Nanomedicine-Based Advances in Brain Cancer Treatment—A Review
Abstract
1. Introduction
1.1. Brain Cancer: Its Types and Prevalence
1.2. Nanomedicine for Overcoming the Limitations of Conventional Treatments
2. Pathophysiology of Brain Cancer and Challenges in Its Treatment
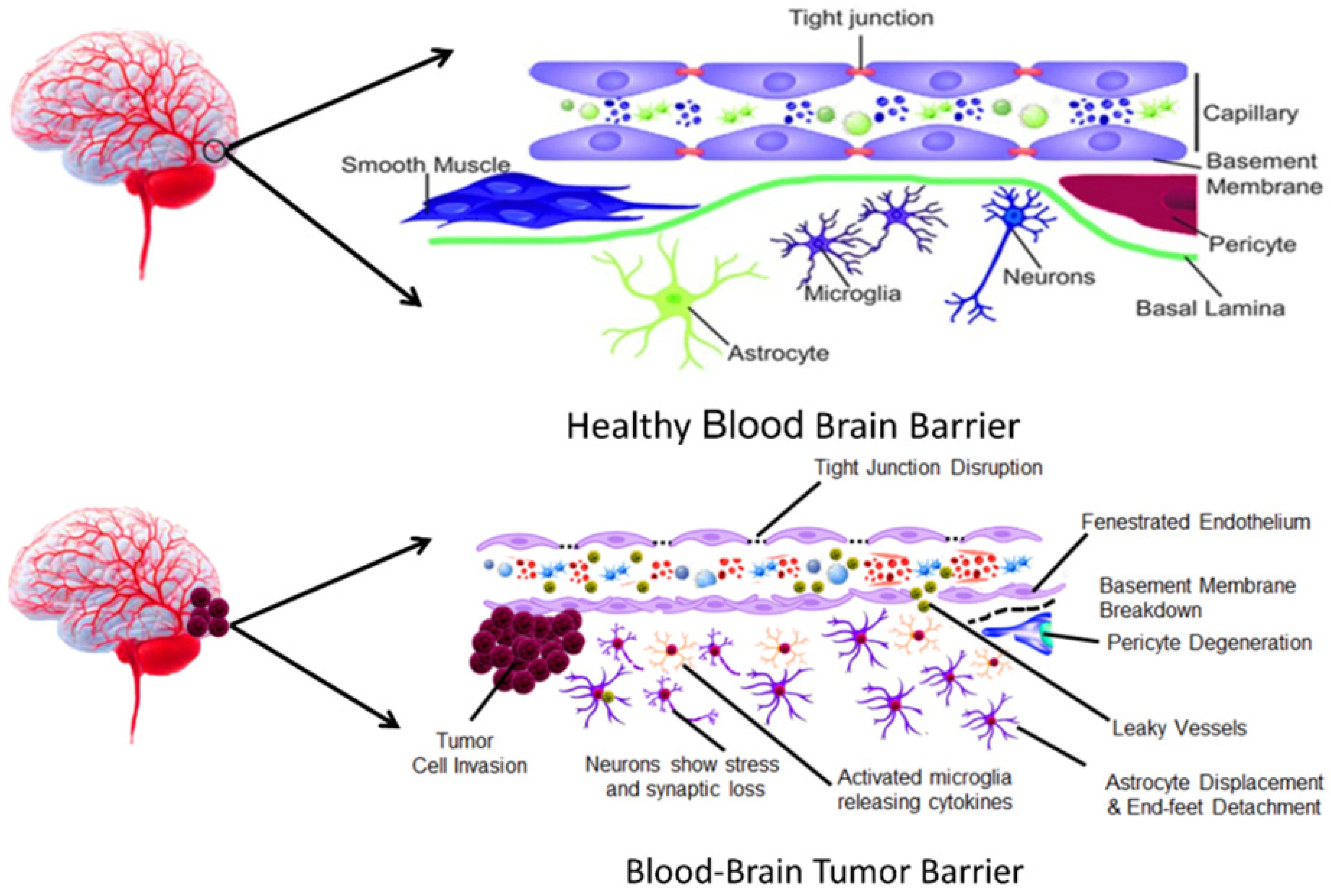
2.1. BBB and Its Role in Drug Delivery Challenges
2.2. Transformation of BBB During Tumor Progression
2.3. Tumor Microenvironment and Its Resistance Mechanisms
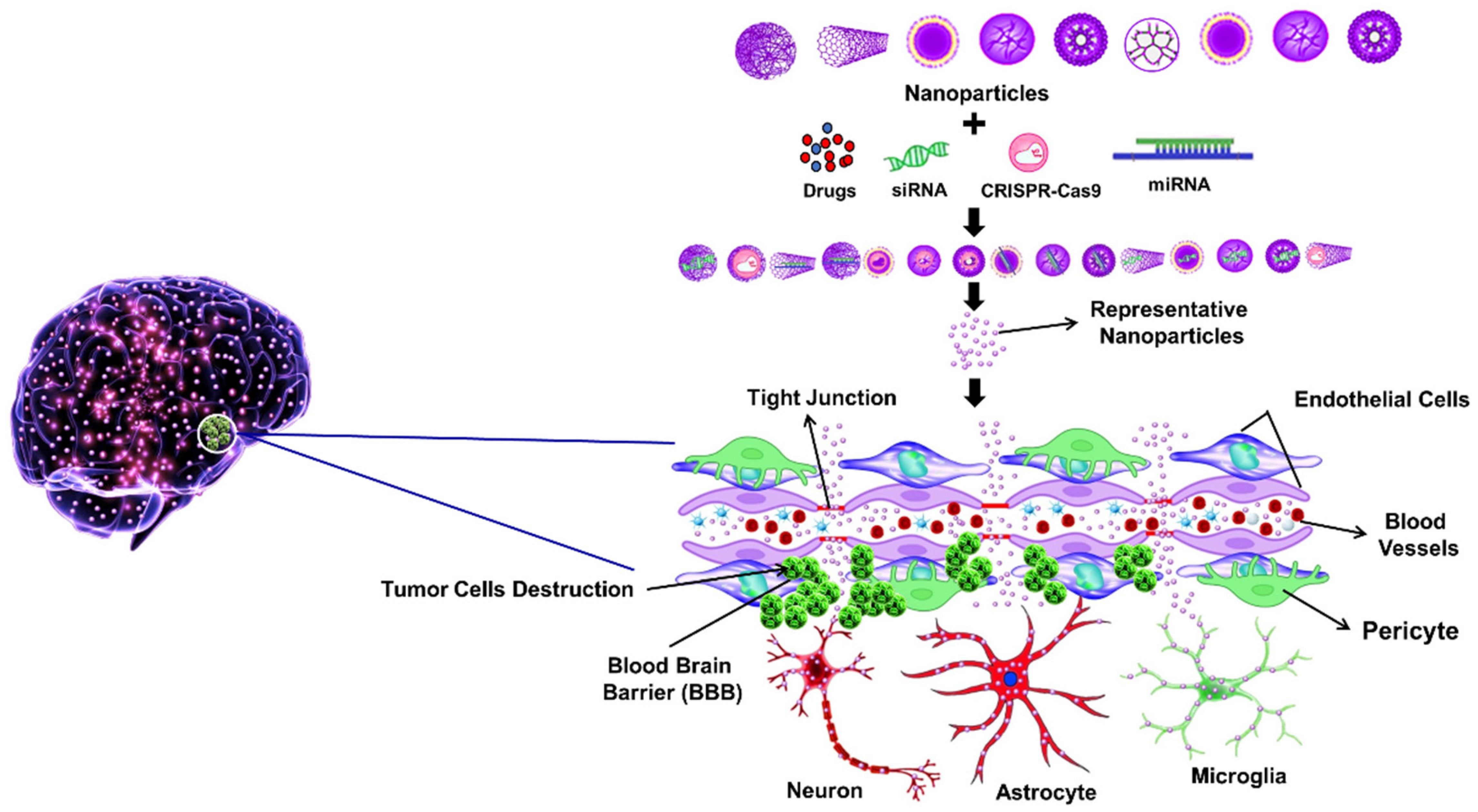
3. BBB Penetration Strategies Using Nanotechnology
3.1. Passive Diffusion Mechanism
3.2. Receptor-Mediated Transport
3.3. Adsorptive-Mediated Transport
3.4. Cell-Mediated Transport
3.5. Intranasal Delivery
3.6. Focused Ultrasound (FUS)
4. Various Nanoparticles Used in the Treatment of Brain Cancer
4.1. Liposomes
4.2. Dendrimers
4.3. Nano-Micelles
4.4. Carbon Nanotubes (CNTs)
4.5. Silver NPs (AgNPs) and Gold NPs (AuNPs)
4.6. Zinc Oxide NPs (ZnONPs)
4.7. Nucleic Acid-Based NPs
4.8. Viromimetic NPs
4.9. Upconversion NPs
4.10. Albumin-Based NPs
4.11. Amorphous Carbon-Based Nanomaterials
4.12. Carbon Dots
4.13. Iron-Oxide–Graphene-Based Hybrid NPs
4.14. Sillica-Based NPs
5. Various Nanoparticles Used in the Diagnosis and Biosensing of Brain Cancer
5.1. Magnetic NPs
5.2. Extracellular Vesicles and Exosomes
5.3. Metallic NPs for Brain Cancer Imaging and Diagnosis
5.4. Quantum Dots (QDs)
5.5. Polymeric Nano-Vehicles for Brain Cancer Imaging
5.6. Biomimetic Nanocomposites for Multimodal Imaging of Glioblastoma
5.7. Iron-Oxide–Graphene-Based Hybrid NPs as Diagnostic Agents
5.8. Silicate Nanocarriers
6. Nanotheranostics: Integrating Diagnosis and Therapy
6.1. NPs in Imaging-Guided Therapy
6.1.1. MRI
Nanomaterials as MRI Contrast Agents
- SPIONs
- Gd-Based NPs
Inorganic NPs for MRI
6.1.2. Positron Emission Tomography (PET)
6.1.3. Fluorescence Imaging
QDs as Fluorescent Probes
AuNPs for Fluorescence Enhancement
Polymer-Encapsulated Organic NPs
6.1.4. Integration of Imaging Modalities
6.2. Therapy and Diagnosis Using Nanoplatforms: Real-Time Monitoring of Treatment Response
Monitoring and Evaluation Techniques
6.3. NPs on Targeted Radiotherapy of Brain Tumors
| Nanoparticle Strategy | Mechanism of Action | Theranostic Function | Advantages | References |
|---|---|---|---|---|
| pH-Responsive NPs (e.g., CaCO3) | Stable at physiological pH; degrade in acidic TME to release drugs | Enables real-time drug release monitoring via enhanced MR imaging | Site-specific drug release, improved imaging contrast, low systemic toxicity | [139] |
| Quantum Dot-Based Platforms (e.g., Ag2S QDs) | Emit fluorescence in the NIR-II window, allowing deep tissue imaging | Real-time in vivo tracking of drug targeting and efficacy | High spatial resolution, deep penetration, non-invasive monitoring | [140] |
| Biomimetic Nanoplatforms (e.g., macrophage membrane-coated) | Mimic natural cells to traverse the BBB and target tumors | Enhanced drug delivery and synergistic chemo-photothermal therapy | Immune evasion, BBB penetration, tumor-specific targeting | [141] |
| Self-Adaptive Nanoplatforms | Dynamically adjust size, charge or surface features based on tumor microenvironment | Improved targeting and controlled drug release in glioblastoma | Responsive delivery, deep tumor penetration, personalized intervention | [142] |
| Upconverting covalent organic frameworks | Generate and monitor ROS during photodynamic therapy | Real-time tracking of therapeutic response and oxidative stress | Photostable, tunable, dual imaging and therapeutic monitoring capability | [143] |
7. Immuno-Nanomedicine for Brain Cancer
7.1. Nanovaccines for Immunotherapy of GBM
7.1.1. Autologous Nanovaccines
7.1.2. Dendritic Cell-Based Nanovaccines
7.1.3. Hydrogel-Based Nanovaccines
7.2. Checkpoint Inhibitors Combined with Nanocarriers
7.3. Engineered NPs for Tumor-Specific Immune Activation
8. Pre-Clinical and Clinical Studies
9. Emerging Nanotechnologies for Brain Cancer Treatment
9.1. CRISPR(Clustered Regularly Interspaced Short Palindromic Repeats) Based Nanomedicine for Gene Editing
9.2. RNA-Based Nano-Delivery for Tumor Suppression
9.3. Smart NPs with Stimuli-Responsive Drug Release
9.3.1. Types of Stimuli
9.3.2. Applications and Efficacy
10. Future Perspectives and Challenges
10.1. Personalized Nanomedicine Approaches for Brain Cancer
10.2. AI and Machine Learning (ML) in Nano Drug Design
10.2.1. Integration of AI and ML
10.2.2. Drug Formulation Design
10.2.3. Autonomous Molecular Design
10.2.4. Improved Predictive Models
10.3. Challenges in Personalized Nanomedicine for Brain Cancer
10.3.1. BBB Crossing Challenges
10.3.2. Tumor Heterogeneity
10.3.3. Clinical Translation and Safety
10.4. Challenges of Using AI and ML in Nano Drug Design
10.4.1. Data Limitations
10.4.2. Scalability and Error Quantification
10.4.3. Physical Explanation of AI Models
10.4.4. Integration with Existing Processes
10.4.5. Model Accuracy and Ethical Considerations
10.5. Ethical Concerns with Nanomedicine for Neuro-Oncology
10.5.1. Biocompatibility and Toxicity
10.5.2. Regulatory and Translational Barriers
10.5.3. Informed Consent and Patient Autonomy
10.5.4. Public Acceptance and Risk
10.5.5. Equity and Access
10.5.6. Privacy and Data Security
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| AgNp | Silver Nanoparticle |
| AGuIX | Activation and Guidance of Irradiation by X-ray |
| AI | Artificial Intelligence |
| ANG-2 | Angiopep-2 |
| ATP | Adenosine Triphosphate |
| AnnV-PLGA-NPs | Annexin A5-functionalized PLGA nanoparticles |
| AuNp | Gold Nanoparticle |
| BBB | Blood–Brain Barrier |
| BBTB | Blood-Brain Tumor Barrier |
| Bcl-2 | B-cell Lymphoma 2 |
| Cas9 | CRISPR-associated protein 9 |
| CD | Cluster of Differentiation |
| cGAMP | Cyclic GMP-AMP |
| CNS | Central Nervous System |
| CNTs | Carbon Nanotubes |
| CPC | Cationic Peptide Carrier |
| CPP | Cell Penetrating Peptide |
| CPPO | Bis(2,4,5-trichloro-6-carbopentoxyphenyl)oxalate |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CT | Computed Tomography |
| Cu | Copper |
| DC | Dendritic cell |
| DNA | Deoxyribonucleic Acid |
| DOx | Doxorubicin |
| DPPC | DOx@PNIPAM-PEI-CPP |
| EGFR | Epidermal Growth Factor Receptor |
| FUS | Focused Ultrasound |
| GBM | Glioblastoma Multiforme |
| Gd | Gadolinium |
| GO | Graphene Oxide |
| HK | Honokiol |
| HMGB1 | High Mobility Group Box 1 |
| HSP70 | Heat Shock Protein 70 |
| IFN-γ | Interferon Gamma |
| JQ1 | A BET Bromodomain Inhibitor |
| MGMT | O6-Methylguanine-DNA Methyltransferase |
| MHC I | Major Histocompatibility Complex I |
| miRNA | MicroRNA |
| ML | Machine Learning |
| MMP | Matrix Metalloproteinases |
| mpa | Mercaptopropionic Acid |
| MPEG-PCL | Methoxy Poly(Ethylene Glycol)-Poly(Ε-Caprolactone) |
| MRI | Magnetic Resonance Imaging |
| mRNA | Messenger RNA |
| MSN | Mesoporous Silica Nanoparticle |
| MTIC | 5-(3-Methyltriazen-1yl)-Imidazole-4-Carboxamide |
| nm | Nanometer |
| NIR | Near-infrared |
| NP | Nanoparticle |
| Pbrm1 | Polybromo 1 |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| PEG | Polyethylene Glycol |
| PET | Positron Emission Tomography |
| pHLIP | pH (Low) Insertion Peptide |
| PLGA | Poly(Lactic-Co-Glycolic Acid) |
| QD | Quantum Dot |
| RGD | Arg-Gly-Asp |
| rGO | Reduced Graphene Oxide |
| RNA | Ribonucleic Acid |
| RNAi | RNA interference |
| ROS | Reactive Oxygen Species |
| siRNA | Small Interfering RNA |
| SPION | Superparamagnetic Iron Oxide Nanoparticle |
| SrNPs | Stimuli-Responsive Nanoparticles |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| STING | Stimulator of Interferon Genes |
| TGF-β | Transforming Growth Factor Beta |
| TLR | Toll-like Receptor |
| TME | Tumour Microenvironment |
| TMZ | Temozolomide |
| TNF-α | Tumor Necrosis Factor alpha. |
| TPP | Triphenylphosphonium |
| US | Ultrasound |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | World Health Organization |
| ZnONP | Zinc Oxide Nanoparticle |
References
- Biratu, E.S.; Schwenker, F.; Ayano, Y.M.; Debelee, T.G. A Survey of Brain Tumor Segmentation and Classification Algorithms. J. Imaging 2021, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Weller, M.; Wen, P.Y.; Kros, J.M.; Aldape, K.; Chang, S. A clinical perspective on the 2016 WHO brain tumor classification and routine molecular diagnostics. Neuro-Oncol. 2017, 19, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Robles, L.A.; Mundis, G.M. Chondromas of the Lumbar Spine: A Systematic Review. Glob. Spine J. 2021, 11, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Pekmezci, M.; Villanueva-Meyer, J.E.; Goode, B.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Bastian, B.C.; Chamyan, G.; Maher, O.M.; Khatib, Z.; et al. The genetic landscape of ganglioglioma. Acta Neuropathol. Commun. 2018, 6, 47. [Google Scholar] [CrossRef]
- Younes, E.; Montava, M.; Bachelard-Serra, M.; Jaloux, L.; Salburgo, F.; Lavieille, J.P. Intracanalicular Vestibular Schwannomas: Initial Clinical Manifestation, Imaging Classification and Risk Stratification for Management Proposal. Otol. Neurotol. 2017, 38, 1345–1350. [Google Scholar] [CrossRef]
- Dehdashti, A.R.; Chakraborty, S. Aggressive pituitary adenomas: Is pathology the only feature of aggressiveness? Acta Neurochir. 2018, 160, 57–58. [Google Scholar] [CrossRef]
- Wilson, D.A.; Awad, A.W.; Brachman, D.; Coons, S.W.; McBride, H.; Youssef, E.; Nakaji, P.; Shetter, A.G.; Smith, K.A.; Spetzler, R.F.; et al. Long-term radiosurgical control of subtotally resected adult pineocytomas. J. Neurosurg. 2012, 117, 212–217. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Chang, S.M. Grade II and III Oligodendroglioma and Astrocytoma. Neurol. Clin. 2018, 36, 467–484. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Farmanfarma, K.K.; Mohammadian, M.; Shahabinia, Z.; Hassanipour, S.; Salehiniya, H. Brain cancer in the world: An epidemiological review. World Cancer Res. J. 2019, 6, e1356. [Google Scholar]
- Ilic, I.; Ilic, M. International patterns and trends in the brain cancer incidence and mortality: An observational study based on the global burden of disease. Heliyon 2023, 9, e18222. [Google Scholar] [CrossRef]
- Loushambam, B.; Yanglem, S.; Krishnaswami, V.; Kumar, M.; Vijayaraghavalu, S. Nanomedicine: Pioneering Advances in Neural Disease, Stroke and Spinal Cord Injury Treatment. Neuroglia 2025, 6, 9. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanotechnology meets oncology: Nanomaterials in brain cancer research, diagnosis and therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Yang, W.; Chen, X.; Gao, J.; Gong, X.; Wang, H.; Duan, Y.; Wei, D.; Chang, J. Nanoparticle-based diagnostic and therapeutic systems for brain tumors. J. Mater. Chem. B 2019, 7, 4734–4750. [Google Scholar] [CrossRef]
- Bai, F.; Deng, Y.; Li, L.; Lv, M.; Razzokov, J.; Xu, Q.; Xu, Z.; Chen, Z.; Chen, G.; Chen, Z. Advancements and challenges in brain cancer therapeutics. Exploration 2024, 4, 20230177. [Google Scholar] [CrossRef]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood-Brain Barrier for the Treatment of Primary Brain Tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef]
- Treps, L.; Perret, R.; Edmond, S.; Ricard, D.; Gavard, J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1359479. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Huang, S.; Fan, B.; Wei, J.; Ouyang, L.; Chen, Z.; Jiang, B. Silencing matrix metalloproteinase 9 exerts a protective effect on astrocytes after oxygen-glucose deprivation and is correlated with suppression of aquaporin-4. Neurosci. Lett. 2020, 731, 135047. [Google Scholar] [CrossRef]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef]
- Badr, C.E.; Silver, D.J.; Siebzehnrubl, F.A.; Deleyrolle, L.P. Metabolic heterogeneity and adaptability in brain tumors. Cell. Mol. Life Sci. 2020, 77, 5101–5119. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.K.; Vaupel, P. Heterogeneity in tissue oxygenation: From physiological variability in normal tissues to pathophysiological chaos in malignant tumours. Adv. Exp. Med. Biol. 2014, 812, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Fortin, D. Drug Delivery Technology to the CNS in the Treatment of Brain Tumors: The Sherbrooke Experience. Pharmaceutics 2019, 11, 248. [Google Scholar] [CrossRef]
- Tripathy, D.K.; Panda, L.P.; Biswal, S.; Barhwal, K. Insights into the glioblastoma tumor microenvironment: Current and emerging therapeutic approaches. Front. Pharmacol. 2024, 15, 1355242. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Meng, W.; Huang, L.; Guo, J.; Xin, Q.; Liu, J.; Hu, Y. Innovative Nanomedicine Delivery: Targeting Tumor Microenvironment to Defeat Drug Resistance. Pharmaceutics 2024, 16, 1549. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.; Faraji, F.; Farhadi, T.; Hesami, O.; Iranpanah, A.; Webber, K.; Bishayee, A. Current Advances in Nanoformulations of Therapeutic Agents Targeting Tumor Microenvironment to Overcome Drug Resistance. Cancer Metastasis Rev. 2023, 42, 959–1020. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Huang, Y.; Zhang, L.; Li, W.; Cao, P.; Min, W.; Li, J.; Jing, W. A Nanogel with effective blood-brain barrier penetration ability through passive and active dual-targeting function. J. Nanomater. 2021, 6623031. [Google Scholar] [CrossRef]
- Cornelissen, F.; Markert, G.; Deutsch, G.; Antonara, M.; Faaij, N.; Bartelink, I.; Noske, D.; Vandertop, W.; Bender, A.; Westerman, B. Explaining blood–brain barrier permeability of small molecules by integrated analysis of different transport mechanisms. J. Med. Chem. 2023, 66, 7253–7267. [Google Scholar] [CrossRef]
- Shi, S.; Ren, H.; Xie, Y.; Yu, M.; Chen, Y.; Yang, L. Engineering advanced nanomedicines against central nervous system diseases. Mater. Today 2023, 69, 355–392. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Ren, Z.; Cong, H.; Shen, Y.; Yu, B. The nanocarrier strategy for crossing the blood-brain barrier in glioma therapy. Chin. Chem. Lett. 2025, 36, 109996. [Google Scholar] [CrossRef]
- Culkins, C.; Adomanis, R.; Phan, N.; Robinson, B.; Slaton, E.; Lothrop, E.; Chen, Y.; Kimmel, B. Unlocking the Gates: Therapeutic Agents for Noninvasive Drug Delivery Across the Blood-Brain Barrier. Mol. Pharm. 2024, 21, 5430–5454. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Yee, G.; Khang, D. Nanoparticle-Based Combinational Strategies for Overcoming the Blood-Brain Barrier and Blood-Tumor Barrier. Int. J. Nanomed. 2024, 19, 2529–2552. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the treatment of brain cancer. A review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef]
- Ruiz-Molina, D.; Mao, X.; Alfonso-Triguero, P.; Lorenzo, J.; Bruna, J.; Yuste, V.J.; Candiota, A.P.; Novio, F. Advances in preclinical/clinical glioblastoma treatment: Can nanoparticles be of help? Cancers 2022, 14, 4960. [Google Scholar] [CrossRef]
- Hare, J.I.; Neijzen, R.W.; Anantha, M.; Dos Santos, N.; Harasym, N.; Webb, M.S.; Allen, T.M.; Bally, M.B.; Waterhouse, D.N. Treatment of colorectal cancer using a combination of liposomal irinotecan (Irinophore C™) and 5-fluorouracil. PLoS ONE 2013, 8, e62349. [Google Scholar] [CrossRef]
- Wadhwa, K.; Chauhan, P.; Kumar, S.; Pahwa, R.; Verma, R.; Goyal, R.; Singh, G.; Sharma, A.; Rao, N.; Kaushik, D. Targeting brain tumors with innovative nanocarriers: Bridging the gap through the blood-brain barrier. Oncol. Res. 2024, 32, 877. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal antibodies in cancer therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, A.V.; Bodyak, N.D.; Yin, M.; Thomas, J.D.; Clardy, S.M.; Conlon, P.R.; Stevenson, C.A.; Uttard, A.; Qin, L.; Gumerov, D.R.; et al. Dolaflexin: A novel antibody–drug conjugate platform featuring high drug loading and a controlled bystander effect. Mol. Cancer Ther. 2021, 20, 885–895. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Dmello, C.; Chen, L.; Arrieta, V.A.; Gonzalez-Buendia, E.; Kane, J.R.; Magnusson, L.P.; Baran, A.; James, C.D.; Horbinski, C.; et al. Ultrasound-mediated delivery of paclitaxel for glioma: A comparative study of distribution, toxicity and efficacy of albumin-bound versus cremophor formulations. Clin. Cancer Res. 2020, 26, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.A.; Galperin, A.; Ratner, B.D. Drug encapsulated polymeric microspheres for intracranial tumor therapy: A review of the literature. Adv. Drug Deliv. Rev. 2015, 91, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.; Alfaidi, M.A.; Batiha, G.E.S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A new race of pharmaceutical nanocarriers. BioMed Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- Kaurav, M.; Ruhi, S.; Al-Goshae, H.A.; Jeppu, A.K.; Ramachandran, D.; Sahu, R.K.; Sarkar, A.K.; Khan, J.; Ashif Ikbal, A.M. Dendrimer: An update on recent developments and future opportunities for the brain tumors diagnosis and treatment. Front. Pharmacol. 2023, 14, 1159131. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Z.; Gao, H.; Rostami, I.; You, Q.; Jia, X.; Wang, C.; Zhu, L.; Yang, Y. Enhanced blood-brain-barrier penetrability and tumor-targeting efficiency by peptide-functionalized poly (amidoamine) dendrimer for the therapy of gliomas. Nanotheranostics 2019, 3, 311–330. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, L.; Sahu, H.; Qayum, A.; Singh, S.K.; Nakhate, K.T.; Ajazuddin; Gupta, U. Chitosan engineered PAMAM dendrimers as nanoconstructs for the enhanced anti-cancer potential and improved in vivo brain pharmacokinetics of temozolomide. Pharm. Res. 2018, 35, 9. [Google Scholar] [CrossRef]
- Chauhan, M.; Singh, R.P.; Yadav, B.; Shekhar, S.; Kumar, A.; Mehata, A.K.; Nayak, A.K.; Dutt, R.; Garg, V.; Kailashiya, V.; et al. Development and characterization of micelles for nucleolin-targeted co-delivery of docetaxel and upconversion nanoparticles for theranostic applications in brain cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 87, 104808. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Gao, X.; Yu, T.; Xu, G.; Guo, G.; Liu, X.; Hu, X.; Wang, X.; Liu, Y.; Mao, Q.; You, C.; et al. Enhancing the anti-glioma therapy of doxorubicin by honokiol with biodegradable self-assembling micelles through multiple evaluations. Sci. Rep. 2017, 7, 43501. [Google Scholar] [CrossRef]
- Huang, X.; Mu, N.; Ding, Y.; Huang, R.; Wu, W.; Li, L.; Chen, T. Tumor microenvironment targeting for glioblastoma multiforme treatment via hybrid cell membrane coating supramolecular micelles. J. Control. Release 2024, 366, 194–203. [Google Scholar] [CrossRef]
- Meher, J.G.; Kesharwani, P.; Chaurasia, M.; Singh, A.; Chourasia, M.K. Carbon nanotubes (CNTs): A novel drug delivery tool in brain tumor treatment. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Kesharwani, P., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 375–396. [Google Scholar]
- Ren, J.; Shen, S.; Wang, D.; Xi, Z.; Guo, L.; Pang, Z.; Qian, Y.; Sun, X.; Jiang, X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with Angiopep-2. Biomaterials 2012, 33, 3324–3333. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-G.; Xu, J.; Li, Z.-G.; Ren, G.-G.; Yang, Z. In vitro toxicity of multi-walled carbon nanotubes in C6 rat glioma cells. Neurotoxicology 2012, 33, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Fu, P.P.; Yu, H.; Ray, P.C. Theranostic nanomedicine for cancer detection and treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Urbańska, K.; Pająk, B.; Orzechowski, A.; Sokołowska, J.; Grodzik, M.; Sawosz, E.; Szmidt, M.; Sysa, P. The effect of silver nanoparticles (AgNPs) on proliferation and apoptosis of in ovo cultured glioblastoma multiforme (GBM) cells. Nanoscale Res. Lett. 2015, 10, 98. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef]
- Shukla, A.; Trivedi, S.P. An in vitro analysis of the rat C6 glioma cells to elucidate the linear alkylbenzene sulfonate induced oxidative stress and consequent G2/M phase cell cycle arrest and cellular apoptosis. Chemosphere 2018, 205, 443–451. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, A.; Wang, S.; Sun, Y.; Chu, L.; Zhou, L.; Yang, X.; Liu, X.; Sha, C.; Sun, K.; et al. Efficacy of temozolomide-conjugated gold nanoparticle photothermal therapy of drug-resistant glioblastoma and its mechanism study. Mol. Pharm. 2022, 19, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Alle, M.; Kim, T.H.; Park, S.H.; Lee, S.-H.; Kim, J.-C. Doxorubicin-carboxymethyl xanthan gum capped gold nanoparticles: Microwave synthesis, characterization and anti-cancer activity. Carbohydr. Polym. 2020, 229, 115511. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Veeranarayanan, S.; Poulose, A.C.; Nagaoka, Y.; Minegishi, H.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Type 1 ribotoxin-curcin conjugated biogenic gold nanoparticles for a multimodal therapeutic approach towards brain cancer. Biochim. Biophys. Acta-Gen. Subj. 2014, 1840, 1657–1669. [Google Scholar] [CrossRef]
- Shim, K.H.; Hulme, J.; Maeng, E.H.; Kim, M.-K.; An, S.S.A. Analysis of zinc oxide nanoparticles binding proteins in rat blood and brain homogenate. Int. J. Nanomed. 2014, 9, 217–224. [Google Scholar] [CrossRef]
- Sharma, A.K.; Singh, V.; Gera, R.; Purohit, M.P.; Ghosh, D. Zinc oxide nanoparticle induces microglial death by NADPH-oxidase-independent reactive oxygen species as well as energy depletion. Mol. Neurobiol. 2017, 54, 6273–6286. [Google Scholar] [CrossRef]
- Wahab, R.; Kaushik, N.K.; Verma, A.K.; Mishra, A.; Hwang, I.; Yang, Y.-B.; Shin, H.-S.; Kim, Y.-S. Fabrication and growth mechanism of ZnO nanostructures and their cytotoxic effect on human brain tumor U87, cervical cancer HeLa and normal HEK cells. JBIC J. Biol. Inorg. Chem. 2011, 16, 431–442. [Google Scholar] [CrossRef]
- Attia, H.; Nounou, H.; Shalaby, M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat’s brain after oral exposure. Toxics 2018, 6, 29. [Google Scholar] [CrossRef]
- Nehra, M.; Uthappa, U.; Kumar, V.; Kumar, R.; Dixit, C.; Dilbaghi, N.; Mishra, Y.; Kumar, S.; Kaushik, A. Nanobiotechnology-assisted therapies to manage brain cancer in personalized manner. J. Control. Release 2021, 338, 407–428. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef]
- Dash, B.; Lu, Y.; Pejrprim, P.; Lan, Y.; Chen, J. Hyaluronic Acid-Modified, IR780-Conjugated and Doxorubicin-Loaded Reduced Graphene Oxide for Targeted Cancer Chemo/Photothermal/Photodynamic Therapy. Biomater. Adv. 2022, 136, 212764. [Google Scholar] [CrossRef]
- Taheriazam, A.; Abad, G.; Hajimazdarany, S.; Imani, M.; Ziaolhagh, S.; Zandieh, M.; Bayanzadeh, S.; Mirzaei, S.; Hamblin, M.; Entezari, M.; et al. Graphene Oxide Nanoarchitectures in Cancer Biology: Nano-Modulators of Autophagy and Apoptosis. J. Control. Release 2023, 356, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.; Lombardo, S.; Chisari, G.; Forte, S.; Sciuto, E.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Hettiarachchi, S.; Graham, R.; Mintz, K.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R. Triple Conjugated Carbon Dots as a Nano-Drug Delivery Model for Glioblastoma Brain Tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef] [PubMed]
- Ostovar, S.; Pourmadadi, M.; Shamsabadipour, A.; Mashayekh, P. Nanocomposite of Chitosan/Gelatin/Carbon Quantum Dots as a Biocompatible and Efficient Nanocarrier for Improving the Curcumin Delivery Restrictions to Treat Brain Cancer. Int. J. Biol. Macromol. 2023, 244, 124986. [Google Scholar] [CrossRef]
- Liyanage, P.; Zhou, Y.; Al-Youbi, A.; Bashammakh, A.; El-Shahawi, M.; Vanni, S.; Graham, R.; Leblanc, R. Pediatric Glioblastoma Target-Specific Efficient Delivery of Gemcitabine across the Blood-Brain Barrier via Carbon Nitride Dots. Nanoscale 2020, 12, 12412–12423. [Google Scholar] [CrossRef]
- Vallejo, F.; Sigdel, G.; Véliz, E.; Leblanc, R.; Vanni, S.; Graham, R. Carbon Dots in Treatment of Pediatric Brain Tumors: Past, Present and Future Directions. Int. J. Mol. Sci. 2023, 24, 9562. [Google Scholar] [CrossRef]
- Yadav, N.; Kannan, D.; Patil, S.; Singh, S.; Lochab, B. Amplified Activity of Artesunate Mediated by Iron Oxide Nanoparticles Loaded on a Graphene Oxide Carrier for Cancer Therapeutics. ACS Appl. Bio Mater. 2020, 3, 6722–6736. [Google Scholar] [CrossRef]
- Deng, L.; Li, Q.; Al-Rehili, S.; Omar, H.; Almalik, A.; Alshamsan, A.; Zhang, J.; Khashab, N. Hybrid Iron Oxide–Graphene Oxide–Polysaccharides Microcapsule: A Micro-Matryoshka for On-Demand Drug Release and Antitumor Therapy In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 6859–6868. [Google Scholar] [CrossRef]
- Moloudi, K.; Samadian, H.; Jaymand, M.; Khodamoradi, E.; Hoseini-Ghahfarokhi, M.; Fathi, F. Iron Oxide/Gold Nanoparticles-Decorated Reduced Graphene Oxide Nanohybrid as the Thermo-Radiotherapy Agent. IET Nanobiotechnol. 2020, 14, 428–432. [Google Scholar] [CrossRef]
- Khan, J.; Jindal, A.; Kumar, A.; Sharma, N. Tuning Stimuli-Triggered Mesoporous Silica Nanoparticles in Novel Way of Brain Cancer Therapy. BioNanoScience 2024, 14, 216–230. [Google Scholar]
- Shadmani, N.; Makvandi, P.; Parsa, M.; Azadi, A.; Nedaei, K.; Mozafari, N.; Poursina, N.; Mattoli, V.; Tay, F.; Maleki, A.; et al. Enhancing Methotrexate Delivery in the Brain by Mesoporous Silica Nanoparticles Functionalized with Cell-Penetrating Peptide Using In Vivo and Ex Vivo Monitoring. Mol. Pharm. 2023, 20, 3412–3425. [Google Scholar] [CrossRef]
- Fei, H.; Jin, Y.; Jiang, N.; Zhou, Y.; Wei, N.; Liu, Y.; Miao, J.; Zhang, L.; Li, R.; Zhang, A.; et al. Gint4.T-siHDGF Chimera-Capped Mesoporous Silica Nanoparticles Encapsulating Temozolomide for Synergistic Glioblastoma Therapy. Biomaterials 2024, 306, 122479. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, J.; Tang, L.; Wang, Y.; Yang, H.; Wang, X.; Fan, W. Custom-Design of Multi-Stimuli-Responsive Degradable Silica Nanoparticles for Advanced Cancer-Specific Chemotherapy. Small 2024, 20, e2400353. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cao, Y.; Singh, R.; Janjua, T.; Popat, A. Silica Nanoparticles for Brain Cancer. Expert Opin. Drug Deliv. 2023, 20, 1749–1767. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, V.; Sugumaran, A.; Perumal, V.; Manavalan, M.; Kondeti, D.P.; Basha, S.K.; Ahmed, M.A.; Kumar, M.; Vijayaraghavalu, S. Nanoformulations—Insights Towards Characterization Techniques. Curr. Drug Targets 2022, 23, 1330–1344. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef]
- Wang, X.; Tu, M.; Tian, B.; Yi, Y.; Wei, Z.; Wei, F. Synthesis of tumor-targeted folate conjugated fluorescent magnetic albumin nanoparticles for enhanced intracellular dual-modal imaging into human brain tumor cells. Anal. Biochem. 2016, 512, 8–17. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Zhang, H.; Tian, B.; Li, R.; Hou, X.; Wei, F. Fluorescent magnetic PEI-PLGA nanoparticles loaded with paclitaxel for concurrent cell imaging, enhanced apoptosis and autophagy in human brain cancer. Colloids Surf. B Biointerfaces 2018, 172, 708–717. [Google Scholar] [CrossRef]
- Du, C.; Liu, X.; Hu, H.; Li, H.; Yu, L.; Geng, D.; Chen, Y.; Zhang, J. Dual-targeting and excretable ultrasmall SPIONs for T1-weighted positive MR imaging of intracranial glioblastoma cells by targeting the lipoprotein receptor-related protein. J. Mater. Chem. B 2020, 8, 2296–2306. [Google Scholar] [CrossRef]
- Hallal, S.; Ebrahimkhani, S.; Shivalingam, B.; Graeber, M.B.; Kaufman, K.L.; Buckland, M.E. The emerging clinical potential of circulating extracellular vesicles for non-invasive glioma diagnosis and disease monitoring. Brain Tumor Pathol. 2019, 36, 29–39. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, D.; Li, W.; Xiang, X.; Zhao, J.; Yu, B.; Wang, C.; He, Z.; Zhu, L.; Yang, Y. Evaluation of serum extracellular vesicles as noninvasive diagnostic markers of glioma. Theranostics 2019, 9, 5347–5358. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, H.M.; Meyers, A.; Rahman, K.; Dyment, N.A.; Sasso, D.; Xue, C.; Oliver, D.L.; Lichtler, A.; Deng, X.; Ridwan, S.M. Intravenously-injected gold nanoparticles (AuNPs) access intracerebral F98 rat gliomas better than AuNPs infused directly into the tumor site by convection enhanced delivery. Int. J. Nanomed. 2018, 13, 3937–3948. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhu, J.; Sun, N.; Song, N.; Xing, Y.; Huang, H.; Zhao, J. Chlorotoxin peptide-functionalized polyethylenimine-entrapped gold nanoparticles for glioma SPECT/CT imaging and radionuclide therapy. J. Nanobiotechnol. 2019, 17, 30. [Google Scholar] [CrossRef]
- Cho, J.-H.; Kim, A.-R.; Kim, S.-H.; Lee, S.-J.; Chung, H.; Yoon, M.-Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017, 47, 182–192. [Google Scholar] [CrossRef]
- Tamba, B.; Streinu, V.; Foltea, G.; Neagu, A.; Dodi, G.; Zlei, M.; Tijani, A.; Stefanescu, C. Tailored surface silica nanoparticles for blood-brain barrier penetration: Preparation and in vivo investigation. Arab. J. Chem. 2018, 11, 981–990. [Google Scholar] [CrossRef]
- Ding, F.; Chen, Z.; Kim, W.Y.; Sharma, A.; Li, C.; Ouyang, Q.; Zhu, H.; Yang, G.; Sun, Y.; Kim, J.S. A nano-cocktail of an NIR-II emissive fluorophore and organoplatinum (ii) metallacycle for efficient cancer imaging and therapy. Chem. Sci. 2019, 10, 7023–7028. [Google Scholar] [CrossRef]
- Huang, N.; Cheng, S.; Zhang, X.; Tian, Q.; Pi, J.; Tang, J.; Huang, Q.; Wang, F.; Chen, J.; Xie, Z. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood–brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomedicine 2017, 13, 83–93. [Google Scholar] [CrossRef]
- Chan, M.H.; Liu, R.S.; Hsiao, M. Light/Ultrasound Responsive 750 nm-Emitted Non-toxic Indium Phosphide Quantum Dots Hybrid Nanobubble for Brain Tumor Imaging. FASEB J. 2019, 33, 662–666. [Google Scholar] [CrossRef]
- Madhankumar, A.; Mrowczynski, O.D.; Patel, S.R.; Weston, C.L.; Zacharia, B.E.; Glantz, M.J.; Siedlecki, C.A.; Xu, L.-C.; Connor, J.R. Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater. 2017, 58, 205–213. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Mansur, A.A.; Carvalho, S.M.; Florentino, R.M.; Mansur, H.S. L-cysteine and poly-L-arginine grafted carboxymethyl cellulose/Ag-In-S quantum dot fluorescent nanohybrids for in vitro bioimaging of brain cancer cells. Int. J. Biol. Macromol. 2019, 133, 739–753. [Google Scholar] [CrossRef]
- Ge, J.; Lan, M.; Zhou, B.; Liu, W.; Guo, L.; Wang, H.; Jia, Q.; Niu, G.; Huang, X.; Zhou, H.; et al. A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 2014, 5, 4596. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, S.P.; Syrgiannis, Z.; Marković, Z.M.; Bonasera, A.; Kepić, D.P.; Budimir, M.D.; Milivojević, D.D.; Spasojević, V.D.; Dramičanin, M.D.; Pavlović, V.B.; et al. Modification of structural and luminescence properties of graphene quantum dots by gamma irradiation and their application in a photodynamic therapy. ACS Appl. Mater. Interfaces 2015, 7, 25865–25874. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, J.; Jiang, C.; Lin, J.; Huang, P. Biodegradable titanium nitride MXene quantum dots for cancer phototheranostics in NIR-I/II biowindows. Chem. Eng. J. 2020, 400, 126009. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Niu, Y.; He, K.; Tang, M. Application of Quantum Dots in Brain Diseases and Their Neurotoxic Mechanism. Nanoscale Adv. 2024, 6, 3733–3746. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Deng, G.; Gao, X.; Chen, Z.; Bao, Y.; et al. Targeted Tumour Theranostics in Mice via Carbon Quantum Dots Structurally Mimicking Large Amino Acids. Nat. Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef]
- Guo, B.; Sheng, Z.; Hu, D.; Liu, C.; Zheng, H.; Liu, B. Through scalp and skull NIR-II photothermal therapy of deep orthotopic brain tumors with precise photoacoustic imaging guidance. Adv. Mater. 2018, 30, 1802591. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, D.; Wang, X.; Zhang, Z.; Yang, Y.; Jiang, L.; Qi, W.; Ye, Z.; He, S. Fluorination enhances NIR-II fluorescence of polymer dots for quantitative brain tumor imaging. Angew. Chem. Int. Ed. 2020, 59, 21049–21057. [Google Scholar] [CrossRef]
- Suárez-García, S.; Arias-Ramos, N.; Frias, C.; Candiota, A.P.; Arús, C.; Lorenzo, J.; Ruiz-Molina, D.; Novio, F. Dual T1/T2 nanoscale coordination polymers as novel contrast agents for MRI: A preclinical study for brain tumor. ACS Appl. Mater. Interfaces 2018, 10, 38819–38832. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wu, M.; Hu, D.; Pan, Y.; Hu, F.; Liu, X.; Thakor, N.; Ng, W.H.; Liu, X.; Sheng, Z. Biomimetic nanocomposites cloaked with bioorthogonally labeled glioblastoma cell membrane for targeted multimodal imaging of brain tumors. Adv. Funct. Mater. 2020, 30, 2004346. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B. Phototheranostics: Active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 2018, 13, 386–398. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Wang, R.; Zhao, P.; Gu, W.; Ye, L. Albumin-mediated synthesis of fluoroperovskite KMnF3 nanocrystals for T1-T2 dual-modal magnetic resonance imaging of brain gliomas with improved sensitivity. Chem. Eng. J. 2020, 395, 125066. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional Graphene Oxide/Iron Oxide Nanoparticles for Magnetic Targeted Drug Delivery, Dual Magnetic Resonance/Fluorescence Imaging and Cancer Sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional NPs for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 319–347. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Wang, W.; Shang, S.; Wang, Y.; Xu, B. Utilization of nanomaterials in MRI contrast agents and their role in therapy guided by imaging. Front. Bioeng. Biotechnol. 2024, 12, 1484577. [Google Scholar] [CrossRef]
- Basiak, B.; Veggel, F.C.J.M.V.; Tomanek, B. Applications of NPs for MRI cancer diagnosis and therapy. J. Nanomater. 2013, 2013, 148578. [Google Scholar] [CrossRef]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide NPs: A versatile platform for targeted molecular imaging, molecular diagnostics and therapy. ACS Chem. Biol. 2011, 6, 109–115. [Google Scholar] [CrossRef]
- Yang, J.; Feng, J.; Yang, S.; Xu, Y.; Shen, Z. Exceedingly small magnetic iron oxide NPs for T1-weighted magnetic resonance imaging and imaging-guided therapy of tumors. Small 2023, 19, 2302856. [Google Scholar] [CrossRef]
- Mao, C. Introduction: Bio and nano imaging and analysis. Microsc. Res. Tech. 2011, 74, 559–562. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic NPs for imaging, targeting and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, G.; Michela, V.; Lauri, C.; Franchi, G.; Pizzichini, P.; Signore, A. Copper-64 labeled nanoparticles for PET imaging: A review of the recent literature. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shi, S.; Liang, C.; Shen, S.; Cheng, L.; Wang, C.; Song, X.; Goel, S.; Barnhart, T.E.; Cai, W.; et al. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano 2015, 9, 10423–10435. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, B. Polymer-encapsulated organic NPs for fluorescence and photoacoustic imaging. Chem. Soc. Rev. 2014, 43, 4167–4185. [Google Scholar] [CrossRef]
- Thomas, G.; Boudon, J.; Maurizi, L.; Moreau, M.; Walker, P.; Sverin, I.; Oudot, A.; Goze, C.; Poty, S.; Vrigneaud, J.; et al. Innovative magnetic NPs for PET/MRI bimodal imaging. ACS Omega 2019, 4, 30218–30227. [Google Scholar] [CrossRef]
- Sarcan, E.T. NPs for dual imaging: PET and fluorescence imaging. Ank. Univ. J. Pharm. Sci. 2024, 59, 67–75. [Google Scholar] [CrossRef]
- Ahmad, F.; Varghese, R.; Panda, S.; Ramamoorthy, S.; Areeshi, M.Y.; Fagoonee, S.; Haque, S. Smart nanoformulations for brain cancer theranostics: Challenges and promises. Cancers 2022, 14, 5389. [Google Scholar] [CrossRef]
- Sonali; Viswanadh, M.K.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Narendra; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70–86. [Google Scholar] [CrossRef]
- Yang, K.; Tang, H.; Zhang, Y.; Wu, Y.; Su, L.; Zhang, X.; Du, W.; Zhang, J.; Wang, G.; Liu, D.; et al. NIR-II ratiometric fluorescent nanoplatform for real-time monitoring and evaluating cancer sonodynamic therapy efficacy. Adv. Opt. Mater. 2024, 12, 2303258. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, J.; Chen, B.; Ge, X.; Zhang, X.; Gao, S.; Ma, Q.; Song, J. NIR-II fluorescent activatable drug delivery nanoplatform for cancer-targeted combined photodynamic and chemotherapy. ACS Appl. Bio Mater. 2022, 5, 711–722. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Ye, Z.; Guan, K.; Teng, L.; Wu, J.; Yin, X.; Song, G.; Zhang, X.-B. Reactive oxygen correlated chemiluminescent imaging of a semiconducting polymer nanoplatform for monitoring chemodynamic therapy. Nano Lett. 2020, 20, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, M.; Liang, B.; Sun, W.; Shao, Y.; Hu, X.; Xing, D. Breaking the Barrier: Nanoparticle-Enhanced Radiotherapy as the New Vanguard in Brain Tumor Treatment. Front. Pharmacol. 2024, 15, 1394816. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Sancey, L.; Dufort, S.; Duc, L.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.; Villa, J.; et al. Treatment of Multiple Brain Metastases Using Gadolinium Nanoparticles and Radiotherapy: NANO-RAD, a Phase I Study Protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, M.; Ghazi, F.; Mehrabifard, M.; Alivirdiloo, V.; Hajiabbasi, M.; Rahimi, F.; Mobed, A.; Taheripak, G.; Farani, M.; Huh, Y.; et al. State-of-the-Art Application of Nanoparticles in Radiotherapy: A Platform for Synergistic Effects in Cancer Treatment. Strahlenther. Und Onkol. 2024, 201, 577–588. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting Brain Metastases with Ultrasmall Theranostic Nanoparticles, a First-in-Human Trial from an MRI Perspective. Sci. Adv. 2020, 6, eaay5279. [Google Scholar] [CrossRef]
- Dong, Z.; Feng, L.; Zhu, W.; Sun, X.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 2016, 110, 60–70. [Google Scholar] [CrossRef]
- Hu, F.; Li, C.; Zhang, Y.; Wang, M.; Wu, D.; Wang, Q. Real-time in vivo visualization of tumor therapy by a near-infrared-II Ag2S quantum dot-based theranostic nanoplatform. Nano Res. 2015, 8, 1637–1647. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Zhou, Y.; Chu, Y.; Shen, J.; Cai, Y.; Sun, X. Near Infrared-Activatable biomimetic nanoplatform for Tumor-Specific drug release, penetration and Chemo-Photothermal synergistic therapy of orthotopic glioblastoma. Int. J. Nanomed. 2024, 19, 6999–7014. [Google Scholar] [CrossRef]
- Cheng, W.; Qu, H.; Yang, J.; Chen, H.; Pan, Y.; Duan, Z.; Xue, X. Hierarchically engineered Self-Adaptive nanoplatform guided intuitive and precision interventions for Deep-Seated glioblastoma. ACS Nano 2025, 19, 129–138. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, F.; Guan, K.; Wang, Y.; Fu, X.; Yang, Y.; Yin, X.; Song, G.; Zhang, X.; Tan, W. In vivo therapeutic response monitoring by a self-reporting upconverting covalent organic framework nanoplatform. Chem. Sci. 2019, 11, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, K.; Zhang, G.; Chen, P.; Xu, X.; Zhang, M.; Mao, J.; Zhao, G.; Qi, C.; Cai, K.; et al. Autologous Nanovaccine Induces Immunogenic Domino Effect to Prevent Postoperative Recurrence of Orthotopic Glioblastoma. Adv. Funct. Mater. 2025, 35, 2412040. [Google Scholar] [CrossRef]
- Wang, T.; Han, M.; Han, Y.; Jiang, Z.; Zheng, Q.; Zhang, H.; Li, Z. Antigen Self-Presented Personalized Nanovaccines Boost the Immunotherapy of Highly Invasive and Metastatic Tumors. ACS Nano 2024, 18, 6333–6347. [Google Scholar] [CrossRef]
- Cheng, F.; Su, T.; Zhou, S.; Liu, X.; Yang, S.; Lin, S.; Guo, W.; Zhu, G. Single-Dose Injectable Nanovaccine-in-Hydrogel for Robust Immunotherapy of Large Tumors with Abscopal Effect. Sci. Adv. 2023, 9, eade6257. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Li, R.; Zhang, C.; Wu, M.; Zhang, X.; Zheng, A.; Liao, N.; Zheng, Y.; Xu, H.; et al. Multifunctional Biomimetic Nanocarriers for Dual-Targeted Immuno-Gene Therapy Against Hepatocellular Carcinoma. Adv. Sci. 2024, 11, 2400951. [Google Scholar] [CrossRef]
- Allen, S.; Liu, X.; Jiang, J.; Liao, Y.; Chang, C.; Nel, A.; Meng, H. Immune Checkpoint Inhibition in Syngeneic Mouse Cancer Models by a Silicasome Nanocarrier Delivering a GSK3 Inhibitor. Biomaterials 2020, 269, 120635. [Google Scholar] [CrossRef]
- Jia, Z.; Yan, X.; Zhou, H.; Wang, W.; Li, C.; Zhang, B. Anti-PD-L1 Checkpoint Inhibitor Combined with Nanocarrier-Mediated Cisplatin Codelivery System for Effective Treatment of Pancreatic Cancer. Mol. Immunol. 2024, 174, 69–76. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, L.; Guo, Y.; Wang, Q.; Li, Y.; Sun, H.; Xie, S.; Liang, Y. Advancements in Stimulus-Responsive Co-Delivery Nanocarriers for Enhanced Cancer Immunotherapy. Int. J. Nanomed. 2024, 19, 3387–3404. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, C.M.; Won, J.E.; Jang, G.-Y.; Lee, J.H.; Park, S.H.; Kang, T.H.; Han, H.D.; Park, Y.-M. Enhancement of Anticancer Immunity by Immunomodulation of Apoptotic Tumor Cells Using Annexin A5 Protein-Labeled Nanocarrier System. Biomaterials 2022, 288, 121677. [Google Scholar] [CrossRef]
- Xie, R.; Wang, Y.; Tong, F.; Yang, W.; Lei, T.; Du, Y.; Wang, X.; Yang, Z.; Gong, T.; Shevtsov, M.; et al. Hsp70-Targeting and Size-Tunable Nanoparticles Combine with PD-1 Checkpoint Blockade to Treat Glioma. Small 2023, 19, e2300570. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Watkins-Schulz, R.; Junkins, R.; David, C.; Johnson, B.; Montgomery, S.; Peine, K.; Darr, D.; Yuan, H.; McKinnon, K.; et al. A Nanoparticle-Incorporated STING Activator Enhances Antitumor Immunity in PD-L1-Insensitive Models of Triple-Negative Breast Cancer. JCI Insight 2018, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Hsu, F.; Qiu, J.; Chern, G.; Lee, Y.; Chang, C.; Huang, Y.; Sung, Y.; Chiang, C.; Huang, R.; et al. Highly Efficient and Tumor-Selective Nanoparticles for Dual-Targeted Immunogene Therapy against Cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.; Cao, S.; Zhang, Q.; Chen, X.; Luo, W.; Tan, J.; Xu, X.; Tian, J.; Saw, P.; et al. Multifunctional Redox-Responsive Nanoplatform with Dual Activation of Macrophages and T Cells for Antitumor Immunotherapy. ACS Nano 2023, 17, 924–937. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Z.; Wang, T.; Wang, J.; Zhang, H.; Li, Z.; Wang, S.; Sheng, F.; Yu, J.; Hou, Y. Biodegradable MnO-Based Nanoparticles with Engineering Surface for Tumor Therapy: Simultaneous Fenton-Like Ion Delivery and Immune Activation. ACS Nano 2022, 16, 9291–9304. [Google Scholar] [CrossRef]
- Choi, J.; Shim, M.; Yang, S.; Hwang, H.; Cho, H.; Kim, J.; Yun, W.; Moon, Y.; Kim, J.; Yoon, H.; et al. Visible-Light-Triggered Prodrug Nanoparticles Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. ACS Nano 2021, 15, 14674–14685. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, S.; Wang, P.; Sun, L.; Wang, Y.; Zhang, L.; Xu, M.; Chen, J.; Wu, R.; Zhang, J.; et al. Genetically Engineering Cell Membrane-Coated BTO Nanoparticles for MMP2-Activated Piezocatalysis-Immunotherapy. Adv. Mater. 2023, 35, e202300964. [Google Scholar] [CrossRef]
- Peter, K.; Kar, S.K.; Gothalwal, R.; Gandhi, P. Curcumin in Combination with Other Adjunct Therapies for Brain Tumor Treatment: Existing Knowledge and Blueprint for Future Research. Int. J. Mol. Cell. Med. 2021, 10, 163–181. [Google Scholar]
- Levy, A.; Blacher, E.; Vaknine, H.; Lund, F.E.; Stein, R.; Mayo, L. CD38 deficiency in the tumor microenvironment attenuates glioma progression and modulates features of tumor-associated microglia/macrophages. Neuro-Oncol. 2012, 14, 1037–1049. [Google Scholar] [CrossRef]
- Shingaki, T.; Hidalgo, I.J.; Furubayashi, T.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Yamashita, S. The transnasal delivery of 5-fluorouracil to the rat brain is enhanced by acetazolamide (the inhibitor of the secretion of cerebrospinal fluid). Int. J. Pharm. 2009, 377, 85–91. [Google Scholar] [CrossRef]
- Azadi, A.; Hamidi, M.; Rouini, M.R. Methotrexate-loaded chitosan nanogels as ‘Trojan Horses’ for drug delivery to brain: Preparation and in vitro/in vivo characterization. Int. J. Biol. Macromol. 2013, 62, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, S.; Yu, Y.; Lv, Y.; Wang, A.; Yan, X.; Li, N.; Sha, C.; Sun, K.; Li, Y. Intranasal Delivery of Temozolomide-Conjugated Gold Nanoparticles Functionalized with Anti-EphA3 for Glioblastoma Targeting. Mol. Pharm. 2021, 18, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.; Jeitany, M.; Andrieux, A.; Junier, M.-P.; Chneiweiss, H.; Boussin, F. Intranasal Administration of Temozolomide Delayed the Development of Brain Tumors Initiated by Human Glioma Stem-Like Cell in Nude Mice. J. Cancer Sci. Ther. 2017, 9, 374–378. [Google Scholar] [CrossRef]
- Chen, T.C.; Da Fonseca, C.O.; Schönthal, A.H. Intranasal Perillyl Alcohol for Glioma Therapy: Molecular Mechanisms and Clinical Development. Int. J. Mol. Sci. 2018, 19, 3905. [Google Scholar] [CrossRef]
- Kim, J.S.; Shin, D.H.; Kim, J.S. Dual-Targeting Immunoliposomes Using Angiopep-2 and CD133 Antibody for Glioblastoma Stem Cells. J. Control. Release 2018, 269, 245–257. [Google Scholar] [CrossRef]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.-L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced Efficacy of Combined Temozolomide and Bromodomain Inhibitor Therapy for Gliomas Using Targeted Nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, L.; Lu, Y.; Sun, T.; Shen, C.; Chen, X.; Chen, Q.; An, S.; He, X.; Ruan, C.; et al. T7 Peptide-Functionalized PEG-PLGA Micelles Loaded with Carmustine for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2016, 8, 27465–27473. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Siew, A.; Chooi, K.W.; Okubanjo, O.; Garrett, N.; Lalatsa, K.; Serrano, D.; Summers, I.; Moger, J.; Stapleton, P.; et al. Lomustine Nanoparticles Enable Both Bone Marrow Sparing and High Brain Drug Levels—A Strategy for Brain Cancer Treatments. Pharm. Res. 2016, 33, 1289–1303. [Google Scholar] [CrossRef]
- Charest, G.; Sanche, L.; Fortin, D.; Mathieu, D.; Paquette, B. Glioblastoma Treatment: Bypassing the Toxicity of Platinum Compounds by Using Liposomal Formulation and Increasing Treatment Efficiency with Concomitant Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 244–249. [Google Scholar] [CrossRef]
- Baklaushev, V.P.; Nukolova, N.N.; Khalansky, A.S.; Gurina, O.I.; Gubarkova, E.V.; Grinenko, N.F.; Melnikov, P.A.; Gulyaev, M.V.; Chekhonin, V.P.; Kabanov, A.V. Treatment of Glioma by Cisplatin-Loaded Nanogels Conjugated with Monoclonal Antibodies Against Cx43 and BSAT1. Drug Deliv. 2015, 22, 276–285. [Google Scholar] [CrossRef]
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection Enhanced Delivery of Cisplatin-Loaded Brain Penetrating Nanoparticles Cures Malignant Glioma in Rats. J. Control. Release 2017, 263, 112–119. [Google Scholar] [CrossRef]
- Timbie, K.F.; McDannold, N.; Zinn, K.R.; Aftab, S.; Huang, R.; Chang, E.; Hynynen, K.; Fogh, S. MR Image-Guided Delivery of Cisplatin-Loaded Brain-Penetrating Nanoparticles to Invasive Glioma with Focused Ultrasound. J. Control. Release 2017, 263, 120–131. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs. Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Todo, T. ATIM-14. Results of Phase II Clinical Trial of Oncolytic Herpes Virus G47Δ in Patients with Glioblastoma. Neuro-Oncology 2019, 21 (Suppl. S6), vi4. [Google Scholar] [CrossRef]
- Land, C.A.; Musich, P.R.; Haydar, D.; Krenciute, G.; Xie, Q. Chimeric Antigen Receptor T-Cell Therapy in Glioblastoma: Charging the T Cells to Fight. J. Transl. Med. 2020, 18, 428. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.-J.; Glantz, M.; Peereboom, D.M.; et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with Temozolomide for Patients with Newly Diagnosed, EGFRvIII-Expressing Glioblastoma (ACT IV): A Randomized, Double-Blind, International Phase 3 Trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Li, X.L.; Zeng, S.; He, H.P.; Zeng, X.; Peng, L.-L.; Chen, L.-G. A Hybrid Glioma Tumor Cell Lysate Immunotherapy Vaccine Demonstrates Good Clinical Efficacy in the Rat Model. OncoTargets Ther. 2020, 13, 8109–8124. [Google Scholar] [CrossRef]
- Kadiyala, P.; Li, D.; Nunez, F.M.; Altshuler, D.; Doherty, R.; Kuai, R.; Yu, M.; Kamran, N.; Edwards, M.; Moon, J.J.; et al. High-Density Lipoprotein-Mimicking Nanodiscs for Chemo-Immunotherapy against Glioblastoma Multiforme. ACS Nano 2019, 13, 1365–1384. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Yang, J.; Shen, Q.; Liu, R.; Li, Y.; Shi, Y.; Chen, J.; Shen, Y.; Xiao, Z.; Weng, J.; et al. Traceable Nanoparticles with Dual Targeting and ROS Response for RNAi-Based Immunochemotherapy of Intracranial Glioblastoma Treatment. Adv. Mater. 2018, 30, 1705054. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.-L.; Iwasaki, A. VEGF-C-Driven Lymphatic Drainage Enables Immunosurveillance of Brain Tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Jiao, M.; Xu, S.; Ismail, M.; Xie, X.; An, Y.; Guo, H.; Qian, R.; Shi, B.; Zheng, M. Brain-targeted CRISPR/Cas9 nanomedicine for effective glioblastoma therapy. J. Control. Release 2022, 341, 68–78. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.; Schubert, M.; Friedmann-Morvinski, D.; Cohen, Z.; Behlke, M.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eaabc9450. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Wu, X.; Zhao, Y.; Liu, Y. Cascade-Responsive Nanoparticles for Efficient CRISPR/Cas9-Based Glioblastoma Gene Therapy. ACS Appl. Mater. Interfaces 2025, 17, 2485–2495. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, X.; Yang, Q.; Zheng, M.; Shimoni, O.; Ruan, W.; Wang, Y.; Zhang, D.; Yin, J.; Huang, X.; et al. Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci. Adv. 2022, 8, eabm8011. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, Y.; Chen, J.; Huang, N.; Wang, Z.; Cheng, Y. Gene Therapy for Drug-Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound. Int. J. Nanomed. 2021, 16, 185–199. [Google Scholar] [CrossRef]
- Fierro, J.; Tran, A.; Factoriza, C.; Chin, B.; Dou, H. TAMI-14. Nanoparticle delivery of PD-L1 CRISPR/Cas9 plasmid DNA for anti-glioblastoma immunotherapy. Neuro-Oncology 2020, 22, ii216. [Google Scholar] [CrossRef]
- Shetty, K.; Yasaswi, S.; Dutt, S.; Yadav, K.S. Multifunctional Nanocarriers for Delivering siRNA and miRNA in Glioblastoma Therapy: Advances in Nanobiotechnology-Based Cancer Therapy. 3 Biotech 2022, 12, 301. [Google Scholar] [CrossRef]
- Guo, Y.; Lee, H.; Fang, Z.; Velalopoulou, A.; Kim, J.; Thomas, M.B.; Liu, J.; Abramowitz, R.G.; Kim, Y.T.; Coskun, A.F.; et al. Single-Cell Analysis Reveals Effective siRNA Delivery in Brain Tumors with Microbubble-Enhanced Ultrasound and Cationic Nanoparticles. Sci. Adv. 2021, 7, eabf7390. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, R.; Wang, Y.; Zhang, X.; Yang, Y.; Zhao, B.; Yang, L. A Simple Self-Assembly Nanomicelle Based on Brain Tumor-Targeting Peptide-Mediated siRNA Delivery for Glioma Immunotherapy via Intranasal Administration. Acta Biomater. 2023, 155, 521–537. [Google Scholar] [CrossRef]
- He, W.; Wang, N.; Wang, Y.; Liu, M.; Qing, Q.; Su, Q.; Zou, Y.; Liu, Y. Engineering Nanomedicine for Non-Viral RNA-Based Gene Therapy of Glioblastoma. Pharmaceutics 2024, 16, 482. [Google Scholar] [CrossRef]
- Petrescu, G.E.D.; Sabo, A.A.; Torsin, L.I.; Calin, G.A.; Dragomir, M.P. MicroRNA-Based Theranostics for Brain Cancer: Basic Principles. J. Exp. Clin. Cancer Res. 2019, 38, 231. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shoorei, H.; Noferesti, L.; Hussen, B.M.; Moghadam, M.H.B.; Taheri, M.; Rashnoo, F. Nanoparticle-Mediated Delivery of MicroRNAs-Based Therapies for Treatment of Disorders. Pathol. Res. Pract. 2023, 248, 154667. [Google Scholar] [CrossRef] [PubMed]
- Gareev, I.; Beylerli, O.; Tamrazov, R.; Ilyasova, T.; Shumadalova, A.; Du, W.; Yang, B. Methods of miRNA Delivery and Possibilities of Their Application in Neuro-Oncology. Non-Coding RNA Res. 2023, 8, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Guo, X.; Yang, T.; Yu, M.; Chen, D.; Wang, J. Brain Tumor-Targeted Therapy by Systemic Delivery of siRNA with Transferrin Receptor-Mediated Core-Shell Nanoparticles. Int. J. Pharm. 2016, 510, 394–405. [Google Scholar] [CrossRef]
- Gu, B.; Zeng, X.; Gong, M.; Li, X.; Yu, Q.; Tan, Z.; Lou, Z.; Jiang, T.; Che, Y.; Ao, Y.; et al. Target Reprogramming the 3′UTR of Tumor-Suppressor Genes by a siRNA Composite Nanoparticle for Glioblastoma Therapy. Adv. Funct. Mater. 2024, 34, 2400837. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Li, H.; Mao, K.; Wang, H.; Meng, X.; Wang, J.; Wu, C.; Chen, H.; Wang, X.; et al. An Optimized Ionizable Cationic Lipid for Brain Tumor-Targeted siRNA Delivery and Glioblastoma Immunotherapy. Biomaterials 2022, 287, 121645. [Google Scholar] [CrossRef]
- Zou, Y.; Sun, X.; Wang, Y.; Yan, C.; Liu, Y.; Li, J.; Zhang, D.; Zheng, M.; Chung, R.; Shi, B. Single siRNA Nanocapsules for Effective siRNA Brain Delivery and Glioblastoma Treatment. Adv. Mater. 2020, 32, e2000416. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, Y.; Liu, Y.; Chen, Y.; Zhao, P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346. [Google Scholar] [CrossRef]
- Ismail, M.; Wang, Y.; Li, Y.; Liu, J.; Zheng, M.; Zou, Y. Stimuli-Responsive Polymeric Nanocarriers Accelerate On-Demand Drug Release to Combat Glioblastoma. Biomacromolecules 2024, 25, 6250–6282. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Ghasemi, A.; Zangabad, P.; Rahighi, R.; Basri, S.; Mirshekari, H.; Amiri, M.; Pishabad, S.; Aslani, A.; Bozorgomid, M.; et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed]
- Maboudi, A.H.; Lotfipour, M.H.; Rasouli, M.; Azhdari, M.H.; MacLoughlin, R.; Bekeschus, S.; Doroudian, M. Micelle-Based Nanoparticles with Stimuli-Responsive Properties for Drug Delivery. Nanotechnol. Rev. 2024, 13, 20230218. [Google Scholar] [CrossRef]
- An, X.; Zhu, A.; Luo, H.; Ke, H.; Chen, H.; Zhao, Y. Rational Design of Multi-Stimuli-Responsive Nanoparticles for Precise Cancer Therapy. ACS Nano 2016, 10, 5947–5958. [Google Scholar] [CrossRef]
- Wu, F.; Qiu, F.; Wai-Keong, S.A.; Diao, Y. The Smart Dual-Stimuli Responsive Nanoparticles for Controlled Anti-Tumor Drug Release and Cancer Therapy. Anti-Cancer Agents Med. Chem. 2021, 21, 1202–1215. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Li, Y. Stimuli-Responsive Nanomedicines for Overcoming Cancer Multidrug Resistance. Theranostics 2018, 8, 1059–1074. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart Drug Delivery Systems for Precise Cancer Therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Lin, Z.; Chou, W.; Cheng, Y.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting Nanoparticle Delivery to Tumors Using Machine Learning and Artificial Intelligence Approaches. Int. J. Nanomed. 2022, 17, 1365–1379. [Google Scholar] [CrossRef]
- Joshi, R.P.; Kumar, N. Artificial Intelligence for Autonomous Molecular Design: A Perspective. Molecules 2021, 26, 6761. [Google Scholar] [CrossRef]
- Bannigan, P.; Aldeghi, M.; Bao, Z.; Häse, F.; Aspuru-Guzik, A.; Allen, C. Machine Learning Directed Drug Formulation Development. Adv. Drug Deliv. Rev. 2021, 175, 113806. [Google Scholar] [CrossRef] [PubMed]
- Noorain; Srivastava, V.; Parveen, B.; Parveen, R. Artificial Intelligence in Drug Formulation and Development: Applications and Future Prospects. Curr. Drug Metab. 2023, 24, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dong, J.; Ouyang, D. AI-Directed Formulation Strategy Design Initiates Rational Drug Development. J. Control. Release 2025, 378, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Rao, L.; Kumar, H.; Bansal, N.; Deep, A.; Parashar, J.; Yadav, M.; Mittal, V.; Kaushik, D. Applications of Nanomedicine in Brain Tumor Therapy: Nanocarrier-Based Drug Delivery Platforms, Challenges and Perspectives. Recent Pat. Nanotechnol. 2023, 18, 99–119. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Torres-Suárez, A.I. Towards Tailored Management of Malignant Brain Tumors with Nanotheranostics. Acta Biomater. 2018, 73, 52–63. [Google Scholar] [CrossRef]
- Khilar, S.; Dembinska-Kenner, A.; Hall, H.; Syrmos, N.; Ligarotti, G.K.I.; Plaha, P.; Apostolopoulos, V.; Chibbaro, S.; Barbagallo, G.M.V.; Ganau, M. Towards a New Dawn for Neuro-Oncology: Nanomedicine at the Service of Drug Delivery for Primary and Secondary Brain Tumours. Brain Sci. 2025, 15, 136. [Google Scholar] [CrossRef]
- Sorrentino, C.; Ciummo, S.L.; Fieni, C.; Di Carlo, E. Nanomedicine for Cancer Patient-Centered Care. MedComm 2024, 5, e767. [Google Scholar] [CrossRef]
- Kuiken, T. Nanomedicine and ethics: Is there anything new or unique. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 111–118. [Google Scholar] [CrossRef]
- Wasti, S.; Lee, I.H.; Kim, S.; Lee, J.-H.; Kim, H. Ethical and legal challenges in nanomedical innovations: A scoping review. Front. Genet. 2023, 14, 1163392. [Google Scholar] [CrossRef]
| Tumor Type | WHO Grade | Nature | Common Location | Typical Age Group | Features | Common Treatment Approaches | References |
|---|---|---|---|---|---|---|---|
| Craniopharyngioma | I | Benign | Near pituitary gland | Children and Adults | Hormonal disruption due to pituitary involvement | Surgical removal, hormone replacement therapy | [2] |
| Chordoma | I/II | Locally malignant | Skull base, spine | 50–60 years | Invasive, compresses nerves; rare | Surgery, proton/carbon ion radiation therapy | [3] |
| Ganglioglioma | I | Benign | Temporal lobes | Children and Young Adults | Causes seizures due to location | Surgery; antiepileptic treatment | [4] |
| Schwannoma | I | Benign | Cranial nerves (esp. VIII) | 20–50 years | Vestibular involvement can lead to hearing loss | Radiosurgery or microsurgical resection | [5] |
| Pituitary Adenoma | I | Mostly benign | Pituitary gland | Adults | Endocrine symptoms due to hormone secretion | Surgery (transsphenoidal), medication | [6] |
| Pineocytoma | I | Benign | Pineal gland | Adults | Slow growing, non-invasive | Surgical removal | [7] |
| Anaplastic Astrocytoma | III | Malignant | Cerebral hemispheres | Adults | Rapidly proliferative, infiltrative | Surgery, radiation therapy, chemotherapy | [8] |
| Anaplastic Oligodendroglioma | III | Malignant | Cortex, white matter | Middle-aged Adults | Derived from myelin-producing cells | Surgery followed by chemo and radiation therapy | [8] |
| Glioblastoma Multiforme | IV | Highly malignant | Cerebrum | 45–70 years | Necrosis, angiogenesis, rapid progression | Maximal resection, radiation therapy, temozolomide, supportive therapies | [9] |
| Aspect | Conventional Strategies | Nanomedicine Strategies | References |
|---|---|---|---|
| BBB Penetration | Conventional therapeutic agents exhibit poor permeability across the BBB, limiting their effectiveness. | Engineered NP’s (typically <100 nm), can traverse the BBB through mechanisms like receptor-mediated or adsorptive transcytosis. | [12] |
| Drug Delivery | Routes include systemic administration, direct implantation, and intranasal delivery, often with low targeting specificity. | Receptor or adsorption mediated transcytosis enables targeted and efficient delivery | [17] |
| Drug Distribution | Erratic, non-uniform distribution; potential off-target toxicity. | Controlled, site-specific delivery minimizes systemic exposure and toxicity. | [14] |
| Dosing Frequency | Frequent dosing needed due to rapid clearance and low bioavailability. | Sustained and controlled release reduces dosing frequency and improves effectiveness. | [12] |
| Invasiveness | Techniques like FUS or intracranial implantation are invasive and risky. | Non-invasive delivery routes such as intravenous or intranasal are safer and more patient-friendly. | [18] |
| Treatment Efficacy | Limited by drug resistance and poor BBB penetration. | Improved via controlled release and tumor receptor targeting. | [12] |
| Patient Compliance | Invasiveness and side effects reduce patient adherence. | Improved compliance due to non-invasive methods and targeted action. | [12] |
| Formulation Flexibility | Limited capability to incorporate diverse drug types or targeting features. | Nanocarriers offer modular design with tunable surface ligands and encapsulated payloads. | [12] |
| Immune System Clearance | Rapid clearance due to recognition by immune cells and RES uptake. | Surface-modified nanoparticles evade immune surveillance and extend circulation. | [14] |
| Theranostics Capability | Diagnosis and therapy are separate; real-time tracking is difficult. | Theranostic nanoparticles integrate imaging and therapy, allowing real-time monitoring. | [13] |
| Strategy | TME Targeted Feature | Mechanism/Approach | References |
|---|---|---|---|
| Oxygen-generating nanomedicines | Hypoxia | Induce reoxygenation, promote ferroptosis, and enhance chemotherapy sensitivity | [29] |
| Extracellular matrix modulation/disruption | Dense extracellular matrix | Facilitate deeper drug penetration and reduce therapeutic resistance | [30] |
| pH/redox/hypoxia-responsive carriers | Acidity, redox, hypoxia | Enable site-specific, stimuli-triggered drug release within the TME | [29] |
| Vascular normalization | Abnormal vasculature | Improve nanoparticle delivery and reduce hypoxia-induced resistance | [30] |
| Immune cell targeting | Immune microenvironment | Reprogram tumor-associated macrophages, fibroblasts, and MDSCs | [30] |
| Combination nanoformulations | Multiple TME components | Achieve synergistic and multitargeted therapeutic effects | [27] |
| Delivery Strategy | Mechanism | Advantages | Limitations | References |
|---|---|---|---|---|
| Passive Diffusion | Relies on physicochemical properties (e.g., lipophilicity, molecular weight, topological polar surface area) to passively cross the BBB | Simplicity; widely utilized for CNS-active drugs; effective with optimized molecular traits | Limited to small, lipophilic drugs; not suitable for large or polar molecules | [31,32] |
| Receptor-mediated Transport | Uses ligands (e.g., transferrin, insulin) on NPs to bind receptors on brain endothelial cells and undergo endocytosis | Targeted delivery with improved brain and glioma specificity; non-invasive | Requires receptor overexpression; potential for receptor saturation and variability | [33,34,35] |
| Adsorptive-mediated Transport | Utilizes electrostatic int eractions between cationic NPs surfaces and anionic BBB endothelium | Broad applicability; does not require specific receptors; promotes high cellular uptake | Lower specificity; potential cytotoxicity due to positive surface charges | [33,35] |
| Cell-mediated Transport | Harnesses immune cells like macrophages and neutrophils as carriers to traverse the BBB and deliver NPs | Enhanced targeting and immune activation; controlled drug release; reduced systemic toxicity | Complex preparation and potential variability in carrier cell function | [36] |
| Intranasal Delivery | Bypasses BBB via nasal mucosa, using olfactory/trigeminal nerve pathways for CNS drug transport | Non-invasive; fast absorption; reduced systemic toxicity; improved patient compliance. | Limited dosing capacity; challenges in consistent delivery and mucosal retention | [34] |
| FUS | Uses US waves and microbubbles to transiently open the BBB for NP delivery | Highly localized, tunable and non-invasive; improves NP penetration | Requires real-time imaging; potential tissue damage; long-term safety under investigation | [36] |
| QD Type | Toxicity Concerns | Clinical Translation Potential | Reference |
|---|---|---|---|
| General QDs | Material and dose-dependent toxicity, risk of reactive oxygen species (ROS) generation, apoptosis and neuroinflammation. | Real-time neuroimaging for tumor, neurodegenerative disorders and therapeutic drug delivery across BBB. | [108] |
| Graphene QDs | Potential oxidative stress, inflammatory responses, and long-term bioaccumulation. | Potential for efficient drug delivery, bioimaging and theranostics. | [105] |
| Functionalized QDs | Metal ion leakage or oxidative stress. | Enhanced targeting, ongoing research. | [106] |
| Cadmium-based QDs | High toxicity, including neurotoxicity attributed to heavy metal (Cd2+) ion release under UV or oxidative conditions, risk of neuroinflammation, oxidative stress, apoptosis, mitochondrial dysfunction and dose dependent neurobehavioral changes. | Despite the safety concerns they are employed in brain tumor imaging, targeted therapy, with functionalization strategies to enhancing specificity. | [108] |
| Carbon-based QDs | Minimal to no detectable toxicity, excellent photo stability and biocompatibility. | High tumor specificity, efficient drug delivery, and dual-modal imaging capability (Near-Infrared fluorescence and Photoacoustic imaging). | [109] |
| Nanomedicine/Therapeutic Modality | Model/System | Findings | References |
|---|---|---|---|
| PD-1 inhibitor (Nivolumab) | CheckMate 143, recurrent GBM patients | No significant overall survival improvement when compared to bevacizumab | [176] |
| Chimeric antigen receptor T-cell therapy | Preclinical and early clinical | Safe, but limited by tumor heterogeneity | [178] |
| Tumor lysate vaccine | Rat glioma model | Increased survival and immune infiltration | [181] |
| VEGF-C modulation | Mouse glioma model | Boosted T-cell trafficking via lymphatics | [184] |
| Curcumin-CD68 conjugate | GL261 glioma-bearing mice | Reduced tumor volume and increased survival via immune modulation | [159] |
| Rhein (CD38 inhibitor) | Wild-type glioma mice | 74% tumor volume reduction and CD38 inhibition in microglia | [160] |
| Perillyl Alcohol | GBM patients | Partial response, disease stabilization, 19% in remission after 4 years | [165] |
| Oncolytic virus G47Δ + TMZ | Phase II trial | 1-year survival rate of 92.3% | [177] |
| ICT-107 DC vaccine | Phase II, newly diagnosed GBM | Improved progression-free survival; overall survival trend | [179] |
| CpG-High Density Lipoprotein nanodiscs | Preclinical glioma model | Enhanced CD4+/CD8+ T-cell activation; prolonged survival | [182] |
| ANG-2 and anti-CD133 immunoliposomes with TMZ | Glioma-bearing mice | Doubled median survival (49.2 vs. 23.3 days) | [166] |
| Transferrin-targeted liposomes with TMZ + JQ1 | U87MG and GL261 mice | Significantly prolonged survival | [167] |
| TMZ + siTGF-β in hybrid nanoparticles | GBM-bearing mice | Survival increased to 36 days | [169] |
| TMZ | Human glioma xenograft models | Delayed tumor growth and prolonged survival in TMZ-sensitive tumors | [164] |
| EGFRvIII nanoclusters + RNAi | Intracranial glioma model | Improved immunochemotherapy; ROS-responsive delivery | [169] |
| Lactoferrin nanoparticles with TMZ | Rodent model | Improved brain delivery and safety | [168] |
| Poly(aspartic acid) NPs with cisplatin | Rats via convection-enhanced delivery | Survival > 100 days vs. 12 days | [174] |
| Cisplatin-loaded NPs + FUS | F98 and 9L gliomas | Survival extended to 35 days | [175] |
| Rindopepimut (EGFRvIII vaccine) + TMZ | ACT IV Phase III trial | No overall survival benefit in EGFRvIII + patients | [180] |
| T7-modified micelles with carmustine | U87 mouse model | Outperformed free carmustine | [170] |
| Lomustine-loaded nanocapsules | Orthotopic GBM model | Increased survival (33 vs. 22.5 days) | [171] |
| TMZ | C6 glioma-bearing rats | Increased survival (31 vs. 20–21.5 days); reduced tumor volume | [168] |
| PEGylated nanogels with cisplatin | Glioma mouse model | Extended survival to 42 days | [173] |
| Methotrexate in chitosan microspheres | Rodent model | Higher brain levels vs. IV; improved brain penetration | [169] |
| Liposomal cisplatin/oxaliplatin | F98 glioma rats | Extended survival (30.2 and 29.6 vs. 13.3 and 21 days) | [172] |
| 5-Fluorouracil + Acetazolamide | Rat model | 2–3× increase in cerebrospinal fluid drug levels; enhanced nose-to-brain delivery | [161] |
| Nanoparticle Type | Features | Therapeutic Outcome | References |
|---|---|---|---|
| Polymeric NPs | ANG-2 decorated, guanidinium and fluorine functionalization for Cas9/gRNA stabilization | Efficient BBB crossing, targeted gene knockout, tumor suppression in glioblastoma models | [185] |
| Lipid NPs | Amino-ionizable lipids for Cas9 mRNA and sgRNA delivery | Up to 70% gene editing, tumor growth inhibition, improved survival | [186] |
| Cascade-Responsive NPs | Environment-sensitive Graphene-Coated Nanoparticles activating CRISPR/Cas9 in tumor microenvironment | PD-L1 targeting, tumor inhibition, prolonged survival with temozolomide | [187] |
| Nanocapsules | Encapsulation of CRISPR/Cas9 complexes for noninvasive delivery | High gene editing efficiency, minimal off-target effects, extended survival | [188] |
| Lipid-Polymer Hybrid NPs | Combined with focused ultrasound for BBB * penetration | MGMT targeting, enhanced sensitivity to temozolomide, improved therapy | [189] |
| Immunotherapy Applications | CRISPR-based PD-L1 knockout via NPs | Enhanced immune response, potential for overcoming immunosuppression | [190] |
| Strategy | Mechanism/Modification | Therapeutic Outcome | References |
|---|---|---|---|
| Transferrin Receptor-Mediated Delivery | Core–shell nanoparticles modified with T7 peptides for EGFR-targeted siRNA delivery across the BBB | Enhanced accumulation in tumor tissue, EGFR downregulation, improved survival | [193] |
| Intranasal Delivery Systems | T7 peptide-modified nanomicelles for non-invasive, BBB-bypassing siRNA administration | Effective glioma targeting, immune modulation, improved therapeutic response | [198] |
| Microbubble-Enhanced US | siRNA-loaded nanoparticles combined with MB-FUS to increase BBB permeability | 10-fold increase in delivery efficiency, enhanced tumor apoptosis | [193] |
| Mesoporous Silica NPs | Mesoporous Silica NPs encapsulating siRNA to reprogram tumor suppressor genes | Improved siRNA stability, tumor inhibition and apoptosis induction | [199] |
| Cationic Lipid Nanoparticles | Optimized cationic lipids for targeting immunoregulatory genes like CD47 and PD-L1 | Boosted T cell-mediated immunity, promising for GBM immunotherapy | [200] |
| ANG-2 Functionalized Nanocapsules | BBB-penetrating nanocapsules for targeted and responsive siRNA release | Efficient tumor targeting, inhibited growth and increased survival in GBM models | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loushambam, B.; Shimray, M.M.K.; Khangembam, R.; Krishnaswami, V.; Vijayaraghavalu, S. Nanomedicine-Based Advances in Brain Cancer Treatment—A Review. Neuroglia 2025, 6, 28. https://doi.org/10.3390/neuroglia6030028
Loushambam B, Shimray MMK, Khangembam R, Krishnaswami V, Vijayaraghavalu S. Nanomedicine-Based Advances in Brain Cancer Treatment—A Review. Neuroglia. 2025; 6(3):28. https://doi.org/10.3390/neuroglia6030028
Chicago/Turabian StyleLoushambam, Borish, Mirinrinchuiphy M. K. Shimray, Reema Khangembam, Venkateswaran Krishnaswami, and Sivakumar Vijayaraghavalu. 2025. "Nanomedicine-Based Advances in Brain Cancer Treatment—A Review" Neuroglia 6, no. 3: 28. https://doi.org/10.3390/neuroglia6030028
APA StyleLoushambam, B., Shimray, M. M. K., Khangembam, R., Krishnaswami, V., & Vijayaraghavalu, S. (2025). Nanomedicine-Based Advances in Brain Cancer Treatment—A Review. Neuroglia, 6(3), 28. https://doi.org/10.3390/neuroglia6030028






