Intra-Aortic Balloon Pump for Patients with Cardiac Conditions: An Update on Available Techniques and Clinical Applications
Abstract
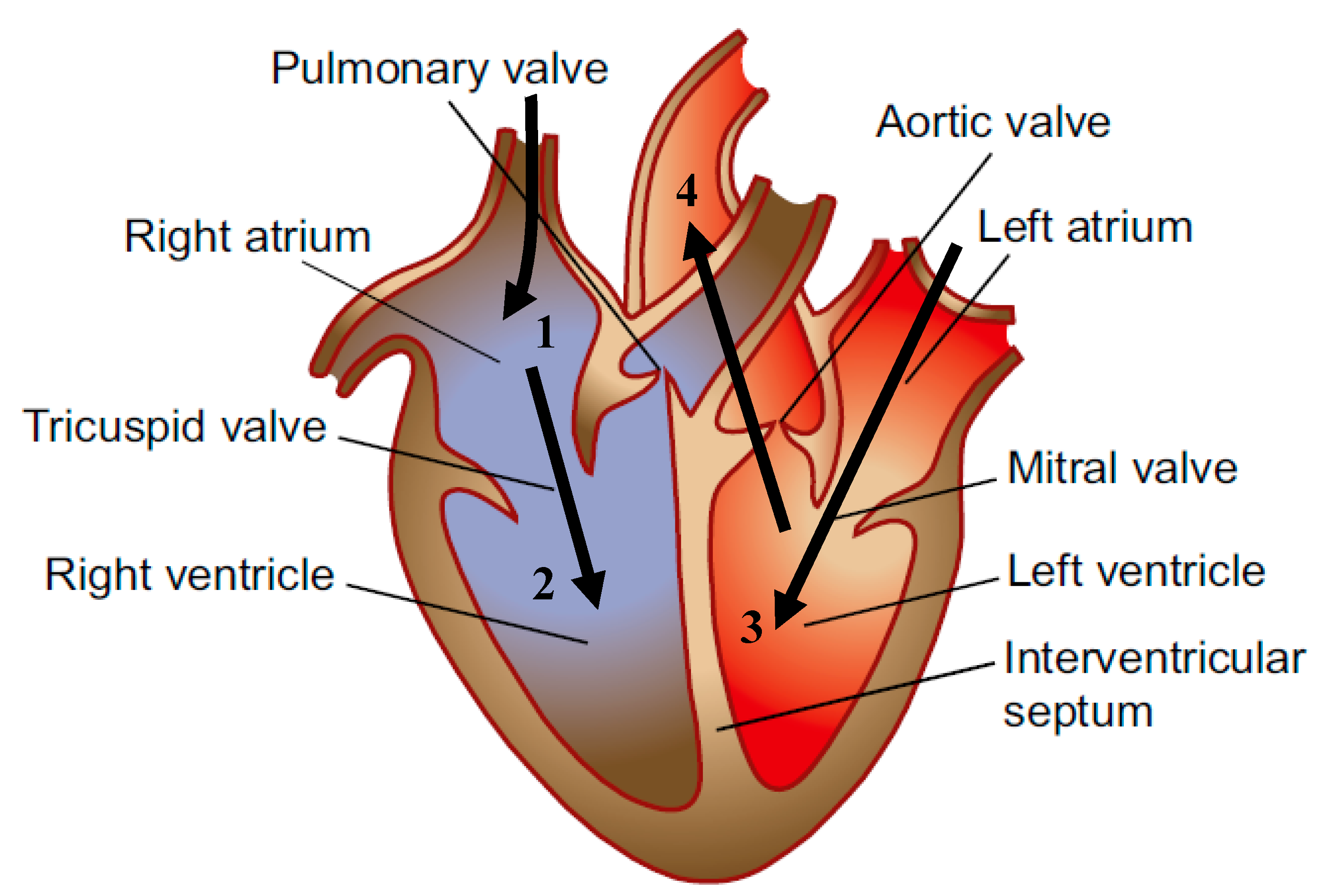
1. Introduction
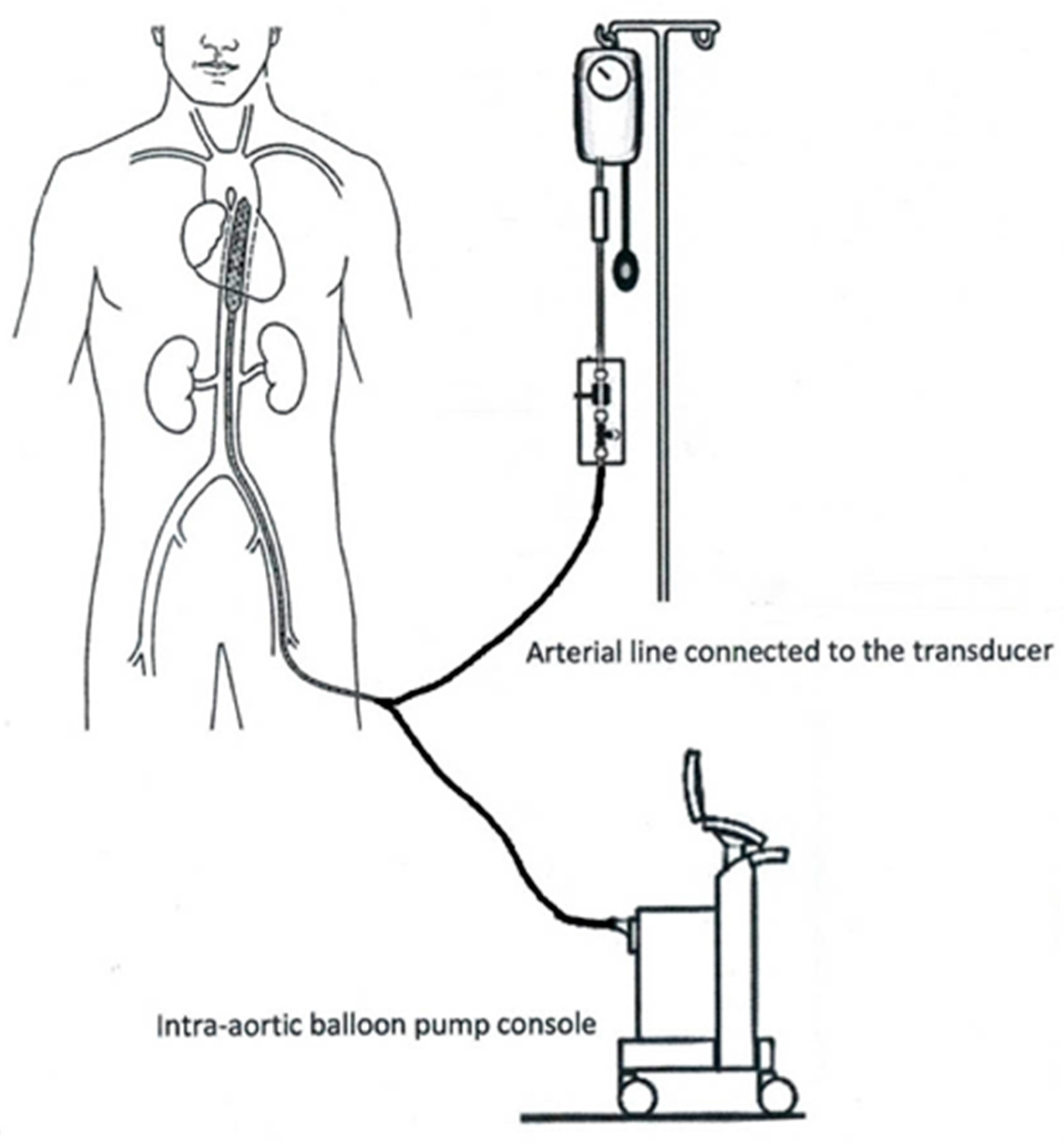
2. IABP Architecture and Indications
2.1. Balloon
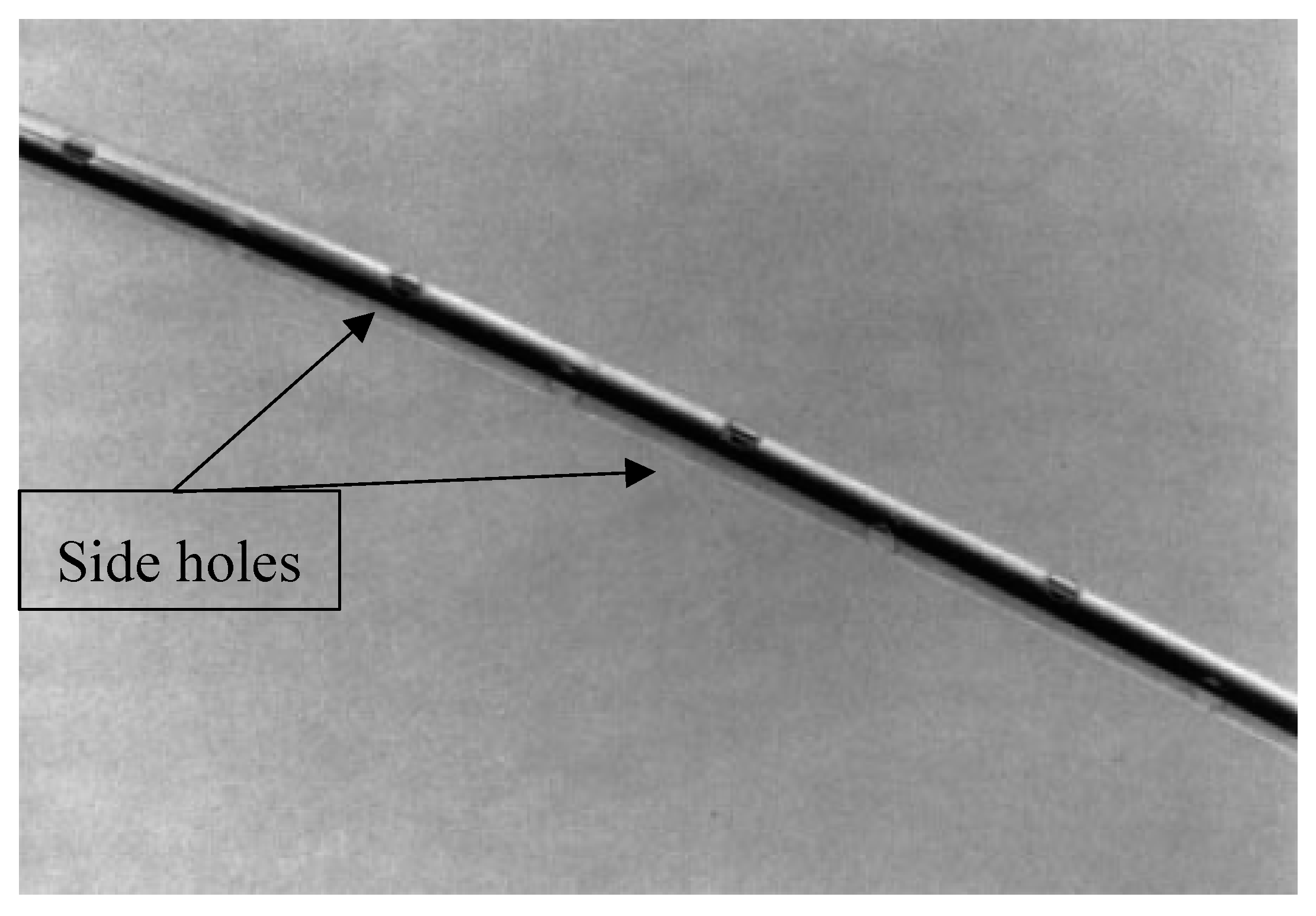
2.2. Catheter
2.3. IABP Console
2.4. Transducers
3. Additional Counterpulsation Techniques
4. Future Use of the IABP
Funding
Conflicts of Interest
References
- Aneta, S. Physics of the human cardiovascular system. Contemp. Phys. 1999, 40, 31–55. [Google Scholar]
- Weber, K.T.; Janicki, J.S. The metabolic demand and oxygen supply of the heart: Physiologic and clinical considerations. Am. J. Cardiol. 1979, 44, 722–729. [Google Scholar] [CrossRef]
- Kawaguchi, O.; Pae, W.E.; Daily, B.B.; Pierce, W.S. Ventriculoarterial coupling with intra-aortic balloon pump in acute ischemic heart failure. J. Thorac Cardiovasc. Surg. 1999, 117, 164–171. [Google Scholar] [CrossRef][Green Version]
- Shah, S.; Gnanasegaran, G.; Sundberg-Cohon, J.; Buscombe, J. The Heart: Anatomy, Physiology and Exercise Physiology. In Integrating Cardiology for Nuclear Medicine Physicians; Movahed, A., Gnanasegaran, G., Buscombe, J., Hall, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Caruso, M.V.; Gramigna, V.; Fragomeni, G. A CFD investigation of intra-aortic balloon pump assist ratio effects on aortic hemodynamics. Biocybern. Biomed. Eng. 2019, 39, 224–233. [Google Scholar] [CrossRef]
- Tremper, R.S. Intra-aortic balloon pump therapy—A primer for perioperative nurses. AORN J. 2006, 84, 33–44. [Google Scholar] [CrossRef]
- Krishna, M.; Zacharowski, K. Principles of intra-aortic balloon pump counterpulsation. Contin. Educ. Anaesth. Crit. Care Pain 2009, 9, 24–28. [Google Scholar] [CrossRef]
- Moulopoulos, S.; Stamatelopoulos, S.; Petrou, P. Intraaortic balloon assistance in intractable cardiogenic shock. Eur. Heart J. 1986, 7, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Clauss, R.H.; Missier, P.; Reed, G.E.; Tice, D. Assisted circulation by counter-pulsation with an intraaortic balloon Methods and effects. In Annual Conference on Engineering in Medicine and Biology; Northwestern University: Evanston, IL, USA, 1962. [Google Scholar]
- Parissis, H.; Graham, V.; Lampridis, S.; Lau, M.; Hooks, G.; Mhandu, P.C. IABP: History-Evolution-Pathophysiology-Indications: What We Need to Know. J. Cardiothorac. Surg. 2016, 11, 122. [Google Scholar] [CrossRef]
- Adrian, K. Origins of intraaortic balloon pumping. Ann. Thorac. Surg. 1990, 50, 672–674. [Google Scholar]
- Weber, K.T.; Janicki, J.S. Intraaortic balloon counterpulsation: A review of physiological principles, clinical results, and device safety. Ann. Thorac. Surg. 1974, 17, 602–636. [Google Scholar] [CrossRef]
- Annamalai, S.K.; Buiten, L.; Esposito, M.L.; Paruchuri, V.; Mullin, A.; Breton, C.; Patel, A.R. Acute Hemodynamic Effects of Intra-Aortic Balloon Counterpulsation Pumps in Advanced Heart Failure. J. Card. Fail. 2017, 23, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Barbara, B.H. Intra-aortic Balloon Pump. Essential 2010, 165, 459–468. [Google Scholar]
- Kantrowitz, A.; Freed, P.S.; Tachi, H.; Suzuki, A. Electrocardiographic Measurement Method for Controlling an Intra-aortic Balloon Pump. U.S. Patent No. 4,809,681, 7 May 1989. [Google Scholar]
- De Waha, S.; Desch, S.; Eitel, I.; Fuernau, G.; Lurz, P.; Sandri, M.; Thiele, H. Reprint of “Intra-aortic balloon counterpulsation—Basic principles and clinical evidence”. Vasc. Pharmacol. 2014, 61, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Connie, L.; Gurung, R. Intra-Aortic Balloon Pump—A Thing of the Past? Kai Tiaki Nurs. N. Z. 2019, 25, 31–33. [Google Scholar]
- Pfluecke, C.; Christoph, M.; Kolschmann, S.; Tarnowski, D.; Forkmann, M.; Jellinghaus, S.; Ibrahim, K. Intra-Aortic Balloon Pump (IABP) Counterpulsation Improves Cerebral Perfusion in Patients with Decreased Left Ventricular Function. Perfusion 2014, 29, 511–516. [Google Scholar] [CrossRef]
- Warshaw, E.M. Latex allergy. J. Am. Acad. Dermatol. 1998, 39, 1–24. [Google Scholar] [CrossRef]
- Schock, R.B.; Williams, J.; Walters, D.A. Intra-Aortic Balloon Catheter Having a Dual Sensor Pressure Sensing System. U.S. Patent No. 6,616,597, 9 September 2003. [Google Scholar]
- EP2049181A1—Catheter Balloons with Integrated Non-Distensible Seals. Google Patents. Google. Available online: https://patents.google.com/patent/EP2049181A1 (accessed on 6 February 2019).
- Perera, D. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: A randomized controlled trial. JAMA 2010, 304, 867–874. [Google Scholar] [CrossRef]
- Joseph, D.L.; Bates, S. Intra Aortic Balloon Pumping: How to Stay on Course. Am. J. Nurs. 1990, 90, 42–47. [Google Scholar] [CrossRef]
- Quick, H.H.; Ladd, M.E.; Hilfiker, P.R.; Paul, G.G.; Ha, S.W.; Debatin, J.F. Autoperfused balloon catheter for intravascular MR imaging. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 1999, 9, 428–434. [Google Scholar] [CrossRef]
- Gatti, G.; Morra, L.; Castaldi, G.; Maschietto, L.; Gripshi, F.; Fabris, E.; Perkan, A.; Benussi, B.; Sinagra, G.; Pappalardo, A. Preoperative Intra-Aortic Counterpulsation in Cardiac Surgery: Insights From a Retrospective Series of 588 Consecutive High-Risk Patients. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2077–2086. [Google Scholar] [CrossRef]
- Majd, A.; Lindsay, J. A Brief Review: History to Understand Fundamentals of Electrocardiography. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 1438310. [Google Scholar]
- Jonathan, W.; Hoff, R.; Kaushansky, Y. Intra-Aortic Balloon Pump Condensation Prevention System. U.S. Patent No. 6,210,319, 3 April 2001. [Google Scholar]
- Zantos, G.N.; Hanlon-Pena, P. Adaptive Real Time ECG Triggering and Uses Thereof. U.S. Patent No. 8,204,582, 19 June 2012. [Google Scholar]
- Papaioannou, T.G.; Stefanadis, C. Basic principles of the Intraaortic balloon pump and mechanisms affecting its performance. ASAIO J. 2005, 51, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Keeling, W.B.; Williams, M.L.; Slaughter, M.S.; Zhao, Y.; Puskas, J.D. Off-pump and on-pump coronary revascularization in patients with low ejection fraction: A report from the society of thoracic surgeons national database. Ann. Thorac. Surg. 2013, 96, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Elghazzawi, Z. Apparatus and Method for Distinguishing Heart Beats from Intra-Aortic Balloon Pump Beats. U.S. Patent No. 5,365,933, 22 November 1994. [Google Scholar]
- Schock, R.B.; Williams, J.; Walters, D.A. US7229403B2—Intra-Aortic Balloon Catheter Having a Dual Sensor Pressure Sensing System. U.S. Patent No 7,229,403, 12 June 2007. [Google Scholar]
- Black, M.C.; Schumer, E.M.; Rogers, M.; Trivedi, J.; Slaughter, M.S. Sunshine Heart C-Pulse: Device for NYHA Class III and ambulatory Class IV heart failure. Future Cardiol. 2016, 12, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Haralabos, P.; Alan, S.; Al-Alao, B. Intra aortic balloon pump: Literature review of risk factors related to complications of the intraaortic balloon pump. J. Cardiothorac. Surg. 2011, 6, 147. [Google Scholar]
- Huu, A.L.; Shum-Tim, D. Intra-Aortic Balloon Pump: Current Evidence & Future Perspectives. Future Cardiol. 2018, 14, 319–328. [Google Scholar] [PubMed]
| Technique | Method | Application | Advantages | Limitations |
|---|---|---|---|---|
| Extracorporeal Counterpulsation | Enhanced External Counterpulsation | Used to relieve angina, improve exercise tolerance, decrease ischemia in the stress test, and improve cardiac output for those with heart failure. | Does not require surgical implantation or anticoagulation regimens. | Patients must go to 35 one-hour sessions a day, five days a week for seven weeks. |
| Percutaneous Counterpulsation | Intra-Aortic Balloon Pump | Used for patients in cardiogenic shock, reversible intracardial mechanical defects that complicate infarction, unstable angina, post-cardiotomy failure. | There are hemodynamic and metabolic benefits with this treatment for patients with acute and chronic cardiac dysfunction. | The location of the catheter in the descending aorta and biocompatibility issues that could occur limit this treatment to short periods. |
| Implantable Counterpulsation | Cardioplus C-pulse Symphony | Used for patients with severe heart failure who still have some cardiac function abilities, but do not qualify for heart transplants due to other conditions. | Placed within the body so patients can move around and are not confined to the hospital. Also great for long term treatment and provides permanent and continuous/ on-demand support. | The placement within the body of these devices could complicate further therapies like implantation of a left ventricular assist device or heart transplantation down the line. |
| Insertion Techniques | Duration of Treatment | Biocompatibility | Advantages of Treatment |
|---|---|---|---|
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limbert, V.M.; Amiri, A.M. Intra-Aortic Balloon Pump for Patients with Cardiac Conditions: An Update on Available Techniques and Clinical Applications. Reports 2019, 2, 19. https://doi.org/10.3390/reports2030019
Limbert VM, Amiri AM. Intra-Aortic Balloon Pump for Patients with Cardiac Conditions: An Update on Available Techniques and Clinical Applications. Reports. 2019; 2(3):19. https://doi.org/10.3390/reports2030019
Chicago/Turabian StyleLimbert, Vanessa M., and Amir M. Amiri. 2019. "Intra-Aortic Balloon Pump for Patients with Cardiac Conditions: An Update on Available Techniques and Clinical Applications" Reports 2, no. 3: 19. https://doi.org/10.3390/reports2030019
APA StyleLimbert, V. M., & Amiri, A. M. (2019). Intra-Aortic Balloon Pump for Patients with Cardiac Conditions: An Update on Available Techniques and Clinical Applications. Reports, 2(3), 19. https://doi.org/10.3390/reports2030019




