Glial Remodeling in the Ventricular–Subventricular Zone and Corpus Callosum Following Hydrocephalus
Abstract
1. Introduction
2. Methodology
3. Glial Remodeling in the Germinal Niche of the Ventricular–Subventricular Zone in Hydrocephalus
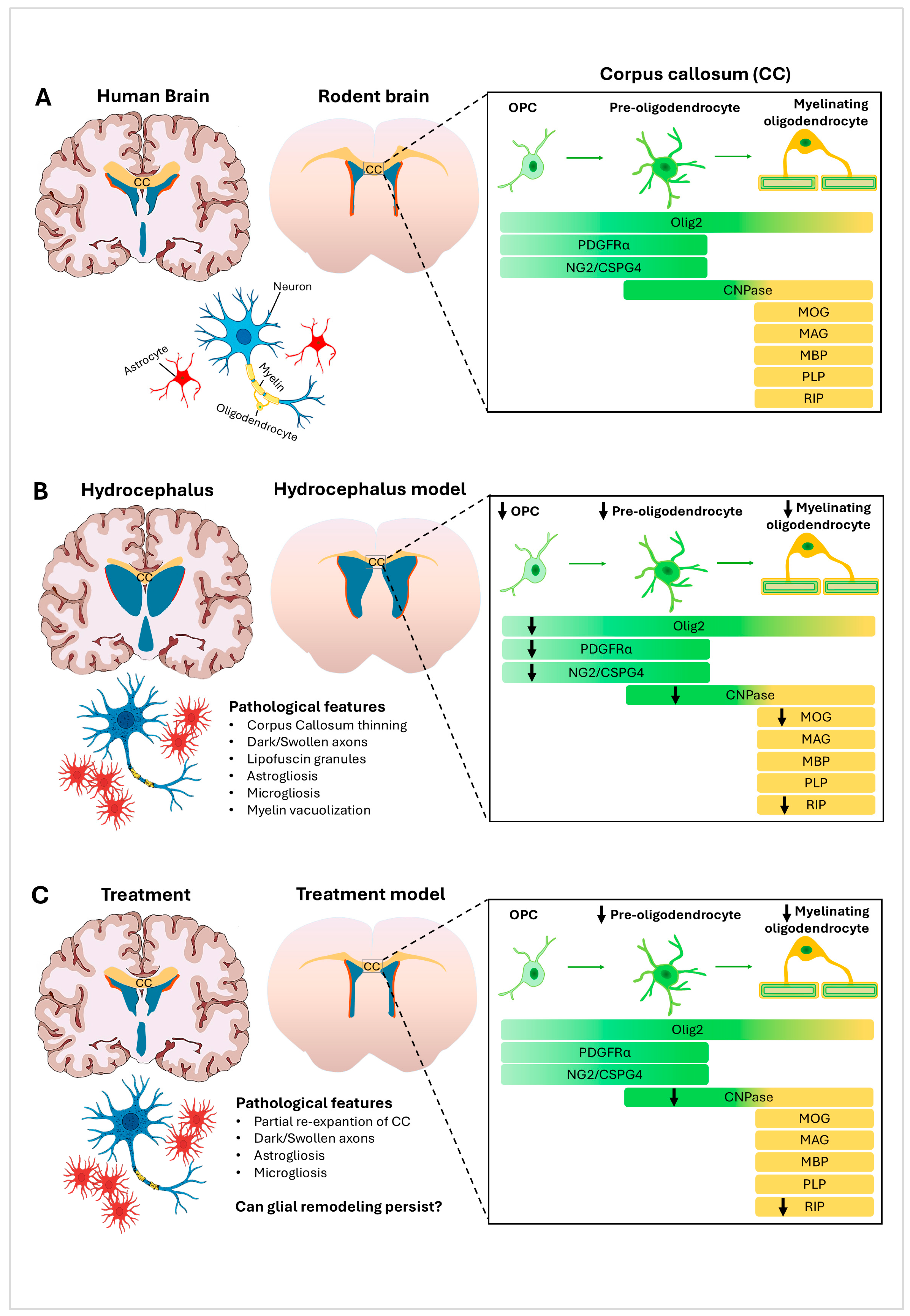
4. Structural and Glial Remodeling in the Corpus Callosum in Hydrocephalus
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Isaacs, A.M.; Riva-Cambrin, J.; Yavin, D.; Hockley, A.; Pringsheim, T.M.; Jette, N.; Lethebe, B.C.; Lowerison, M.; Dronyk, J.; Hamilton, M.G. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS ONE 2018, 13, e0204926. [Google Scholar] [CrossRef]
- Yamada, S.; Ishikawa, M.; Nozaki, K. Exploring mechanisms of ventricular enlargement in idiopathic normal pressure hydrocephalus: A role of cerebrospinal fluid dynamics and motile cilia. Fluids Barriers CNS 2021, 18, 20. [Google Scholar] [CrossRef]
- Persad, A.R.; Bass, V.; Meguro, K. Asymptomatic hydrocephalus. Can. Med. Assoc. J. 2021, 193, E480. [Google Scholar] [CrossRef]
- Kousi, M.; Katsanis, N. The Genetic Basis of Hydrocephalus. Annu. Rev. Neurosci. 2016, 39, 409–435. [Google Scholar] [CrossRef]
- Tullberg, M.; Toma, A.K.; Yamada, S.; Laurell, K.; Miyajima, M.; Watkins, L.D.; Wikkelsø, C. Classification of Chronic Hydrocephalus in Adults: A Systematic Review and Analysis. World Neurosurg. 2024, 183, 113–122. [Google Scholar] [CrossRef]
- Saldarriaga-Cantillo, A.; Yepes-Gaviria, V.; Rivas, J.C. Normal pressure hydrocephalus: Diagnostic delay. Biomédica 2020, 40, 656–663. [Google Scholar] [CrossRef]
- Garegnani, L.; Franco, J.V.; Ciapponi, A.; Garrote, V.; Vietto, V.; Portillo Medina, S.A. Ventriculo-peritoneal shunting devices for hydrocephalus. Cochrane Database Syst. Rev. 2020, 2020, CD012726. [Google Scholar] [CrossRef]
- Adil, D.; Duerden, E.G.; Eagleson, R.; De Ribaupierre, S. Structural Alterations of the Corpus Callosum in Children With Infantile Hydrocephalus. J. Child Neurol. 2024, 39, 66–76. [Google Scholar] [CrossRef]
- Moshref, R.; Algethmi, R.A. Systemic Review: Neurological Deficits following Ventriculoperitoneal Shunt (VPS) Insertion. Asian J. Neurosurg. 2023, 18, 444–453. [Google Scholar] [CrossRef]
- Chen, J.; Xian, J.; Wang, F.; Zuo, C.; We, L.; Chen, Z.; Hu, R.; Feng, H. Long-term outcomes of ventriculoperitoneal shunt therapy in idiopathic normal pressure hydrocephalus. BMC Surg. 2025, 25, 157. [Google Scholar] [CrossRef]
- Campos-Ordoñez, T.; Herranz-Pérez, V.; Chaichana, K.L.; Rincon-Torroella, J.; Rigamonti, D.; García-Verdugo, J.M.; Quiñones-Hinojosa, A.; Gonzalez-Perez, O. Long-term hydrocephalus alters the cytoarchitecture of the adult subventricular zone. Exp. Neurol. 2014, 261, 236–244. [Google Scholar] [CrossRef]
- Mataro, M.; Matarin, M.; Poca, M.A.; Pueyo, R.; Sahuquillo, J.; Barrios, M.; Junque, C. Functional and magnetic resonance imaging correlates of corpus callosum in normal pressure hydrocephalus before and after shunting. J. Neurol. Neurosurg. Psychiatry 2006, 78, 395–398. [Google Scholar] [CrossRef]
- Su, S.; McArdle, D.; Gaillard, F. Post-shunting corpus callosal signal change and review of the literature. J. Clin. Neurosci. 2020, 72, 466–468. [Google Scholar] [CrossRef]
- Quiñones-Hinojosa, A.; Sanai, N.; Soriano-Navarro, M.; Gonzalez-Perez, O.; Mirzadeh, Z.; Gil-Perotin, S.; Romero-Rodriguez, R.; Berger, M.S.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J. Comp. Neurol. 2006, 494, 415–434. [Google Scholar] [CrossRef]
- Lopez-Virgen, V.; Gonzalez-Morales, O.; Gonzalez-Perez, O. The ventricular-subventricular, subgranular and subcallosal zones: Three niches of neural stem cells in the postnatal brain. Exp. Brain Res. 2023, 241, 1463–1470. [Google Scholar] [CrossRef]
- Galvez-Contreras, A.Y.; Gonzalez-Castaneda, R.E.; Campos-Ordonez, T.; Luquin, S.; Gonzalez-Perez, O. Phenytoin enhances the phosphorylation of epidermal growth factor receptor and fibroblast growth factor receptor in the subventricular zone and promotes the proliferation of neural precursor cells and oligodendrocyte differentiation. Eur. J. Neurosci. 2016, 43, 139–147. [Google Scholar] [CrossRef]
- Yu, Q.; Guan, T.; Guo, Y.; Kong, J. The Initial Myelination in the Central Nervous System. ASN Neuro 2023, 15, 17590914231163039. [Google Scholar] [CrossRef]
- MacRae, C.; Varma, H. Chronic Hydrocephalus Following Mumps Encephalitis: Neuropathological Correlates and Review. J. Neuropathol. Exp. Neurol. 2020, 79, 113–117. [Google Scholar] [CrossRef]
- Hänninen, J.J.; Nakajima, M.; Vanninen, A.; Hytönen, S.; Rummukainen, J.; Koivisto, A.M.; Jääskeläinen, J.E.; Soininen, H.; Sutela, A.; Vanninen, R.; et al. Neuropathological findings in possible normal pressure hydrocephalus: A post-mortem study of 29 cases with lifelines. Free Neuropathol. 2022, 3, 2. [Google Scholar] [CrossRef]
- Rodríguez, E.M.; Guerra, M.M.; Vío, K.; González, C.; Ortloff, A.; Bátiz, L.F.; Rodríguez, S.; Jara, M.C.; Muñoz, R.I.; Ortega, E.; et al. A cell junction pathology of neural stem cells leads to abnormal neurogenesis and hydrocephalus. Biol. Res. 2012, 45, 231–241. [Google Scholar] [CrossRef]
- Domínguez-Pinos, M.D.; Páez, P.; Jiménez, A.-J.; Weil, B.; Arráez, M.-A.; Pérez-Fígares, J.-M.; Rodríguez, E.-M. Ependymal Denudation and Alterations of the Subventricular Zone Occur in Human Fetuses With a Moderate Communicating Hydrocephalus. J. Neuropathol. Exp. Neurol. 2005, 64, 595–604. [Google Scholar] [CrossRef]
- Bannister, C.M.; Chapman, S.A. Ventricular Ependyma of Normal and Hydrocephalic Subjects: A Scanning Electronmicroscopic Study. Dev. Med. Child Neurol. 1980, 22, 725–735. [Google Scholar] [CrossRef]
- Konishi, S.; Tanaka, N.; Mashimo, T.; Yamamoto, T.; Sakuma, T.; Kaneko, T.; Tanaka, M.; Izawa, T.; Yamate, J.; Kuwamura, M. Pathological characteristics of Ccdc85c knockout rats: A rat model of genetic hydrocephalus. Exp. Anim. 2020, 69, 26–33. [Google Scholar] [CrossRef]
- Sevensky, R.; Newville, J.C.; Tang, H.L.; Robinson, S.; Jantzie, L.L. Cumulative Damage: Cell Death in Posthemorrhagic Hydrocephalus of Prematurity. Cells 2021, 10, 1911. [Google Scholar] [CrossRef]
- Roales-Buján, R.; Páez, P.; Guerra, M.; Rodríguez, S.; Vío, K.; Ho-Plagaro, A.; García-Bonilla, M.; Rodríguez-Pérez, L.-M.; Domínguez-Pinos, M.-D.; Rodríguez, E.-M.; et al. Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathol. 2012, 124, 531–546. [Google Scholar] [CrossRef]
- Shook, B.A.; Lennington, J.B.; Acabchuk, R.L.; Halling, M.; Sun, Y.; Peters, J.; Wu, Q.; Mahajan, A.; Fellows, D.W.; Conover, J.C. Ventriculomegaly associated with ependymal gliosis and declines in barrier integrity in the aging human and mouse brain. Aging Cell 2014, 13, 340–350. [Google Scholar] [CrossRef]
- Simon, M.J.; Iliff, J.J. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 442–451. [Google Scholar] [CrossRef]
- Vidovic, D.; Davila, R.A.; Gronostajski, R.M.; Harvey, T.J.; Piper, M. Transcriptional regulation of ependymal cell maturation within the postnatal brain. Neural Develop. 2018, 13, 2. [Google Scholar] [CrossRef]
- De Laurentis, C.; Cristaldi, P.; Arighi, A.; Cavandoli, C.; Trezza, A.; Sganzerla, E.P.; Giussani, C.G.; Di Cristofori, A. Role of aquaporins in hydrocephalus: What do we know and where do we stand? A systematic review. J. Neurol. 2021, 268, 4078–4094. [Google Scholar] [CrossRef]
- Brown, F.N.; Iwasawa, E.; Shula, C.; Fugate, E.M.; Lindquist, D.M.; Mangano, F.T.; Goto, J. Early postnatal microglial ablation in the Ccdc39 mouse model reveals adverse effects on brain development and in neonatal hydrocephalus. Fluids Barriers CNS 2023, 20, 42. [Google Scholar] [CrossRef]
- Di Curzio, D.L. Animal Models of Hydrocephalus. Open J. Mod. Neurosurg. 2018, 8, 57–71. [Google Scholar] [CrossRef]
- Campos-ordonez, T.; Gonzalez-perez, O. Characterization of a mouse model of chronic hydrocephalus induced by partial occlusion of the aqueduct of Sylvius in the adult brain. J. Neurosci. Methods 2021, 362, 109294. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Chen, R.; Wang, Y.; Tan, C.; Liu, J.; Zhang, Q.; Xiao, G. Novel therapeutic modulators of astrocytes for hydrocephalus. Front. Mol. Neurosci. 2022, 15, 932955. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- Khodadadei, F.; Arshad, R.; Morales, D.M.; Gluski, J.; Marupudi, N.I.; McAllister, J.P.; Limbrick, D.D.; Harris, C.A. The effect of A1 and A2 reactive astrocyte expression on hydrocephalus shunt failure. Fluids Barriers CNS 2022, 19, 78. [Google Scholar] [CrossRef]
- Deren, K.E.; Packer, M.; Forsyth, J.; Milash, B.; Abdullah, O.M.; Hsu, E.W.; McAllister, J.P. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp. Neurol. 2010, 226, 110–119. [Google Scholar] [CrossRef]
- Campos-Ordoñez, T.; González-Granero, S.; Eudave-Patiño, M.; Buriticá, J.; Herranz-Pérez, V.; García-Verdugo, J.M.; Gonzalez-Perez, O. Normal pressure hydrocephalus decreases the proliferation of oligodendrocyte progenitor cells and the expression of CNPase and MOG proteins in the corpus callosum before behavioral deficits occur. Exp. Neurol. 2023, 365, 114412. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Y.; Chen, L.; Wang, J.; Zhang, J.; Zhang, H.; Tian, S.; Zhang, A.; Zhang, J.; Zhang, J.H.; et al. Cerebrospinal fluid markers of neuroinflammation and coagulation in severe cerebral edema and chronic hydrocephalus after subarachnoid hemorrhage: A prospective study. J. Neuroinflamm. 2024, 21, 237. [Google Scholar] [CrossRef]
- Crews, L.; Wyss-Coray, T.; Masliah, E. Insights into the Pathogenesis of Hydrocephalus from Transgenic and Experimental Animal Models. Brain Pathol. 2004, 14, 312–316. [Google Scholar] [CrossRef]
- Hasan-Olive, M.M.; Enger, R.; Hansson, H.-A.; Nagelhus, E.A.; Eide, P.K. Pathological mitochondria in neurons and perivascular astrocytic endfeet of idiopathic normal pressure hydrocephalus patients. Fluids Barriers CNS 2019, 16, 39. [Google Scholar] [CrossRef]
- Czubowicz, K.; Głowacki, M.; Fersten, E.; Kozłowska, E.; Strosznajder, R.P.; Czernicki, Z. Levels of selected pro- and anti-inflammatory cytokines in cerebrospinal fluid in patients with hydrocephalus. Folia Neuropathol. 2017, 55, 301–307. [Google Scholar] [CrossRef]
- Goulding, D.S.; Vogel, R.; Pandya, C.D.; Shula, C.; Gensel, J.C.; Mangano, F.T.; Goto, J.; Miller, B.A. Neonatal hydrocephalus leads to white matter neuroinflammation and injury in the corpus callosum of Ccdc39 hydrocephalic mice. J. Neurosurg. Pediatr. PED 2020, 25, 476–483. [Google Scholar] [CrossRef]
- Di Curzio, D.L. Neuropathological Changes in Hydrocephalus—A Comprehensive Review. Open J. Mod. Neurosurg. 2018, 08, 81009. [Google Scholar] [CrossRef][Green Version]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Anwar, F.; Zhang, K.; Sun, C.; Pang, M.; Zhou, W.; Li, H.; He, R.; Liu, X.; Ming, D. Hydrocephalus: An update on latest progress in pathophysiological and therapeutic research. Biomed. Pharmacother. 2024, 181, 117702. [Google Scholar] [CrossRef]
- Baig, S.; Nadaf, J.; Allache, R.; Le, P.U.; Luo, M.; Djedid, A.; Nkili-Meyong, A.; Safisamghabadi, M.; Prat, A.; Antel, J.; et al. Identity and nature of neural stem cells in the adult human subventricular zone. iScience 2024, 27, 109342. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Bonsu, J.M.; Redmond, K.J.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. Implications of irradiating the subventricular zone stem cell niche. Stem Cell Res. 2016, 16, 387–396. [Google Scholar] [CrossRef]
- Fukumizu, M.; Takashima, S.; Becker, L.E. Glial reaction in periventricular areas of the brainstem in fetal and neonatal posthemorrhagic hydrocephalus and congenital hydrocephalus. Brain Dev. 1996, 18, 40–45. [Google Scholar] [CrossRef]
- Mistry, A.M.; Dewan, M.C.; White-Dzuro, G.A.; Brinson, P.R.; Weaver, K.D.; Thompson, R.C.; Ihrie, R.A.; Chambless, L.B. Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J. Neurooncol. 2017, 132, 341–349. [Google Scholar] [CrossRef]
- Matarredona, E.R.; Zarco, N.; Castro, C.; Guerrero-Cazares, H. Editorial: Neural Stem Cells of the Subventricular Zone: From Neurogenesis to Glioblastoma Origin. Front. Oncol. 2021, 11, 750116. [Google Scholar] [CrossRef]
- Quiñones-Hinojosa, A.; Chaichana, K. The human subventricular zone: A source of new cells and a potential source of brain tumors. Exp. Neurol. 2007, 205, 313–324. [Google Scholar] [CrossRef]
- Roth, J.; Constantini, S. Hydrocephalus and Brain Tumors. In Cerebrospinal Fluid Disorders; Limbrick, D.D., Leonard, J.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 199–217. [Google Scholar] [CrossRef]
- Garcia-Bonilla, M.; Castaneyra-Ruiz, L.; Zwick, S.; Talcott, M.; Otun, A.; Isaacs, A.M.; Morales, D.M.; Limbrick, D.D.; McAllister, J.P. Acquired hydrocephalus is associated with neuroinflammation, progenitor loss, and cellular changes in the subventricular zone and periventricular white matter. Fluids Barriers CNS 2022, 19, 17. [Google Scholar] [CrossRef]
- Li, Y.; Wu, D.; Wu, C.; Qu, Z.; Zhao, Y.; Li, W.; Wang, J.; Li, Z. Changes in neural stem cells in the subventricular zone in a rat model of communicating hydrocephalus. Neurosci. Lett. 2014, 578, 153–158. [Google Scholar] [CrossRef]
- Mercier, F. Fractones: Extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell. Mol. Life Sci. 2016, 73, 4661–4674. [Google Scholar] [CrossRef]
- Nascimento, M.A.; Sorokin, L.; Coelho-Sampaio, T. Fractone Bulbs Derive from Ependymal Cells and Their Laminin Composition Influence the Stem Cell Niche in the Subventricular Zone. J. Neurosci. 2018, 38, 3880–3889. [Google Scholar] [CrossRef]
- Sato, Y.; Kiyozumi, D.; Futaki, S.; Nakano, I.; Shimono, C.; Kaneko, N.; Ikawa, M.; Okabe, M.; Sawamoto, K.; Sekiguchi, K. Ventricular–subventricular zone fractones are speckled basement membranes that function as a neural stem cell niche. Mol. Biol. Cell 2019, 30, 56–68. [Google Scholar] [CrossRef]
- Norton, E.S.; Whaley, L.A.; Ulloa-Navas, M.J.; García-Tárraga, P.; Meneses, K.M.; Lara-Velazquez, M.; Zarco, N.; Carrano, A.; Quiñones-Hinojosa, A.; García-Verdugo, J.M.; et al. Glioblastoma disrupts the ependymal wall and extracellular matrix structures of the subventricular zone. Fluids Barriers CNS 2022, 19, 58. [Google Scholar] [CrossRef]
- Kerever, A.; Yamada, T.; Suzuki, Y.; Mercier, F.; Arikawa-Hirasawa, E. Fractone aging in the subventricular zone of the lateral ventricle. J. Chem. Neuroanat. 2015, 66–67, 52–60. [Google Scholar] [CrossRef]
- Nowak, M.M.; Fersten, E.; Głowacki, M. Executive functioning pattern as a prognostic indicator for shunt implantation surgery in patients with normal pressure hydrocephalus—A preliminary report. Neurol. Neurochir. Pol. 2016, 50, 98–100. [Google Scholar] [CrossRef]
- Eskandari, R.; McAllister, J.P.; Miller, J.M.; Ding, Y.; Ham, S.D.; Shearer, D.M.; Way, J.S. Effects of hydrocephalus and ventriculoperitoneal shunt therapy on afferent and efferent connections in the feline sensorimotor cortex. J. Neurosurg. 2004, 101, 196–210. [Google Scholar] [CrossRef]
- Suryaningtyas, W.; Arifin, M.; Rantam, F.A.; Bajamal, A.H.; Dahlan, Y.P.; Dewa Gede Ugrasena, I.; Maliawan, S. Erythropoietin protects the subventricular zone and inhibits reactive astrogliosis in kaolin-induced hydrocephalic rats. Childs Nerv. Syst. 2019, 35, 469–476. [Google Scholar] [CrossRef]
- Sampaio-Baptista, C.; Johansen-Berg, H. White Matter Plasticity in the Adult Brain. Neuron 2017, 96, 1239–1251. [Google Scholar] [CrossRef]
- Polis, B.; Polis, L.; Zeman, K.; Paśnik, J.; Nowoslawska, E. CSF levels of myelin basic protein in pediatric patients with entriculoperitoneal shunt infection. Cent. Eur. J. Immunol. 2020, 45, 48–55. [Google Scholar] [CrossRef]
- Gupta, R.; Barolia, D.; Goyal, M. Congenital hydrocephalus, corpus callosum agenesis, and prosencephalic cyst with supernumerary nostril: A neurocristopathy. Asian J. Neurosurg. 2018, 13, 1239–1243. [Google Scholar] [CrossRef]
- Tripathi, R.B.; Jackiewicz, M.; McKenzie, I.A.; Kougioumtzidou, E.; Grist, M.; Richardson, W.D. Remarkable Stability of Myelinating Oligodendrocytes in Mice. Cell Rep. 2017, 21, 316–323. [Google Scholar] [CrossRef]
- Kondziella, D.; Sonnewald, U.; Tullberg, M.; Wikkelso, C. Brain metabolism in adult chronic hydrocephalus. J. Neurochem. 2008, 106, 1515–1524. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Myelin damage and repair in pathologic CNS: Challenges and prospects. Front. Mol. Neurosci. 2015, 8, 35. [Google Scholar] [CrossRef]
- Marton, R.M.; Miura, Y.; Sloan, S.A.; Li, Q.; Revah, O.; Levy, R.J.; Huguenard, J.R.; Pașca, S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019, 22, 484–491. [Google Scholar] [CrossRef]
- Lane, J.I.; Luetmer, P.H.; Atkinson, J.L. Corpus Callosal Signal Changes in Patients with Obstructive Hydrocephalus after Ventriculoperitoneal Shunting. Am. J. Neuroradiol. 2001, 22, 158–162. [Google Scholar]
- Kanno, S.; Saito, M.; Kashinoura, T.; Nishio, Y.; Iizuka, O.; Kikuchi, H.; Takagi, M.; Iwasaki, M.; Takahashi, S.; Mori, E. A change in brain white matter after shunt surgery in idiopathic normal pressure hydrocephalus: A tract-based spatial statistics study. Fluids Barriers CNS 2017, 14, 1. [Google Scholar] [CrossRef]
- Hofmann, E.; Becker, T.; Jackel, M.; Metzner, D.; Schneider, M.; Meixensberger, J.; Reichmann, H. The corpus callosum in communicating and noncommunicating hydrocephalus. Neuroradiology 1995, 37, 212–218. [Google Scholar] [CrossRef]
- Serulle, Y.; Pawar, R.V.; Eubig, J.; Fieremans, E.; Kong, S.E.; George, I.C.; Morley, C.; Babb, J.S.; George, A.E. Diffusional kurtosis imaging in hydrocephalus. Magn. Reson. Imaging 2015, 33, 531–536. [Google Scholar] [CrossRef]
- Tullberg, M.; Jensen, C.; Ekholm, S.; Wikkelsø, C. Normal pressure hydrocephalus: Vascular white matter changes on MR images must not exclude patients from shunt surgery. Am. J. Neuroradiol. 2001, 22, 1665–1673. [Google Scholar]
- Naureen, I.; Waheed, K.A.I.; Rathore, A.W.; Victor, S.; Mallucci, C.; Goodden, J.R.; Chohan, S.N.; Miyan, J.A. Fingerprint changes in CSF composition associated with different aetiologies in human neonatal hydrocephalus: Glial proteins associated with cell damage and loss. Fluids Barriers CNS 2013, 10, 34. [Google Scholar] [CrossRef]
- Shibata, Y.; Mashiko, R. Clinical Value of the Measurement of Myelin Basic Protein in the Cerebrospinal Fluid of Patients with Idiopathic Normal Pressure Hydrocephalus. Adv. Clin. Transl. Res. 2019, 3, 100017. [Google Scholar]
- Bigio, M.R.D.; Wilson, M.J.; Enno, T. Chronic hydrocephalus in rats and humans: White matter loss and behavior changes. Ann. Neurol. 2003, 53, 337–346. [Google Scholar] [CrossRef]
- Carter, C.S.; Vogel, T.W.; Zhang, Q.; Seo, S.; Swiderski, R.E.; Moninger, T.O.; Cassell, M.D.; Thedens, D.R.; Keppler-Noreuil, K.M.; Nopoulos, P.; et al. Abnormal development of NG2+ PDGFR-α+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat. Med. 2012, 18, 1797–1804. [Google Scholar] [CrossRef]
- Olopade, F.E.; Shokunbi, M.T.; Azeez, I.A.; Andrioli, A.; Scambi, I.; Bentivoglio, M. Neuroinflammatory Response in Chronic Hydrocephalus in Juvenile Rats. Neuroscience 2019, 419, 14–22. [Google Scholar] [CrossRef]
- Olopade, F.E.; Shokunbi, M.T.; Sirén, A.L. The relationship between ventricular dilatation, neuropathological and neurobehavioural changes in hydrocephalic rats. Fluids Barriers CNS 2012, 9, 19. [Google Scholar] [CrossRef]
- Santos, M.V.; Garcia, C.A.B.; Jardini, E.O.; Romeiro, T.H.; Lopes, L.d.S.; Machado, H.R.; de Oliveira, R.S. Ventricular-subcutaneous shunt for the treatment of experimental hydrocephalus in young rats: Technical note. Childs Nerv. Syst. 2016, 32, 1507–1511. [Google Scholar] [CrossRef]
- Beggiora, P.D.S.; Da Silva, S.C.; Rodrigues, K.P.; Almeida, T.A.D.L.; Sampaio, G.B.; Silva, G.A.P.D.M.; Machado, H.R.; Lopes, L.D.S. Memantine associated with ventricular-subcutaneous shunt promotes behavioral improvement, reduces reactive astrogliosis and cell death in juvenile hydrocephalic rats. J. Chem. Neuroanat. 2022, 125, 102165. [Google Scholar] [CrossRef]
- Bigio, M.D.; Kanfer, J.N.; Zhang, Y.W. Myelination delay in the cerebral white matter of immature rats with kaolin-induce hydrocephalus is reversible. J. Neuropathol. Exp. Neurol. 1997, 56, 1053–1066. [Google Scholar] [CrossRef]
- Del Bigio, M.R.; Di Curzio, D.L. Nonsurgical therapy for hydrocephalus: A comprehensive and critical review. Fluids Barriers CNS 2015, 13, 3. [Google Scholar] [CrossRef]
- Garcia-Bonilla, M.; Harris, C.A.; Bandyopadhyay, S.; Moore, J.; Horbatiuk, J.; Limbrick, D.D.; Swarup, R.; Crouthamel, J.; Jones, A.; Khasawneh, A.; et al. Reduction of cell surface attachment in experimental hydrocephalus using a novel ventricular catheter with modified tethered liquid perfluorocarbon. J. Neurosurg. 2024, 140, 627–638. [Google Scholar] [CrossRef]
- Dutra, M.; Covas Da Silva, S.; Da Silva Beggiora Marques, P.; Oliveira Amaral, I.; Funo De Souza, S.N.; Dutra, L.A.; Volpon Santos, M.; Machado, H.R.; Da Silva Lopes, L. Celecoxib attenuates neuroinflammation, reactive astrogliosis and promotes neuroprotection in young rats with experimental hydrocephalus. J. Chem. Neuroanat. 2023, 133, 102344. [Google Scholar] [CrossRef]
- Sampaio, G.B.; Da Silva, S.C.; Romeiro, T.H.; Beggiora, P.D.S.; Machado, H.R.; Lopes, L.D.S. Evaluation of the effects of quercetin on brain lesions secondary to experimental hydrocephalus in rats. Childs Nerv. Syst. 2019, 35, 2299–2306. [Google Scholar] [CrossRef]
- Volpon Santos, M.; da Silva Lopes, L.; Machado, H.R.; Santos de Oliveira, R. Behavioral and Biochemical Features of the Course and Surgical Treatment of Experimental Obstructive Hydrocephalus in Young Rats. Dev. Neurosci. 2019, 41, 34–43. [Google Scholar] [CrossRef]
- Catalão, C.H.R.; Correa, D.A.L.; Saito, S.T.; Lopes, L.D.S. Camellia sinensis neuroprotective role in experimentally induced hydrocephalus in Wistar rats. Childs Nerv. Syst. 2014, 30, 591–597. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal Stem Cells Prevent Hydrocephalus After Severe Intraventricular Hemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef]

| Treatment | Animal Model | Result after Treatment | Potential Functional Role | Reference |
|---|---|---|---|---|
| Erythropoietin administration | Rat | Decreased GFAP and Iba1 expression in V-SVZ | Attenuation of glial scar/inflammatory response | [65] |
| CSF diversion | Mouse | Decreased GFAP expression in V-SVZ | Attenuation of glial scar/inflammatory response | [32] |
| Tethered liquid perfluorocarbon (TLP) + CSF diversion | Pig | No differences in GFAP expression in VZ | - | [89] |
| CSF diversion | Rat | Decreased GFAP expression in CC | Attenuation of glial scar/inflammatory response | [85] |
| CSF diversion | Mouse | Restablishment of Olig2, PDGFrα, NG2, and MOG expression in CC | Partial OPCs and myelin repair | [38] |
| CSF diversion | Mouse | No differences in GFAP or Iba1 expression in CC | - | [38] |
| Memantine administration + CSF diversion | Rat | Decreased GFAP expression in CC | Attenuation of glial scar/inflammatory response | [86] |
| Celecoxib administration + CSF diversion | Rat | No differences in GFAP expression in CC | - | [90] |
| Quercetin administration | Rat | No differences in GFAP expression in CC | - | [91] |
| CSF diversion | Rat | No differences in GFAP expression in CC | - | [92] |
| Camellia sinensis administration | Rat | Decreased GFAP expression in CC | Attenuation of glial scar/inflammatory response | [93] |
| Mesenchymal Stem Cells transplantation | Rat | Decreased GFAP expression in CC | Attenuation of glial scar/inflammatory response | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Ordoñez, T.; Ortega-Valles, B.N.; González-Pérez, O. Glial Remodeling in the Ventricular–Subventricular Zone and Corpus Callosum Following Hydrocephalus. Neuroglia 2025, 6, 29. https://doi.org/10.3390/neuroglia6030029
Campos-Ordoñez T, Ortega-Valles BN, González-Pérez O. Glial Remodeling in the Ventricular–Subventricular Zone and Corpus Callosum Following Hydrocephalus. Neuroglia. 2025; 6(3):29. https://doi.org/10.3390/neuroglia6030029
Chicago/Turabian StyleCampos-Ordoñez, Tania, Brenda Nayeli Ortega-Valles, and Oscar González-Pérez. 2025. "Glial Remodeling in the Ventricular–Subventricular Zone and Corpus Callosum Following Hydrocephalus" Neuroglia 6, no. 3: 29. https://doi.org/10.3390/neuroglia6030029
APA StyleCampos-Ordoñez, T., Ortega-Valles, B. N., & González-Pérez, O. (2025). Glial Remodeling in the Ventricular–Subventricular Zone and Corpus Callosum Following Hydrocephalus. Neuroglia, 6(3), 29. https://doi.org/10.3390/neuroglia6030029






