Clinical Importance of Drug Adherence during Tyrosine Kinase Inhibitor Therapy for Chronic Myelogenous Leukemia in Chronic Phase
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Adverse Events
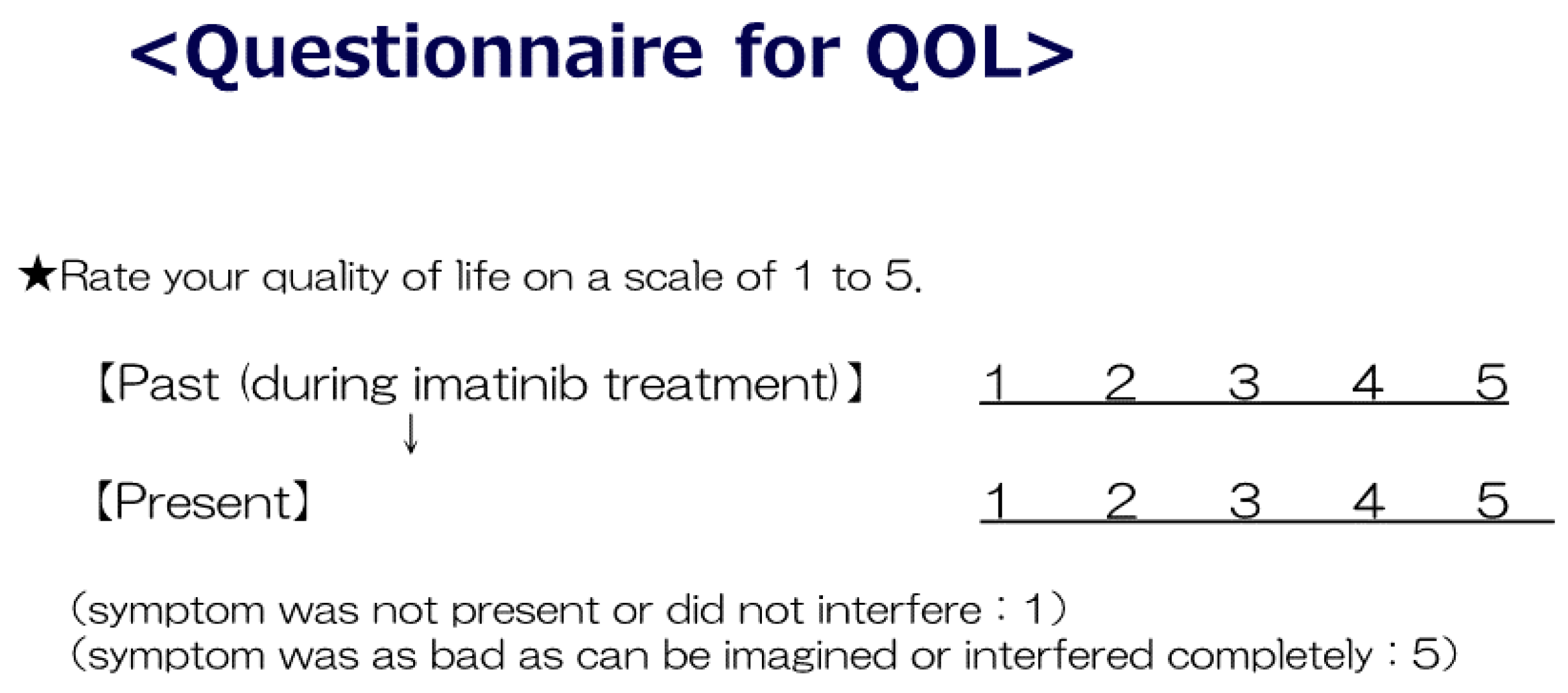
2.3. Quality of Life (QoL)
2.4. Assessment of Adherence and Clinical Outcome
2.5. Statistical Analysis
2.6. Ethics Committee Approval and Patient Consent
3. Results
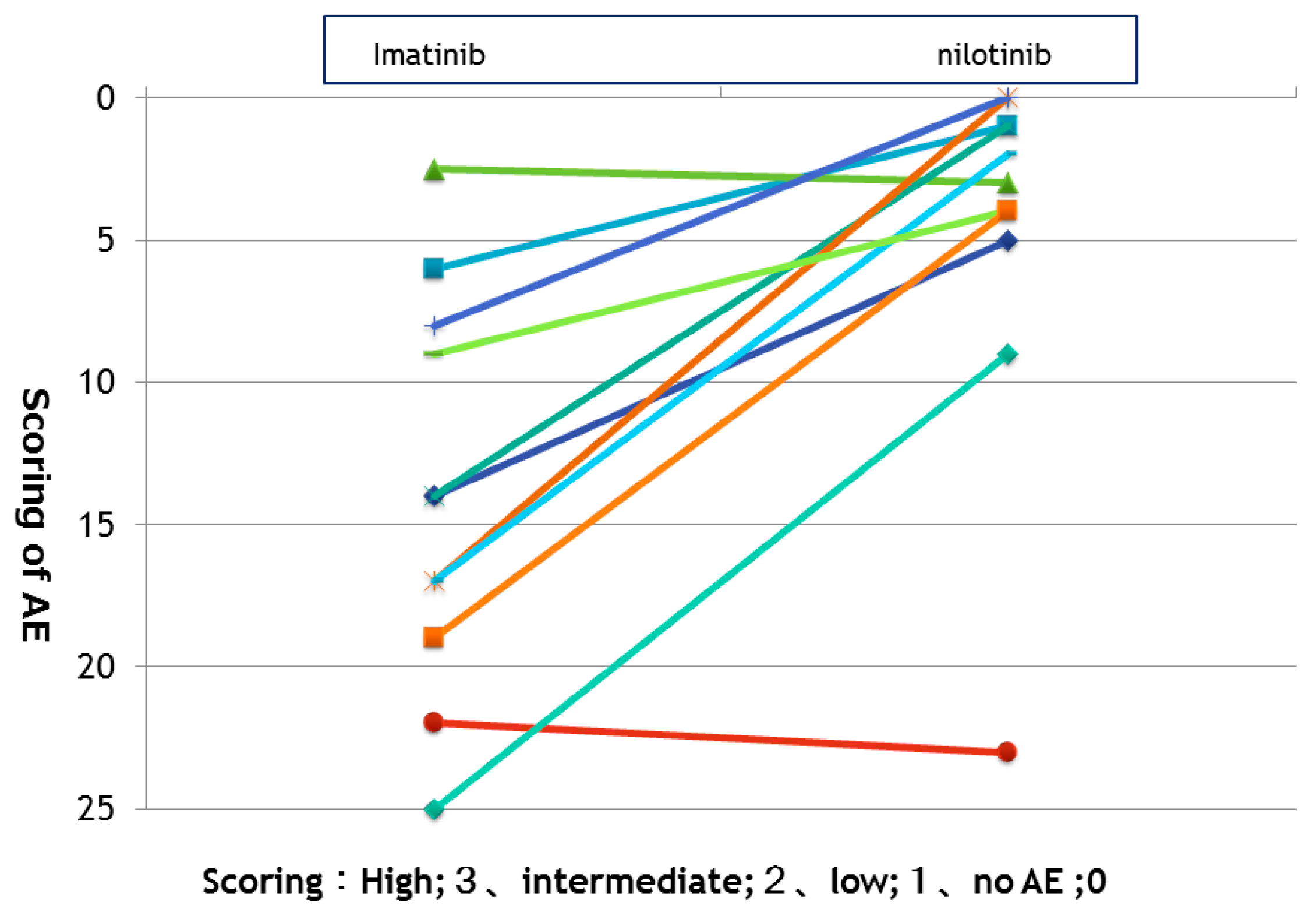
3.1. Comparison of ADEs between IM and NILO Treatments
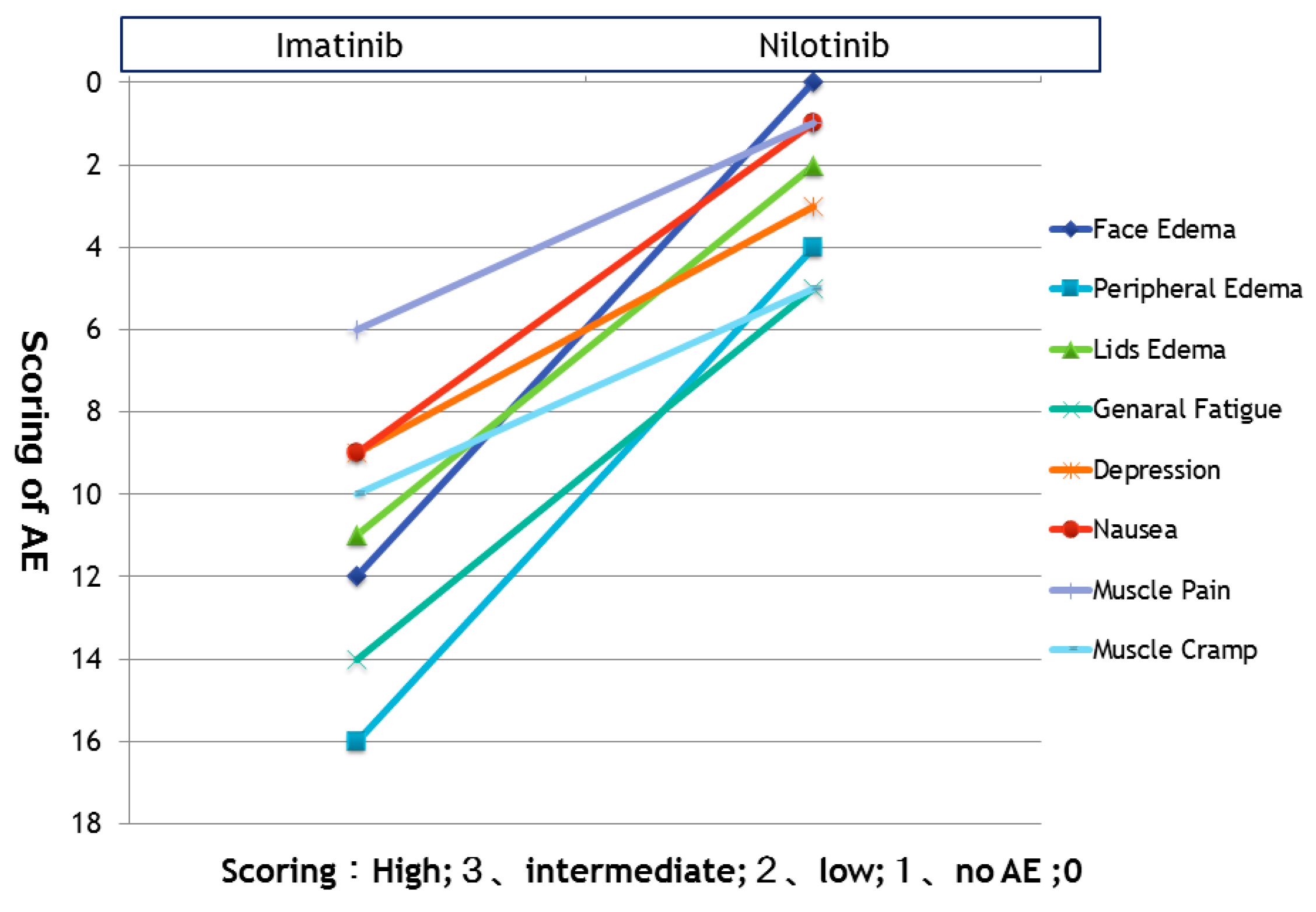
3.2. Improved Symptoms after Switching from Imatinib to Nilotinib
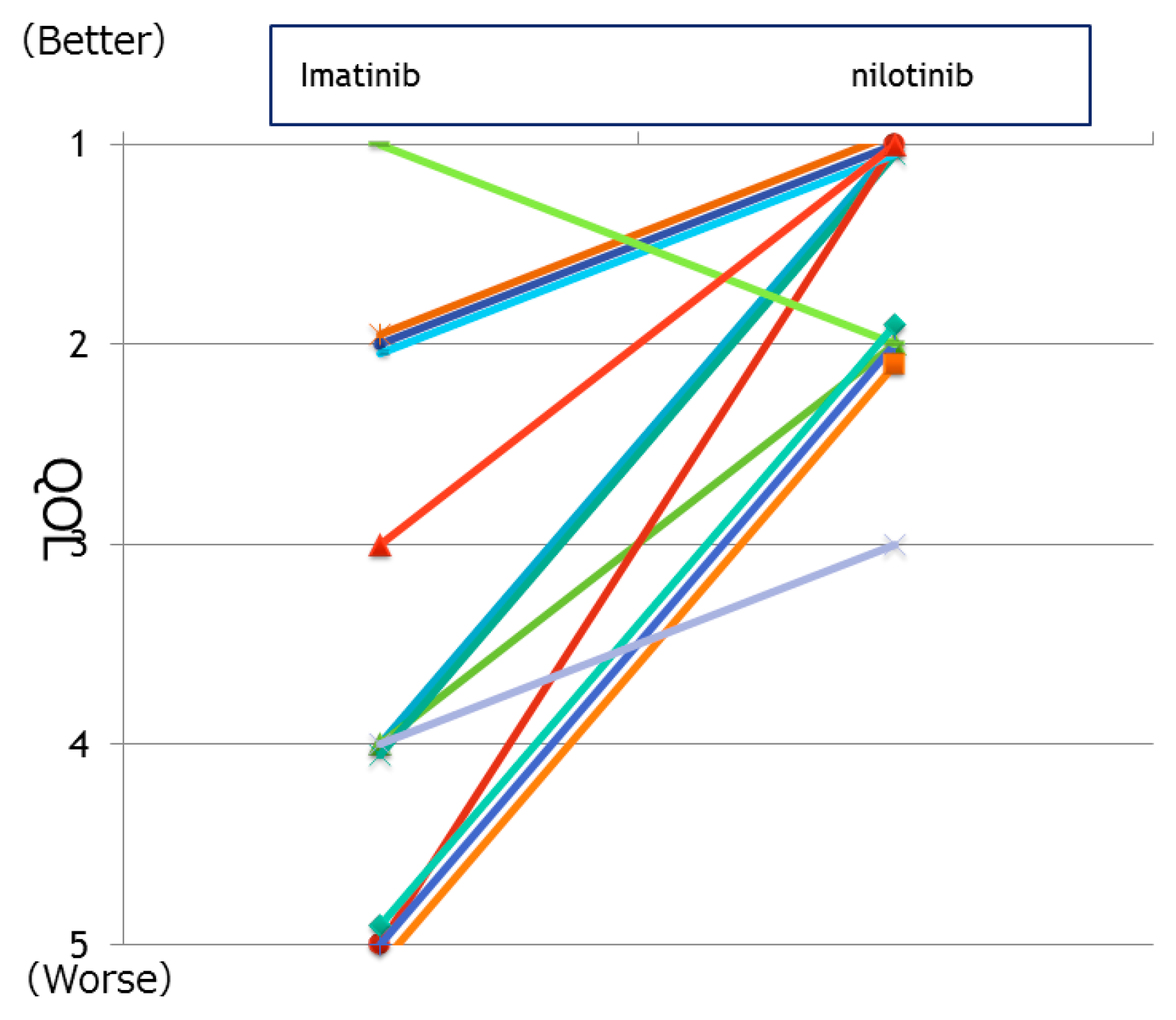
3.3. Alteration in QoL upon Switching from Imatinib to Second-Generation TKI
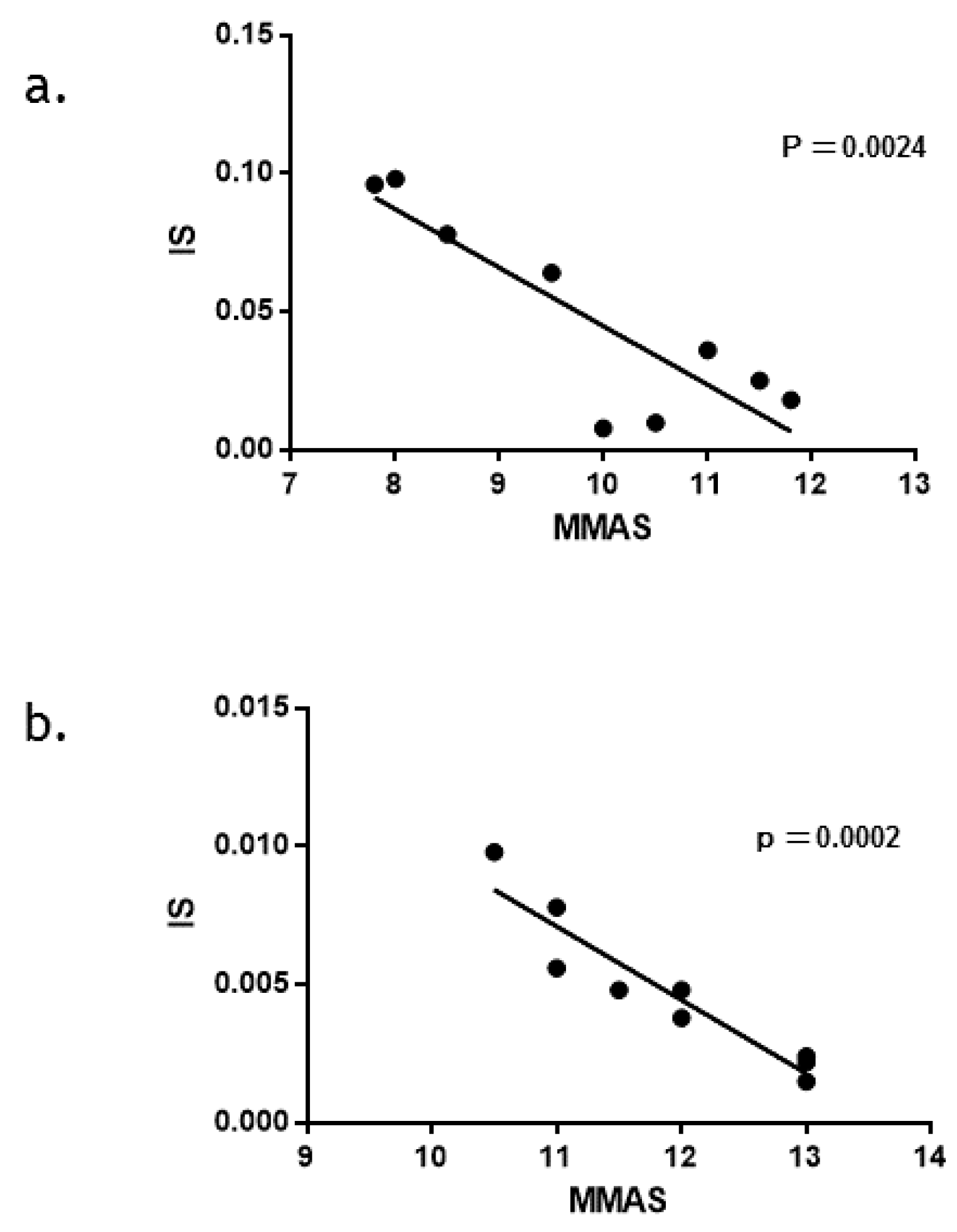
3.4. Relationship between Clinical Outcome (IS) and Drug Adherence (MMAS) in the NILO Group Compared to That in the IM Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CML | Chronic myeloid leukemia |
| TKI | Tyrosine kinase inhibitors |
| ADE | Adverse drug events |
| QoL | Quality of life |
| MMAS | Morisky Medication Adherence Scale |
| IM | Imatinib |
| NILO | Nilotinib |
References
- Baccarani, M.; Cortes, J.; Pane, F.; Niederwieser, D.; Saglio, G.; Apperley, J.; Cervantes, F.; Deininger, M.; Gratwohl, A.; Guilhot, F.; et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 2009, 27, 6041–6051. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, M.; Atallah, E. Practical considerations for the management of patients in the tyrosine kinase inhibitor era. Semin. Hematol. 2009, 46, S16–S21. [Google Scholar] [CrossRef] [PubMed]
- Rosti, G.; Castagnetti, F.; Gugliotta, G.; Palandri, F.; Baccarani, M. Physician’s guide to the clinical management of adverse events on nilotinib therapy for the treatment of CML. Cancer Treat. Rev. 2012, 38, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Santoleri, F.; Sorice, P.; Lasala, R.; Rizzo, R.C.; Costantini, A. Patient adherence and persistence with Imatinib, Nilotinib, Dasatinib in clinical practice. PLoS ONE 2013, 8, e56813. [Google Scholar] [CrossRef]
- Marin, D.; Bazeos, A.; Mahon, F.X.; Eliasson, L.; Milojkovic, D.; Bua, M.; Apperley, J.F.; Szydlo, R.; Desai, R.; Kozlowski, K.; et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J. Clin. Oncol. 2010, 28, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A. Educational session: Managing chronic myeloid leukemia as a chronic disease. Am. Soc. Hematol. Educ. Program. 2011, 2011, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Guilhot, F.; Coombs, J.; Szczudlo, T.; Zernovak, O.; Paolantonio, M.; Bender, C.; Macdonald, N.J.; Shapiro, A. The patient journey in chronic myeloid leukemia patients on tyrosine kinase inhibitor therapies: Qualitative insights using a global ethnographic approach. Patient 2013, 6, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, S.; Olsson, B.; Söderberg, J.; Wadenvik, H. Good adherence to imatinib therapy among patients with chronic myeloid leukemia—A single-center observational study. Ann. Hematol. 2012, 91, 679–685. [Google Scholar] [CrossRef]
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive validity of a medication adherence measure in an outpatient setting. J. Clin. Hypertens. 2008, 10, 348–354. [Google Scholar] [CrossRef]
- Guérin, A.; Chen, L.; Wu, E.Q.; Ponce de Leon, D.; Griffin, J.D. A retrospective analysis of therapy adherence in imatinib resistant or intolerant patients with chronic myeloid leukemia receiving nilotinib or dasatinib in a real-world setting. Curr. Med. Res. Opin. 2012, 28, 1155–1162. [Google Scholar] [CrossRef]
- Trivedi, D.; Landsman-Blumberg, P.; Darkow, T.; Smith, D.; McMorrow, D.; Mullins, C.D. Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. J. Manag. Care Spec. Pharm. 2014, 20, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, E.Q. Adherence and persistence among chronic myeloid leukemia patients during second-line tyrosine kinase inhibitor treatment. J. Manag. Care Spec. Pharm. 2015, 21, 1088. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.H.; Pagnano, K.B.; Vigorito, A.C.; Lorand-Metze, I.; de Souza, C.A. Adherence to tyrosine kinase inhibitor therapy for chronic myeloid leukemia: A Brazilian single-center cohort. Acta Haematol. 2013, 130, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Fang, G.; Richards, K.L.; Walko, C.M.; Earnshaw, S.R.; Happe, L.E.; Blalock, S.J. Comparative evaluation of patients newly initiating first-generation versus second-generation tyrosine kinase inhibitors for chronic myeloid leukemia and medication adherence, health services utilization, and healthcare costs. Curr. Med. Res. Opin. 2015, 31, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, J.; Agrawal, N.; Ahmed, R.; Sharma, S.K.; Gupta, A.; Bhurani, D. Factors influencing adherence to imatinib in Indian chronic myeloid leukemia patients. A cross-sectional Study. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015013. [Google Scholar] [CrossRef]
- Cortes, J.E.; Apperley, J.F.; DeAngelo, D.J.; Deininger, M.W.; Kota, V.K.; Rousselot, P.; Gambacorti-Passerini, C. Management of adverse events associated with bosutinib treatment of chronic-phase chronic myeloid leukemia: Expert panel review. J. Hematol. Oncol. 2008, 11, 143. [Google Scholar] [CrossRef]
- Kekäle, M.; Peltoniemi, M.; Airaksinen, M. Patient-reported adverse drug reactions and their influence on adherence and quality of life of chronic myeloid leukemia patients on per oral tyrosine kinase inhibitor treatment. Patient Prefer. Adherence 2015, 9, 1733–1740. [Google Scholar] [CrossRef]
- Rychter, A.; Jerzmanowski, P.; Hołub, A.; Specht-Szwoch, Z.; Kalinowska, V.; Tęgowska, U.; Seferyńska, I.; Kołkowska-Leśniak, A.; Lech-Marańda, E.; Góra-Tybor, J. Treatment adherence in chronic myeloid leukaemia patients receiving tyrosine kinase inhibitors. Med. Oncol. 2017, 34, 104. [Google Scholar] [CrossRef]
- Maeda, Y.; Okamoto, A.; Kawaguchi, S.I.; Konishi, A.; Yamamoto, K.; Eguchi, G.; Kanai, Y.; Yamaguchi, T. Improved drug adherence in patients with chronic myeloid leukemia in the chronic phase by switching to second-generation tyrosine kinase inhibitors. Acta Haematol. 2017, 138, 140–142. [Google Scholar] [CrossRef]
- Winn, A.N.; Keating, N.L.; Dusetzina, S.B. Factors associated with tyrosine kinase inhibitor initiation and adherence among medicare beneficiaries with chronic myeloid leukemia. J. Clin. Oncol. 2016, 34, 4323–4328. [Google Scholar] [CrossRef]
- Mulu Fentie, A.; Tadesse, F.; Engidawork, E.; Gebremedhin, A. Prevalence and determinants of non-adherence to Imatinib in the first 3-months treatment among newly diagnosed Ethiopian’s with chronic myeloid leukemia. PLoS ONE 2019, 14, e0213557. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, Y.; Okamoto, A.; Yamamoto, K.; Eguchi, G.; Kanai, Y. Clinical Importance of Drug Adherence during Tyrosine Kinase Inhibitor Therapy for Chronic Myelogenous Leukemia in Chronic Phase. Reports 2019, 2, 25. https://doi.org/10.3390/reports2040025
Maeda Y, Okamoto A, Yamamoto K, Eguchi G, Kanai Y. Clinical Importance of Drug Adherence during Tyrosine Kinase Inhibitor Therapy for Chronic Myelogenous Leukemia in Chronic Phase. Reports. 2019; 2(4):25. https://doi.org/10.3390/reports2040025
Chicago/Turabian StyleMaeda, Yasuhiro, Atsushi Okamoto, Kenta Yamamoto, Go Eguchi, and Yoshitaka Kanai. 2019. "Clinical Importance of Drug Adherence during Tyrosine Kinase Inhibitor Therapy for Chronic Myelogenous Leukemia in Chronic Phase" Reports 2, no. 4: 25. https://doi.org/10.3390/reports2040025
APA StyleMaeda, Y., Okamoto, A., Yamamoto, K., Eguchi, G., & Kanai, Y. (2019). Clinical Importance of Drug Adherence during Tyrosine Kinase Inhibitor Therapy for Chronic Myelogenous Leukemia in Chronic Phase. Reports, 2(4), 25. https://doi.org/10.3390/reports2040025





