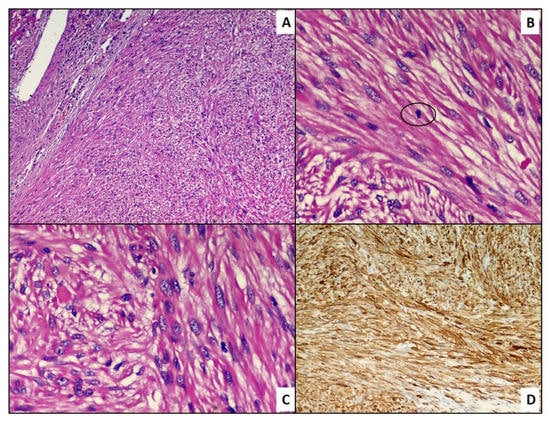
Figure 3.
(A) Histological preparation including a proliferation of mesenchymal cells more atypical than the previous finding. Note the jagged and less clear and defined edges of the lesion (hematoxylin–eosin, original magnification: 10×). (B) Histological detail of a circled atypical mitotic figure (hematoxylin–eosin, original magnification: 40×). (C) Histological detail of the lesion: note the greater cellular crowding and nuclear irregularities (hematoxylin–eosin, original magnification: 40×). (D) Positive immunostaining for smooth muscle actin (immunohistochemistry, original magnification: 10×).
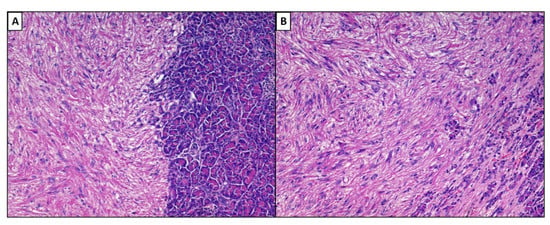
Figure 4.
(A,B). Histological preparation including metastases in the pancreatic parenchyma (left) of PHLeiomyosarcoma. Note the same growth pattern as the primary lesion (hematoxylin–eosin, original magnification: 10× (A) and 20× (B).
3.3. Review of Literature
We identified 64 publications over a period of time between 1926 and 2020, almost entirely consisting of a single case report each (n = 61) except for three papers comprising 2 cases [6], 3 cases [49], and 34 cases [19]. We included a total of 100 cases, consisting of 24 cases (24%) of primary hepatic leiomyoma, 1 case (1%) of atypical leiomyoma, and the remaining 75 cases (75%) of primary hepatic leiomyosarcoma.
A total of 19 cases (29.6%) consisted of primary hepatic leiomyomas [3,5,12,13,14,18,21,32,39,44,50,52,54,57,58,60,64,65,66], whereas 1 case (1.56%) was of leiomyoma in a patient with HIV [25], and 1 case (1.56%) was of leiomyoma in a transplant recipient [28]. Another single case was a myxoid leiomyoma [29], and another case was a leiomyoma close to a hepatobiliary cystadenoma [30]. Finally, 1 case consisted of a giant leiomyoma (>10 cm in maximum diameter) [34] and 1 case consisted of “atypical” leiomyoma [15].
More than half of the lesions (both leiomyomas and leiomyosarcomas) were located in the right lobe of the liver, with the remaining cases located in the left lobe. The symptoms most complained of by patients were right upper abdominal pain, with one case of associated fever [27] and one case of intestinal obstruction due to the size of the lesion [34].
4. Discussion
Primary hepatic leiomyoma is a rare type of mesenchymal tumor originating from the liver, thought to originate from the smooth muscle layer of the intrahepatic blood vessel wall or of the biliar tree [64]. Similarly, primary leiomyosarcoma of the liver is even rarer, with about 70 cases reported in the literature [63]. However, the case of a PHL evolved into PHLeiomyosarcoma has never been reported. In this paper, we report our experience, and, probably, the first case ever described. The first author to describe a case of primary leiomyoma of the liver was Demel [3] in 1926, presenting the case of a patient with a 12 cm lesion to the right lobe of the liver. Conversely, the first official report of primitive leiomyosarcoma of the liver occurred in 1926 by Beaird [4] who described a lesion likely departing from the muscular wall of the portal vein. From that moment, there have been different descriptions with cases rather simple to diagnose, frankly benign, and cases that appeared immediately malignant.
Interestingly, some authors have highlighted how immunodeficiency conditions (congenital or acquired, such as AIDS) can cause or underlie the development of mesenchymal lesions starting from the liver. For example, Ross J.S. [22] and Prevot S. [25] in 1992 and 1994, respectively, reported two cases of primary leiomyosarcoma and leiomyoma of the liver in previously HIV-positive patients. The authors postulated how immune dysregulation could affect the immune system’s own immune surveillance function, allowing more such neoplasms to manifest themselves.
Curiously, in 1998, Yoon et al. [29] reported a case of primary myxoid leiomyoma of the liver, providing elements of the morphological analysis of this lesion that had not yet been highlighted in this situation, and tried to explain these modifications as regressive rather than neoplastic progression events.
From careful study of the literature, no one has ever described a malignant transformation of a PHL into a PHleiomyosarcoma, except for the paper by Lee [15] which, in 1990, described a case of atypical leiomyoma of the liver, borrowing certain analogies with lesions of other body districts such as soft tissues or the female gynecological system. This provides a scientific basis for our experience with the reported case.
Traditionally, the histopathological diagnosis of PHL does not require particular effort: a good and clear morphology together with a small number of immunohistochemical markers (actin smooth muscle and desmin) are sufficient to perform the diagnosis. Similarly, even in the case of an overt PHleiomyosarcoma [67], atypia, necrosis and mitotic figures, together with a few markers of IHC, are sufficient. On the other hand, case such as this one presented by us can turn out to be more difficult. Already in the first histological stage, although the lesion did not meet the requirements to satisfy a diagnosis of leiomyosarcoma, characters such as focal necrosis were described, which required a careful follow-up of the lesion. In fact, after a few years, despite the previous removal, progression of the disease took place, with the development of a full-blown picture of PHleiomyosarcoma, recently also metastasized to the pancreas.
5. Conclusions
The meaning of our work is to draw attention to the possibility that in unusual sites, such as the liver, a very rare neoplasm, may, exceptionally, evolve into something frankly malignant. The rarity in the rarity should not be ignored.
Author Contributions
Conceptualization, A.C. and G.C.; methodology, G.C.; software, G.C.; validation, A.C., L.R. and L.V.; formal analysis, V.A.; investigation, S.F.; resources, A.C.; data curation, G.I.; writing—original draft preparation, G.C.; writing—review and editing, L.V.; visualization, G.I.; supervision, L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Digestive System Tumours: WHO Classification of Tumours; World Health Organization (WHO): Geneva, Switzerland, 2019; Volume 1.
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Demel, R. Ein operierter Fall von Leber-Myom. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1926, 261, 881–884. [Google Scholar] [CrossRef]
- Beaird, J.B.; Scofield, G.F. Budd-Chiari syndrome. Hepatic vein occlusion due to leiomyosarcoma primary in the inferior vena cava. Arch. Intern. Med. 1962, 110, 435–441. [Google Scholar] [CrossRef]
- Rios-Dalenz, J.L. Leiomyoma of the Liver. Arch Pathol. 1965, 79, 54–56. [Google Scholar]
- Masur, H.; Sussman, E.B.; Molander, D.W. Primary hepatic leiomyosarcoma: A report of two cases. Gastroenterology 1975, 69, 994–997. [Google Scholar] [CrossRef]
- Bloustein, P.A. Hepatic leiomyosarcoma: Ultrastructural study and review of the differential diagnosis. Hum. Pathol. 1978, 9, 713–715. [Google Scholar] [CrossRef]
- Maki, H.S.; Hubert, B.C.; Sajjad, S.M.; Kirchner, J.P.; Kuehner, M.E. Primary hepatic leiomyosarcoma. Arch. Surg. 1987, 122, 1193–1196. [Google Scholar] [CrossRef]
- Griffin, A.S.; Sterchi, J.M. Primary leiomyosarcoma of the inferior vena cava: A case report and review of the literature. J. Surg. Oncol. 1987, 34, 53–60. [Google Scholar] [CrossRef]
- Shurbaji, M.S.; Olson, J.L.; Kuhajda, F.P. Thorotrast-associated hepatic leiomyosarcoma and cholangiocarcinoma in a single patient. Hum. Pathol. 1987, 18, 524–526. [Google Scholar] [CrossRef]
- Kinoshita, A.; Sakon, M.; Monden, M.; Gotoh, M.; Kobayashi, K.; Okuda, H.; Kuroda, C.; Sakurai, M.; Okamura, J.; Mori, T. Triple synchronous malignant tumors. Hepatic leiomyosarcoma, splenic hemangiosarcoma and sigmoid colon cancer. Case report. Acta Chir. Scand. 1988, 154, 477–479. [Google Scholar]
- Rummeny, E.; Weissleder, R.; Stark, D.D.; Saini, S.; Compton, C.C.; Bennett, W.; Hahn, P.F.; Wittenberg, J.; Malt, R.A.; Ferrucci, J.T. Primary liver tumors: Diagnosis by MR imaging. AJR Am. J. Roentgenol. 1989, 152, 63–72. [Google Scholar] [CrossRef]
- Herzberg, A.J.; MacDonald, J.A.; Tucker, J.A.; Humphrey, P.A.; Meyers, W.C. Primary leiomyoma of the liver. Am. J. Gastroenterol. 1990, 85, 1642–1645. [Google Scholar]
- Little, J.M.; Kenny, J.; Hollands, M.J. Hepatic incidentaloma: A modern problem. World J. Surg. 1990, 14, 448–451. [Google Scholar] [CrossRef]
- Lee, P.K.; Teixeira, O.H.; Simons, J.A.; Goodman, R.L.; Brais, M.P.; Barber, G.G.; Dunlap, H.J.; Walley, V.M. Atypical hepatic vein leiomyoma extending into the right atrium: An unusual cause of the Budd-Chiari syndrome. Can. J. Cardiol. 1990, 6, 107–110. [Google Scholar]
- Spagliardi, E.; Longo, A.; Blanco, G.F.; Ruggeri, C.; Buscaglia, M.; Torelli, P. Rare primary hepatic neoplasms. Our experience in 2 cases: A primary lymphoma and a leiomyosarcoma of the liver. Minerva Chir. 1990, 45, 95–102. [Google Scholar]
- Sundaresan, M.; Kelly, S.B.; Benjamin, I.S.; Akosa, A.B. Primary hepatic vascular leiomyosarcoma of probable portal vein origin. J. Clin. Pathol. 1990, 43, 1036. [Google Scholar] [CrossRef]
- Bartoli, S.; Alò, P.; Leporelli, P.; Puce, E.; Di Tondo, U.; Thau, A. Primary hepatic leiomyoma. Minerva Chir. 1991, 46, 777–779. [Google Scholar]
- Ishii, H.; Nakayama, T.; Hiyama, Y. Primary hepatic leiomyosarcoma: The investigation of domestic and foreign 34 cases. Nihon Shokakibyo Gakkai Zasshi 1991, 88, 1256–1263. [Google Scholar]
- Korbi, S.; Aouini, M.T.; Remadi, S.; el Ajmi, S.; Mokni, M.; Ben Ayed, F.; Gannouni, A. Primary hepatic leiomyosarcoma. A case report with immunohistochemical studies and review of the literature. J. Submicrosc. Cytol. Pathol. 1991, 23, 643–647. [Google Scholar]
- Reinertson, T.E.; Fortune, J.B.; Peters, J.C.; Pagnotta, I.; Balint, J.A. Primary leiomyoma of the liver. A case report and review of the literature. Dig. Dis. Sci. 1992, 37, 622–627. [Google Scholar] [CrossRef]
- Ross, J.S.; Del Rosario, A.; Bui, H.X.; Sonbati, H.; Solis, O. Primary hepatic leiomyosarcoma in a child with the acquired immunodeficiency syndrome. Hum. Pathol. 1992, 23, 69–72. [Google Scholar] [CrossRef]
- Baur, M.; Pötzi, R.; Lochs, H.; Neuhold, N.; Walgram, M.; Gangl, A. Primary leiomyosarcoma of the liver—A case report. Z. Gastroenterol. 1993, 31, 20–23. [Google Scholar]
- Saint-Paul, M.C.; Gugenheim, J.; Hofman, P.; Arpurt, J.P.; Fabiani, P.; Michiels, J.F.; Fujita, N.; Goubeaux, B.; Loubière, R.; Delmont, J.; et al. Leiomyosarcoma of the liver: A case treated by transplantation. Gastroenterol. Clin. Biol. 1993, 17, 218–222. [Google Scholar]
- Prévot, S.; Néris, J.; de Saint Maur, P.P. Detection of Epstein Barr virus in an hepatic leiomyomatous neoplasm in an adult human immunodeficiency virus 1-infected patient. Virchows Arch. 1994, 425, 321–325. [Google Scholar] [CrossRef]
- Hiyama, Y. Primary hepatic leiomyosarcoma and rhabdomyosarcoma. Ryoikibetsu Shokogun Shirizu 1995, 7, 463–466. [Google Scholar]
- Abdelli, N.; Thiefin, G.; Diebold, M.D.; Bouche, O.; Aucouturier, J.P.; Zeitoun, P. Primary leiomyosarcoma of the liver 37 years after successful treatment of hereditary retinoblastoma. Gastroenterol. Clin. Biol. 1996, 20, 502–505. [Google Scholar]
- Davidoff, A.M.; Hebra, A.; Clark, B.J.; Tomaszewski, J.E.; Montone, K.T.; Ruchelli, E.; Lau, H.T. Epstein-Barr virus-associated hepatic smooth muscle neoplasm in a cardiac transplant recipient. Transplantation 1996, 61, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.S.; Kang, G.H.; Kim, O.J. Primary myxoid leiomyoma of the liver. Arch. Pathol. Lab. Med. 1998, 122, 1112–1115. [Google Scholar] [PubMed]
- Yanase, M.; Ikeda, H.; Ogata, I.; Ohno, A.; Moriya, A.; Miura, N.; Kimura, S.; Mori, M.; Oka, T.; Ohtomo, K.; et al. Primary smooth muscle tumor of the liver encasing hepatobiliary cystadenoma without mesenchymal stroma. Am. J. Surg. Pathol. 1999, 23, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.; Hayashi, D.; Inokuchi, T.; Okamura, K.; Takahashi, T.; Noshima, S.; Morita, N.; Esato, K. Combined right hepatic and retrohepatic caval resection with reconstruction using a polytetrafluoroethylene graft for primary leiomyosarcoma of the liver: Report of case. Surg. Today 1999, 29, 67–70. [Google Scholar] [CrossRef]
- Mesenas, S.J.; Ng, K.Y.; Raj, P.; Ho, J.M.; Ng, H.S. Primary leiomyoma of the liver. Singap. Med. J. 2000, 41, 129–131. [Google Scholar]
- Tsuji, M.; Takenaka, R.; Kashihara, T.; Hadama, T.; Terada, N.; Mori, H. Primary hepatic leiomyosarcoma in a patient with hepatitis C virus-related liver cirrhosis. Pathol. Int. 2000, 50, 41–47. [Google Scholar] [CrossRef]
- Belli, G.; Ciciliano, F.; Lannelli, A.; Marano, I. Hepatic resection for primary giant leiomyoma of the liver. HPB 2001, 3, 11–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Linares Torres, P.; Vivas Alegre, S.; Castañón López, C.; Domínguez Carbajo, A.B.; Honrado Franco, E.; Espinel Díez, J.; Jorquera Plaza, F.; Olcoz Goñi, J.L. Primary hepatic leiomyosarcoma in a patient with gastric non-Hodgkin lymphoma. Gastroenterol. Hepatol. 2002, 25, 452–454. [Google Scholar] [CrossRef]
- Fujita, H.; Kiriyama, M.; Kawamura, T.; Ii, T.; Takegawa, S.; Dohba, S.; Kojima, Y.; Yoshimura, M.; Kobayashi, A.; Ozaki, S.; et al. Primary hepatic leiomyosarcoma in a woman after renal transplantation: Report of a case. Surg. Today 2002, 32, 446–449. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.L.; Choi, W.H. A case of primary myxoid leiomyosarcoma of the liver. Korean J. Intern. Med. 2002, 17, 278–282. [Google Scholar] [CrossRef]
- Baek, I.; Kim, J.H.; Lee, M.S.; Baik, G.H.; Hahn, T.; Park, H.J.; Park, S.H.; Chang, W.K.; Kim, W.J.; Park, C.K. A case of primary leiomyosarcoma of the liver. Taehan Kan Hakhoe Chi 2002, 8, 481–485. [Google Scholar] [PubMed]
- Beuzen, F.; Roudie, J.; Moali, I.; Maitre, S.; Barthelemy, P.; Smadja, C. Primary leiomyoma of the liver: A rare benign tumor. Gastroenterol. Clin. Biol. 2004, 28, 1169–1172. [Google Scholar] [CrossRef]
- Maruta, K.; Sonoda, Y.; Saigo, R.; Yoshioka, T.; Fukunaga, H. A patient with von Recklinghausen’s disease associated with polymyositis, asymptomatic pheochromocytoma, and primary hepatic leiomyosarcoma. Nihon Ronen Igakkai Zasshi 2004, 41, 339–343. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Lee, H.L.; Sohn, J.H.; Kim, J.B.; Han, D.S.; Jeon, Y.C.; Hahm, J.S.; Lee, D.H.; Kee, C.S.; Park, Y.W. A case of primary hepatic leiomyosarcoma presenting with multiple subcutaneous scalp mass. Korean J. Gastroenterol. 2005, 46, 233–236. [Google Scholar]
- Kwon, K.M.; Jang, B.K.; Chung, W.J.; Park, K.S.; Cho, K.B.; Hwang, J.S.; Kang, K.J.; Kang, Y.N.; Kwon, J.H. A case of primary hepatic leiomyosarcoma with intrahepatic and abdominal subcutaneous metastasis in Behcet’s disease. Korean J. Hepatol. 2005, 11, 386–391. [Google Scholar] [PubMed]
- Surendrababu, N.R.; Rao, A.; Samuel, R. Primary hepatic leiomyosarcoma in an infant. Pediatr. Radiol. 2006, 36, 366. [Google Scholar] [CrossRef]
- Marin, D.; Catalano, C.; Rossi, M.; Guerrisi, A.; Di Martino, M.; Berloco, P.; Passariello, R. Gadobenate dimeglumine-enhanced magnetic resonance imaging of primary leiomyoma of the liver. J. Magn. Reson. Imaging 2008, 28, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Tsiatis, A.C.; Atkinson, J.B.; Wright, J.K.; Cates, J.M. Primary hepatic myxoid leiomyosarcoma: A case report and review of the literature. Ultrastruct. Pathol. 2008, 32, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Jeong, T.Y.; Kim, Y.S.; Park, K.J.; Lee, J.S.; Huh, J.G.; Ryu, S.H.; Lee, J.H.; Moon, J.S. A case of primary leiomyosarcoma of the liver presenting with acute bleeding. Korean J. Gastroenterol. 2008, 51, 194–198. [Google Scholar]
- Giuliante, F.; Sarno, G.; Ardito, F.; Pierconti, F. Primary hepatic leiomyosarcoma in a young man after Hodgkin’s disease: Diagnostic pitfalls and therapeutic challenge. Tumori J. 2009, 95, 374–377. [Google Scholar] [CrossRef]
- Liang, X.; Shi, X.-M.; Xie, J.-P.; Yang, J.-Y.; Zhang, X.-J.; Fu, Z.-R.; Ding, G.-S.; Li, R.-D. Liver transplantation for primary hepatic leiomyosarcoma: A case report and review of the literatures. Med. Oncol. 2010, 27, 1269–1272. [Google Scholar] [CrossRef]
- Shamseddine, A.; Faraj, W.; Mukherji, D.; El Majzoub, N.; Khalife, M.; Soubra, A.; Shamseddine, A. Unusually young age distribution of primary hepatic leiomyosarcoma: Case series and review of the adult literature. World J. Surg. Oncol. 2010, 8, 56. [Google Scholar] [CrossRef]
- Santos, I.; Valls, C.; Leiva, D.; Serrano, T.; Martinez, L.; Ruiz, S. Primary hepatic leiomyoma: Case report. Abdom. Imaging 2011, 36, 315–317. [Google Scholar] [CrossRef]
- Shivathirthan, N.; Kita, J.; Iso, Y.; Hachiya, H.; Kyunghwa, P.; Sawada, T.; Kubota, K. Primary hepatic leiomyosarcoma: Case report and literature review. World J. Gastrointest. Oncol. 2011, 3, 148–152. [Google Scholar] [CrossRef]
- Tsai, P.S.; Yeh, T.C.; Shih, S.L. Primary hepatic leiomyosarcoma in a 5-month-old female infant. Acta Radiol. Short Rep. 2013, 2, 2047981613498722. [Google Scholar] [CrossRef] [PubMed]
- Raber, E.L.; Cheng, A.L.; Dong, W.F.; Sutherland, F. Primary hepatic leiomyoma in a transplant patient: Characterization with magnetic resonance imaging. Transplantation 2012, 93, e4–e5. [Google Scholar] [CrossRef] [PubMed]
- Chelimilla, H.; Badipatla, K.; Ihimoyan, A.; Niazi, M. A rare occurrence of primary hepatic leiomyosarcoma associated with epstein barr virus infection in an AIDS patient. Case Rep. Gastrointest. Med. 2013, 2013, 691862. [Google Scholar] [CrossRef]
- Metta, H.; Corti, M.; Trione, N.; Masini, D.; Monestes, J.; Rizzolo, M.; Carballido, M. Primary hepatic leiomyosarcoma—A rare neoplasm in an adult patient with AIDS: Second case report and literature review. J. Gastrointest. Cancer 2014, 45 (Suppl. S1), 36–39. [Google Scholar] [CrossRef]
- Luo, X.Z.; Ming, C.S.; Chen, X.P.; Gong, N.Q. Epstein-Barr virus negative primary hepatic leiomyoma: Case report and literature review. World J. Gastroenterol. 2013, 19, 4094–4098. [Google Scholar] [CrossRef]
- Vyas, S.; Psica, A.; Watkins, J.; Yu, D.; Davidson, B. Primary hepatic leiomyoma: Unusual cause of an intrahepatic mass. Ann. Transl. Med. 2015, 3, 73. [Google Scholar] [PubMed]
- Lv, W.F.; Han, J.K.; Cheng, D.L.; Tang, W.J.; Lu, D. Imaging features of primary hepatic leiomyosarcoma: A case report and review of literature. Oncol. Lett. 2015, 9, 2256–2260. [Google Scholar] [CrossRef]
- Navarro, C.; Hamidian Jahromi, A.; Donato, M.; Caliri, N.; Tempra, A.; Sangster, G. Primary Leiomyoma of the Liver: Case Report and Review of the Literature. J. La. State Med. Soc. 2015, 167, 129–133. [Google Scholar] [PubMed]
- Ter-Ovanesov, M.D.; Valkin, D.L.; Gaboyan, A.S.; Kukosh, M.Y.; Ronzin, A.V.; Andrianova, V.S.; Larin, A.L.; Zhuk, A.I. Hepatic leiomyosarcoma: A case report. Vopr Onkol. 2016, 62, 857–862. [Google Scholar]
- Iida, T.; Maeda, T.; Amari, Y.; Yurugi, T.; Tsukamoto, Y.; Nakajima, F. Primary hepatic leiomyosarcoma in a patient with autosomal dominant polycystic kidney disease. CEN Case Rep. 2017, 6, 74–78. [Google Scholar] [CrossRef]
- Blas Laina, J.L.; González Ruiz, Y.; Gonzalvo González, E.; Sanz Moncasi, M.P.; Rodríguez Borobia, A. Primary hepatic leiomyoma: A rare liver mass. Gastroenterol. Hepatol. 2017, 40, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Feretis, T.; Kostakis, I.D.; Damaskos, C.; Garmpis, N.; Mantas, D.; Nonni, A.; Kouraklis, G.; Dimitroulis, D. Primary Hepatic Leiomyosarcoma: A Case Report and Review of the Literature. Acta Med. 2018, 61, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Jin, Z.; Gao, P.; Liu, Y. Primary hepatic leiomyoma in a Chinese female patient without underlying disease: A case report. BMC Surg. 2019, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.; Caetano Oliveira, R.; Terracciano, L.; Silva, M.R.; Cipriano, M.A. Hepatic Myxoid Leiomyoma: A Very Rare Tumor. GE Port. J. Gastroenterol. 2020, 27, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Coletta, D.; Parrino, C.; Nicosia, S.; Manzi, E.; Pattaro, G.; Oddi, A.; D’Annibale, M.; Marino, M.; Grazi, G.L. Primary leiomyoma of the liver in an immunocompetent patient. Intractable Rare Dis. Res. 2020, 9, 251–255. [Google Scholar] [CrossRef]
- Chi, M.; Dudek, A.Z.; Wind, K.P. Primary hepatic leiomyosarcoma in adults: Analysis of prognostic factors. Onkologie 2012, 35, 210–214. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).