Acute Pancreatitis as a Complication of Sickle Cell Anaemia
Abstract
1. Introduction
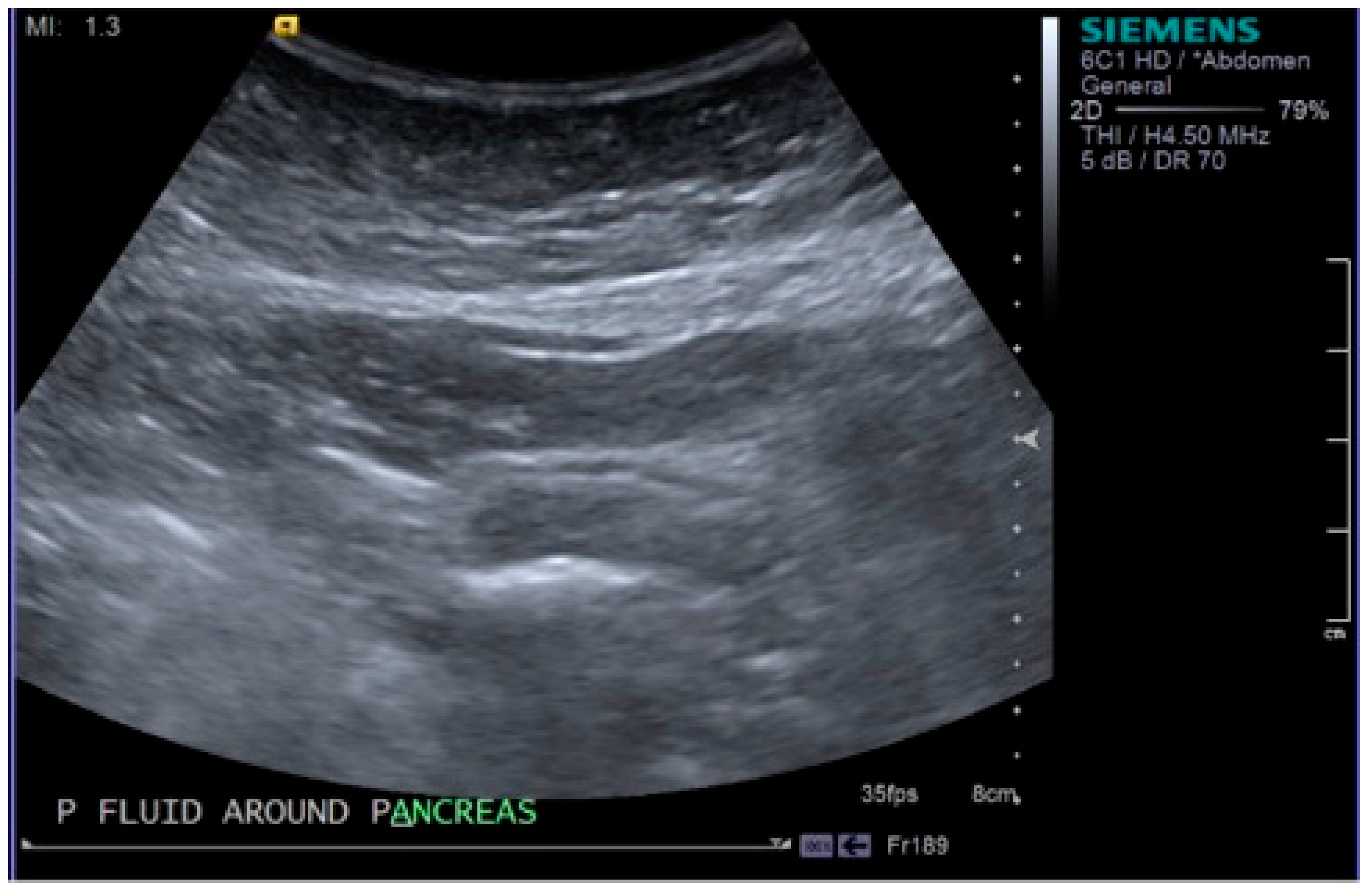
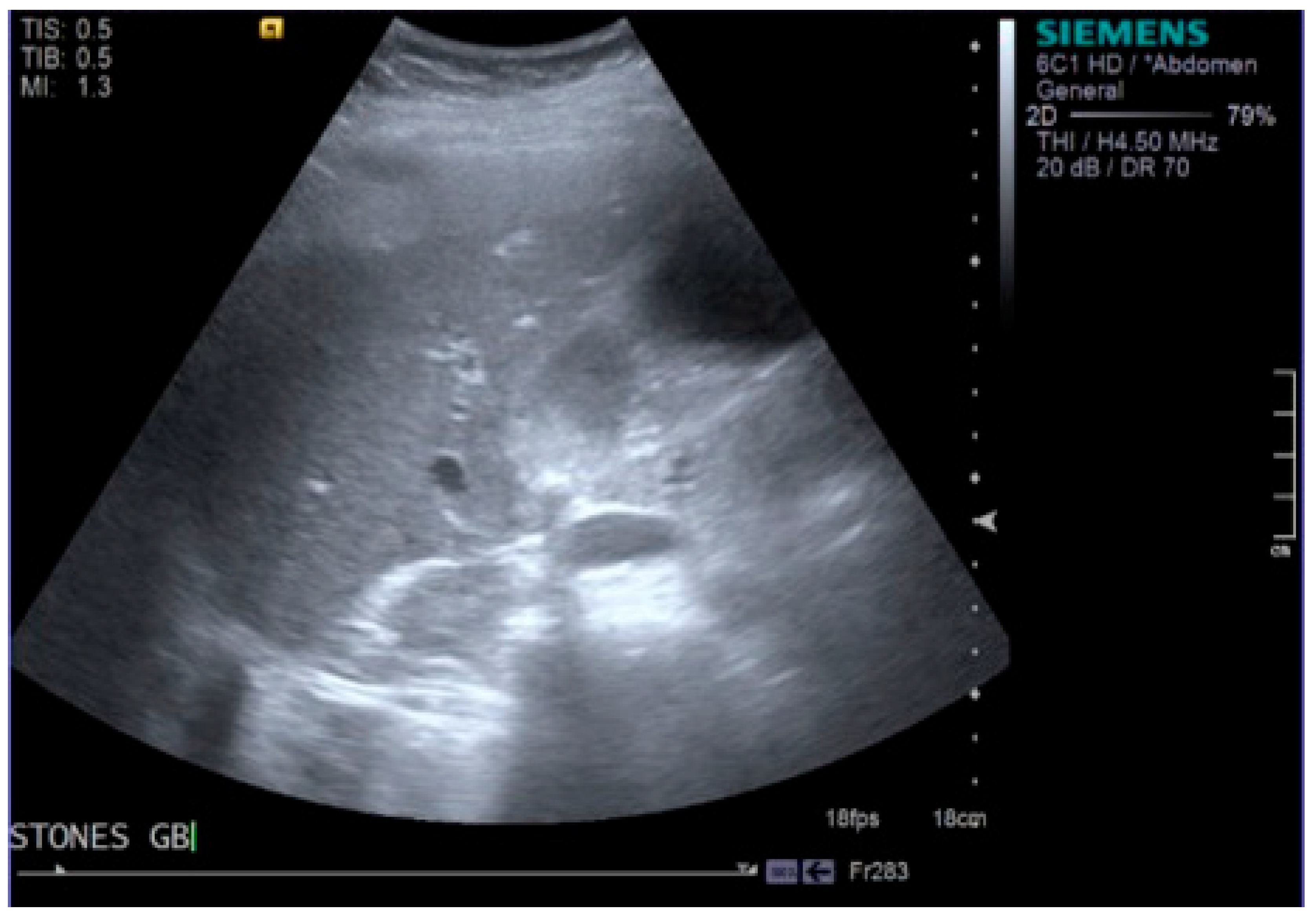
2. Case Report
3. Discussion
4. Conclusions
Conflicts of Interest
References
- Forsmark, C.E.; Vege, S.S.; Wilcox, C.M. Acute Pancreatitis. N. Engl. J. Med. 2016, 375, 1972–1981. [Google Scholar] [CrossRef]
- Lankisch, P.G.; Apte, M.; Banks, P.A. Acute pancreatitis. Lancet 2015, 386, 85–96. [Google Scholar] [CrossRef]
- Shah, A.P.; Mourad, M.M.; Bramhall, S.R. Acute pancreatitis: Current perspectives on diagnosis and management. J. Inflamm. Res. 2018, 11, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Dumnicka, P.; Maduzia, D.; Ceranowicz, P.; Olszanecki, R.; Drozdz, R.; Kusnierz-Cabala, B. The Interplay between Inflammation, Coagulation and Endothelial Injury in the Early Phase of Acute Pancreatitis: Clinical Implications. Int. J. Mol. Sci. 2017, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Rodrigues, J. Comparison of APACHE II and Imrie Scoring Systems in predicting the severity of Acute Pancreatitis. World J. Emerg. Surg. 2007, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Siddiqui, A.; Siddiqui, R.; Kimpo, M.; Russo, L.; Mattana, J. Acute pancreatitis during painful sickle cell vaso-occlusive crisis. Am. J. Hematol. 2003, 73, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Hasan, B.; Asif, T.; Braun, C.; Bahaj, W.; Dosokey, E.; Pauly, R. Pancreatitis in the Setting of Vaso-occlusive Sickle Cell Crisis: A Rare Encounter. Cureus 2017, 9, 1193. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Taborda, C.; Chawla, S. Acute and chronic hepatobiliary manifestations of sickle cell disease: A Review. J. Gastrointest. Pathophysiol. 2017, 8, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Al-Abkari, H.; Abdulnabi, H.; Al-Jamah, A.; Meshikhes, A. Laparoscopic cholecystectomy in patients with Sickle Cell Disease. Saudi Med. J. 2001, 22, 681–685. [Google Scholar] [PubMed]
- Dhiman, R.; Yusif, R.; Nabar, U.; Albaqali, A. Gastrointestinal: Ischemic enteritis and sickle cell disease. J. Gastroenterol. Hepatol. 2004, 19, 1318. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Gladwin, M.T.; Steinberg, M.H. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Presley, T.; Bain, L.; Ballas, S.; Nichols, J.; Sabio, H.; Gladwin, M.; Kato, G.T.; Kim-Shapiro, D. The Mechanism of Hemolysis in Sickle Cell Anemia. Blood 2008, 112, 1439. [Google Scholar]
- Ziegler, D.W.; Long, J.A.; Philippart, A.I.; Klein, M.D. Pancreatitis in Childhood—Experience with 49 Patients. Ann. Surg. 1988, 207, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Posner, G.; Marsh, F.; Bellvue, R.; Dosik, H. Acute-Pancreatitis in Sickle-Cell Crisis. J. Natl. Med. Assoc. 1989, 81, 91–92. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moori, P.L.; Dosis, A.; Ahmad, Z.; Kausar, A.; Triantafyllopoulou, D. Acute Pancreatitis as a Complication of Sickle Cell Anaemia. Reports 2018, 1, 19. https://doi.org/10.3390/reports1030019
Moori PL, Dosis A, Ahmad Z, Kausar A, Triantafyllopoulou D. Acute Pancreatitis as a Complication of Sickle Cell Anaemia. Reports. 2018; 1(3):19. https://doi.org/10.3390/reports1030019
Chicago/Turabian StyleMoori, Parisa L., Alexios Dosis, Zoheb Ahmad, Ambareen Kausar, and Diana Triantafyllopoulou. 2018. "Acute Pancreatitis as a Complication of Sickle Cell Anaemia" Reports 1, no. 3: 19. https://doi.org/10.3390/reports1030019
APA StyleMoori, P. L., Dosis, A., Ahmad, Z., Kausar, A., & Triantafyllopoulou, D. (2018). Acute Pancreatitis as a Complication of Sickle Cell Anaemia. Reports, 1(3), 19. https://doi.org/10.3390/reports1030019




