Abstract
Background: The Notch signaling pathway is an important regulator of stem cell activity in various tissues, including the central nervous system. It has been implicated in neurodevelopmental processes, including neuronal differentiation and synaptic plasticity. Research suggests that its expression may be associated with certain epileptogenic lesions, particularly those with neurodevelopmental origin. The aim of this study was to investigate the expression of Notch-1 in brain biopsies from various cases of pharmacoresistant epilepsy. Methods: Here, we used immunohistochemistry staining to retrospectively analyze 128 developmental lesions associated with pharmacoresistant epilepsy, including 13 cases with focal cortical dysplasia (FCD) type I, 39 with FCD type II, 37 with hippocampal sclerosis (HS), 23 with FCD IIIc, 9 with mild malformations of cortical development (MCD), 4 cases with mild malformation of cortical development with oligodendroglial hyperplasia and epilepsy (MOGHE), and 3 with tuberous sclerosis (TS). The tissues were stained for Neurofilament protein, Vimentin, S-100 protein, NeuN, and GFAP, as well as the stem cell marker Notch-1. Tissue that stained positively for Notch-1 was further characterized. Results: A positive Notch-1 reaction was found in all cases of FCD type IIb and TS, where it appeared in balloon cells but not in dysmorphic neurons, and in a single case of meningioangiomatosis (FCD IIIc), where it stained spider-like cells. Notch-1-positive cells showed a stem-like, glio-neuronal precursor immunophenotype. No staining was observed in the remaining cases with FCD type I, type III, HS, mild MCD, and MOGHE. Conclusions: Notch-1 displays a distinct pattern of expression in some epileptogenic lesions, potentially highlighting a stem cell-like origin or neurodevelopmental abnormalities contributing to pharmacoresistant epilepsy; however, it is not a general marker of such lesions. Its differential expression may prove useful in distinguishing between different types of FCD or other cortical malformations, which could assist in both their diagnosis and potentially in the development of more targeted therapeutic approaches. Further studies with different stem cell markers are needed in this direction.
1. Introduction
Up to one-third of people with epilepsy do not achieve seizure control despite the use of appropriate anti-seizure medications []. Cortical malformations are a common structural cause of intractable epilepsy. They are present in a substantial proportion of patients with pharmacoresistant epilepsy, with a prevalence of ∼25% ranging up to 50% depending on the studied population [,,]. Cortical malformations encompass a variety of developmental disorders of the cerebral cortex, common examples of which are focal cortical dysplasia (FCD) and tuberous sclerosis (TS) [,]. These often result from abnormal neuronal migration, differentiation, or organization during fetal brain development and can vary in severity and location. Nonetheless, their etiopathogenesis is not always clear [,]. Their diagnosis depends on histopathology, imaging, and genetic findings; however, there is sometimes overlap between different types of cortical malformations.
FCD is classified into various types according to tissue architecture hallmarks []. FCD type II arises from mutations in genes involved in the mechanistic target of rapamycin (mTOR) pathway, leading to disruptions in neocortical development [,]. FCD type II is further classified into two subtypes: FCD IIa, which is marked by the presence of dysmorphic neurons (DNs), and FCD IIb, which also includes balloon cells (BCs) alongside DNs [,]. TS is also linked to mTOR pathway mutations. Interestingly, the cortical tubers seen in TS exhibit an identical cellular phenotype to those found in FCD type IIb []. Both conditions display cortical dyslamination with various cell types, including DNs, reactive astrocytes, and giant cells [,]. The DNs in TS tubers are indistinguishable from those observed in FCD type IIa and IIb, and similarly, the giant cells in TS closely resemble the BCs found in FCD IIb, with large cell bodies and an opalescent, eosinophilic cytoplasm. BCs in FCD and TS show a dual expression of neuronal and glial markers, suggesting a failure of these cells to differentiate before migration into the cortex [,].
Stem cells are essential for the proper development of the cerebral cortex, where their differentiation and proliferation play crucial roles in shaping cortical architecture []. Notch signaling is a key pathway that governs stem cell fate decisions, and emerging evidence suggests that it may interact with the mTOR pathway, which is vital for regulating cell growth and metabolism and has been implicated in the pathophysiology of cortical malformations [,]. Dysregulation of Notch signaling can disrupt the balance between stem cell maintenance and differentiation, while mTOR signaling influences the cellular responses to nutrient availability and growth cues. Interplay between the Notch and mTOR signaling pathways could significantly impact cell survival and proliferation. There is evidence of Notch-1 indirectly promoting the activation of the PI3K-AKT-mTOR1 pathway in various tumor types, including ones affecting the nervous system [,,,]. However, a tumor-suppressive role of Notch-1 has also been reported [,]. The precise relationship between Notch signaling and mTOR, as well as their collective involvement in cortical malformations, remain incompletely understood.
The Notch family consists of several transmembrane receptors involved in cell differentiation and development. Specifically, Notch-1 signaling has been implicated in experimental models of epilepsy, both as a consequence of and as a contributor to abnormal neuronal excitation. Research on animal models with acute induced or chronic seizures has demonstrated altered activity and expression of Notch-1 [,]. Moreover, an exogenous pharmacological activation of Notch-1 has been reported to decrease the latency to and increase the frequency and amplitude of epileptic discharges, suggesting the receptor may play a role in the susceptibility, onset, and progression of epileptic disorders [,]. There is also conflicting evidence suggesting Notch-1 might have a neuroprotective role in rats with induced chronic epilepsy [].
In this study, we sought to determine whether human brain tissue from developmental epileptogenic lesions express the stem cell marker Notch-1 and to investigate its potential role in the pathophysiology of the lesions.
2. Materials and Methods
2.1. Patients
In this study, immunohistochemistry (IHC) was employed to retrospectively analyze a cohort of 128 developmental lesions associated with pharmacoresistant epilepsy. The cohort included a range of focal cortical malformations, specifically 13 cases of FCD type I, 39 cases of FCD type II (26 with type IIa and 13 with type IIb), 37 cases of hippocampal sclerosis (HS), 23 cases of FCD type IIIc, 9 cases of mild malformations of cortical development (MCD), 4 cases of MOGHE (mild malformation of cortical development with oligodendroglial hyperplasia and epilepsy), and 3 cases of TS, including 2 autopsy cases of therapeutic abortion due to imaging findings and 1 biopsy from a young patient with long-term epilepsy. With the exception of the two prenatal cases of TS, tissue was obtained through therapeutic surgical removal of the epileptogenic focus.
2.2. Immunohistochemistry
To evaluate their histopathological features and the potential stem-like cell populations involved in the pathogenesis of these lesions, tissue samples from these lesions were stained using the following IHC markers: Neurofilament protein (to identify neurons and assess their integrity), Vimentin (to stain glial and balloon cells and identify reactive astrocytes), S-100 protein (to visualize astrocytic cells and certain glial cells), NeuN (to label mature neurons and assess neuronal differentiation), CD34 (to mark endothelial cells), GFAP (to assess astrocyte activation and gliosis), and, finally, Notch-1 (a stem cell marker used to identify stem-like or undifferentiated cells). An established IHC protocol was employed []. Briefly, brain specimens were fixed in 10% formalin for 24 h at room temperature, after which they were dehydrated in graded ethanol, cleared in xylene, embedded in paraffin, and sectioned at 4 µm using a microtome. Tissue sections were deparaffinized by immersion in xylene and rehydrated. After epitope retrieval, blocking of endogenous peroxidase activity, and non-specific binding, sections were incubated overnight at 4 °C with the following primary antibodies: Anti-Neurofilament protein (clone SMI32): mouse monoclonal antibody (ready-to-use; Zeta Corp.; cat# Z2091MP, Sierra Madre, CA, USA); Anti-Vimentin (clone V9): mouse monoclonal antibody (ready-to-use; Dako; cat# IR630, Santa Clara, CA, USA); Anti-S100: rabbit polyclonal antibody (ready-to-use; Dako, cat# IR504, Santa Clara, CA, USA); Anti-NeuN (clone A60): mouse monoclonal antibody (ready-to-use; Zeta Corp.; cat# Z2178MP, Sierra Madre, CA, USA); Anti-Human CD34 (clone QBEnd/10): mouse monoclonal antibody (ready-to—use; Dako, cat# IR632, Santa Clara, CA, USA); Anti-GFAP (clone EP672Y): rabbit monoclonal antibody (ready-to-use, Cell Marque, cat# 258R-28, Rocklin, CA, USA); and Notch-1: rabbit polyclonal antibody, (1:200; Elabscience; cat# E-AB-12815, Wuhan, China). After washing, the sections were incubated with appropriate biotinylated secondary antibodies—goat anti-mouse (ready-to-use, Merck, cat# 21538-M, Darmstadt, Hesse, Germany) or goat anti-rabbit IgG (ready-to-use, Merck, cat# 21537, Darmstadt, Hesse, Germany) for 30 min at room temperature. The chromogen DAB was used for visualization and slides were counterstained with hematoxylin (ready-to-use, Leica Biosystems, cat#AR9915, Nussloch, Germany).
2.3. Image Analysis
Micrographs of the stained lesion sections were captured using an Olympus BX43 inverted microscope and an Olympus EP50 wireless digital camera (Olympus, Melville, NY, USA). Staining was assessed by a well-trained histopathologist analyzing 3 lesion sections from each case. HALO® Image analysis software, v3.4.2986 (IndicaLabs, Albuquerque, NM, USA) was used for quantification of stem cells in the lesions using the Cell density module.
2.4. Classification of Different Malformations
Diagnosis and classification of the excised lesions was performed by an experienced histopathologist based on the most recent diagnostic criteria [,] using hematoxylin–eosin (HE) and appropriate immunostaining and taking into account cortical architecture, neuronal integrity, glial activation, associated or adjacent malformations, and the involvement of the white matter. In the prenatal cases of TS, the condition was first identified by ultrasound and later confirmed genetically (TSC2).
3. Results
We found a distinct expression pattern of Notch-1 in some epileptogenic lesions. The biomarker was expressed in BCs but not in DNs in FCD type IIb and TS. Furthermore, it stained spider-like cells in the single case of meningioangiomatosis (FCD IIIc with vascular malformation). The stained cells appeared to have a stem-like, glio-neuronal immunophenotype. These findings point towards the possibility that Notch-1 could serve as a marker for BC in these specific epileptogenic conditions, potentially highlighting a stem cell-like origin or neurodevelopmental abnormalities contributing to pharmacoresistant epilepsy.
Notch-1 expression was not detected in other epileptogenic lesions like FCD type I, HS, and the remaining cases of FCD type IIIc, mild MCD, and MOGHE.
The tissue samples that stained positively for Notch-1 were further characterized and described in detail to identify the role of these cells in the pathophysiology of the lesions, particularly in terms of their possible contribution to pharmacoresistant epilepsy.
3.1. Focal Cortical Dysplasia Type II
In our series, there were 39 cases of FCD type II, aged 1–43 years old, with an almost equal gender ratio (19:20). The predominant lesion localization was frontal, accounting for over half of the cases, followed by temporal and, the least common, parietal. There were no cases with posterior–occipital localization. The detailed distribution of cases of FCD types IIa and IIb by sex, age, and lesion localization is presented in Table 1.

Table 1.
Distribution of cases of FCD types IIa and IIb by sex, age, and lesion localization.
DNs in FCD IIa (Figure 1) and IIb cases (Figure 2) were characterized by large soma, abnormal shapes (Figure 1A), aggregated Nissl substance (Figure 2A), and an accumulation of non-phosphorylated heavy neurofilaments (Figure 1B and Figure 2B). BCs, which are characteristic only of type IIb, but not IIa, were also unusually large, with an abundant eosinophilic cytoplasm and large vesicular nuclei, often eccentrically located (Figure 2A,B). Among them, binucleated and even multinucleated cellular forms were common. BCs were positively stained with Vimentin and S-100 protein (Figure 2C,D). All cases with FCD IIa are negative for IHC staining with the biomarker Notch-1 (Figure 1D), while this marker highlights ballooned cells in FCD IIb (Figure 2E,F). Immunostaining with Notch-1 affects the cytoplasm and membrane of the balloon cells, with some cases showing more intensely stained cells with multiple cytoplasmic processes (spider-like cells) (Figure 2F).
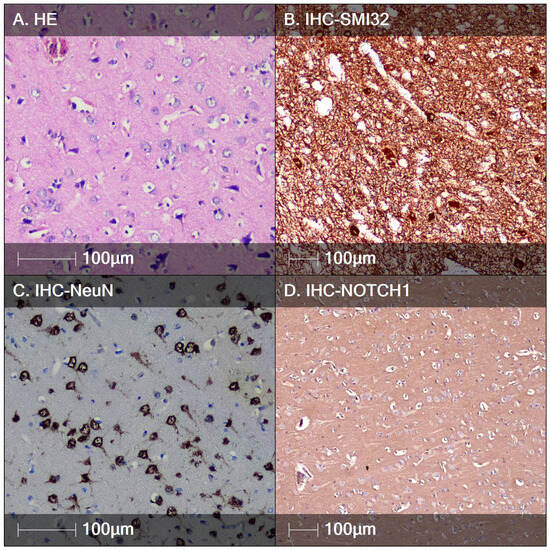
Figure 1.
FCD type IIa. (A) HE staining showing DNs with enlarged cell bodies and nuclei with visible nucleoli; (B) characteristic immunostaining of DNs demonstrating accumulation of non-phosphorylated heavy neurofilaments, IHC staining, SMI32; (C) DNs positive for the marker NeuN, IHC staining; (D) DNs negative for Notch-1; IHC staining.
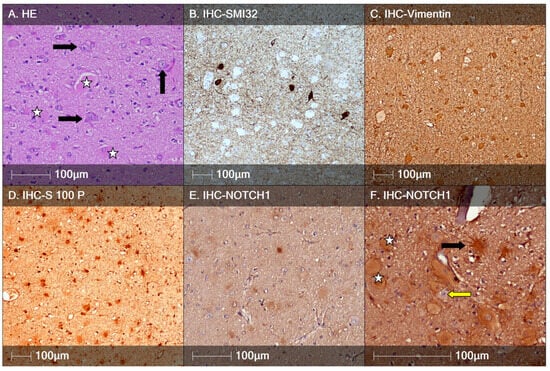
Figure 2.
FCD type IIb. (A) HE staining showing DNs with enlarged cell bodies and nuclei and aggregated Nissl substance (black arrows) and balloon cells (white stars); (B) characteristic immunostaining of DNs positive for SMI32 and SMI32-negative large round BCs; (C) Vimentin-positive DNs; (D) DNs positive for S-100 protein; (E) BCs positive for the marker Notch-1, IHC staining; (F) higher-magnification Notch-1 IHC staining showing positively marked balloon (white star) and spider-like cells (black arrow), but not dysmorphic neurons (yellow arrow).
3.2. Tuberous Sclerosis (TS)
Autopsy Case 1: A 25-year-old female patient was admitted to the hospital for the termination of a spontaneous first pregnancy in the 30th gestational week after imaging revealed hyperechogenic lesions in the fetal heart, which had increased in size compared to previous echocardiographic studies. Fetal screening had revealed multiple hyperechogenic lesions in the fetal cortex with ventriculomegaly (the lower horns of the lateral ventricles measured 12 mm in diameter) and a diminished signal from the cavum septum pellucidum structure. These cortical lesions also showed a tendency to increase in size. Pathological examination of the fetus and placenta showed that the fetus was male, with a length of 42 cm and a weight of 1800 g. The placenta measured 17 × 13 × 3.5 cm, with a para-marginally attached umbilical cord, 19 cm in length, with three visible vessels. The amniotic membranes were gray, elastic, and non-brittle. Autopsy of the fetal internal organs revealed multiple intramural unencapsulated, grayish nodular formations in the heart, ranging from millimeters to 1 cm in size, visible in the heart chambers. Histologically, they consisted of enlarged vacuolated cells with light to pale eosinophilic cytoplasm due to abundant glycogen deposition. The pathological examination of the brain showed signs of histological immaturity, with numerous cortical tubers made up of cells with abundant eosinophilic cytoplasm and large vesicular nuclei (Figure 3A). These cell elements were identical to ballooned cells seen in FCD Type IIb cases. All other organs showed histological signs of immaturity, with some autolysis present. Although the parents declined genetic testing, the observed pathological features of the myocardium and brain were indicative of cardiac rhabdomyomas and cortical tubers, both characteristic of TS.
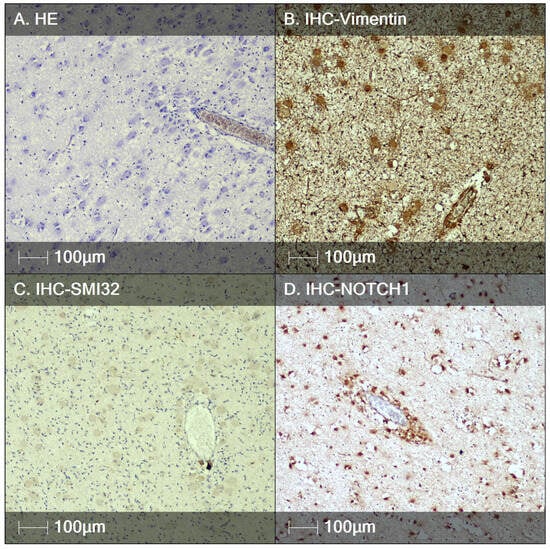
Figure 3.
Tuberous sclerosis, fetal autopsy case I. (A) HE staining showing a tendency for perivascular orientation of BCs; (B) immunostaining of BCs with the marker Vimentin; (C) BCs are negative for neurofilament proteins (IHC study with SMI32); (D) the biomarker NOTCH1 labels the BCs, again demonstrating a tendency for perivascular localization.
Autopsy Case 2: A 36-year-old female patient with a spontaneous second pregnancy was admitted for pregnancy termination in the 29th gestational week after imaging showed multifocal rhabdomyomatosis and tubers in the brain of the fetus. A pathogenic mutation in the TSC2 gene was confirmed. Pathological examination of the fetus and placenta revealed a female fetus, 40 cm in length and 1480 g in weight. The placenta measured 15 × 10 × 3 cm, with a centrally attached umbilical cord, 30 cm in length, and three visible vessels. The amniotic membranes were gray, elastic, and non-brittle. Autopsy of the fetal internal organs revealed numerous intramural unencapsulated nodules in the heart. Histopathologically, they exhibited features of rhabdomyomas. The brain cortex showed immaturity with dyslamination, and tubers made of numerous cells with ballooned eosinophilic cytoplasm and vesicular, sometimes eccentrically located, nuclei (Figure 4A).
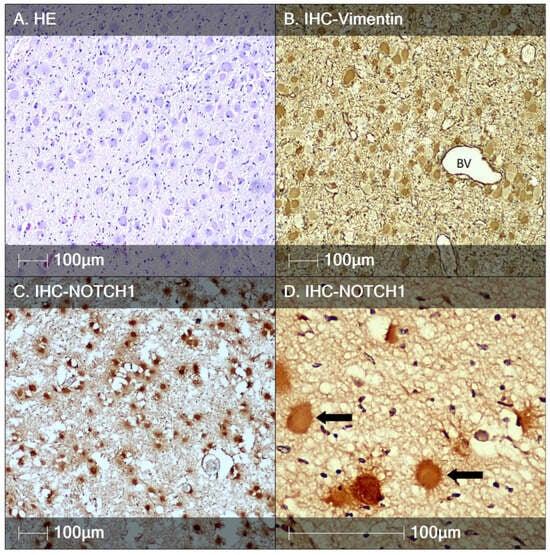
Figure 4.
Tuberous sclerosis, fetal autopsy case II. (A) HE staining showing BCs; (B) immunostaining of BCs with the marker Vimentin, BV—blood vessel; (C) BCs are positive for Notch-1; (D) higher magnification showing the marker NOTCH1 labels cells with a spider-like morphology (black arrows).
In both prenatal cases, BCs showed positive reactions for the markers Vimentin and S-100 protein (Figure 3B,C and Figure 4B). Interestingly, there was no evidence of DNs, even after directed IHC testing with the marker SMI32 (Figure 3C). All BCs expressed the stem cell biomarker Notch-1 (Figure 3D). Their perivascular arrangement further supports their stem cell origin. Additionally, more complex branching of their processes was observed, again immunostained with Notch-1, compared to the BCs in the FCD type IIb cases from our series (Figure 2F and Figure 4D).
Case of TS with longstanding epilepsy: A 10-year-old patient, born via cesarean section from a second pregnancy, was treated with oxygen therapy for respiratory distress syndrome and phototherapy for severe neonatal jaundice. The patient had normal psychomotor development until 7 months of age, when the parents noticed that she preferred using her left hand, while the right hand remained more rigid. At one year, the patient began therapy with antiepileptic drugs due to initial seizures involving the right hand and blinking of the right eye. MRI showed delayed myelination and cortical dysplasia in the left parietal region. Due to the presence of skin lesions, including those in the mother, genetic counseling was performed, revealing a TSC2 mutation. Epilepsy developed typically for TS, with refractory seizures. Video EEG monitoring revealed some asymmetry in the epileptogenic zones, with a massive tuber in the motor zone, almost indistinguishable from the largest tuber located in the left parietal region. This tuber was resected. Pathological examination revealed cortical dyslamination with the presence of ballooned cells and DNs. All BCs showed positive immunostaining with the Notch-1 marker, while DNs were negative (Figure 5C,D).
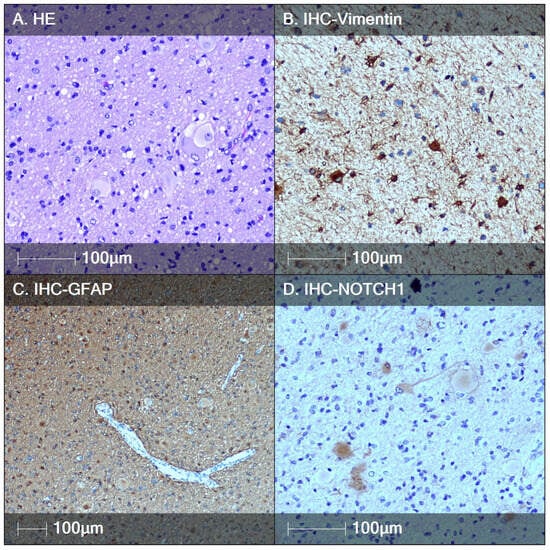
Figure 5.
Tuberous sclerosis, biopsy case. (A) HE staining showing a tendency for back-to-back arrangement of BCs; (B) immunostaining of BCs with the marker Vimentin; (C) BCs are positive for the marker GFAP; (D) biomarker NOTCH1 labels the BCs, showing a tendency for two patterns of immunoreactivity.
3.3. Case of Meningioangiomatosis
The only case of meningioangiomatosis in our series was diagnosed in a 14-year-old patient. The lesion was localized in the temporal lobe. Clinically, it manifested as pharmacoresistant epilepsy. Histologically, the tumor-like formation was represented by meningiovascular proliferation—perivascularly located spindle cells and neurofibrillary tangles (Figure 6A,B). CD34 marked the endothelial cells of the vessels (Figure 6D). There were no signs of increased proliferative activity. Among the described meningiendothelial proliferations, there were cells with a morphology resembling that of DNs and cells with an intermediate phenotype in cases of FCD type IIb. The dysmorphic cells were positive for the markers Neurofilament protein, GFAP, Vimentin, and NeuN (Figure 6C,E–G), which corresponds to a glio-neuronal precursor immunophenotype. A smaller spider-like cell type with multiple processes showed positive expression for the stem cell biomarker Notch-1 (Figure 6H).
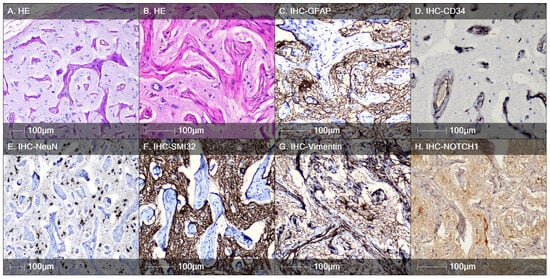
Figure 6.
Meningioangiomatosis. (A) H&E staining demonstrating hamartomatous meningeal–angiomatous proliferation; (B) in some areas, individual cells with a morphology identical to that of DNs are observed; (C) positive immunostaining with GFAP; (D) positive immunohistochemical reaction for CD34, marking the angiomatous component; (E) positive immunohistochemical reaction for NeuN, marking the neuronal cell composition; (F) dysmorphic cells show identical immunostaining for neurofilament proteins similar to DNs in FCD type II (IHC analysis with SMI32 marker); (G) IHC analysis with the Vimentin marker, marking the cellular elements between the meningeal-angiomatous proliferation; (H) positive reaction for the stem cell biomarker Notch-1 in individual cells with irregular branching projections (spider-like cells), as seen in cases of FCD type IIb.
3.4. Quantification Analysis
The Notch-1-positive cells in each lesion type were quantified using HALO® Image Analysis software and are presented as cell densities in Table 2. As can be seen, the greatest density of stem cells positive for the studied marker was found in the fetal autopsy cases of TS and the lowest density of cells was detected in the meningioangiomatosis case. No Notch-1- positive cells were detected in any of the FCD IIa cases.

Table 2.
Quantities of Notch-1-positive cells in the studied lesions.
4. Discussion
BCs characterize FCD IIb but not IIa, though both subtypes show cellular dysplasia []. The pathogenesis of BCs, also typically found in TS and hemimegaloencephaly (HME), is incompletely understood, but they often show mixed cellular lineage, expressing both glial and neuronal proteins, as well as a strong expression of primitive cellular proteins such as Vimentin. We found immunoreactivity for the stem cell marker Notch-1 in BCs, which may contribute to abnormal neuronal development, differentiation, and maturation and network activity.
In cases of FCD type II, there is not only histoarchitectural remodeling but also an abnormal cytoarchitecture. Microtubules are essential for neuronal maturation in terms of polarity, growth, morphology, and migration during cytological maturation []. The dysmorphic, megalocytic neurons in FCD II may be a fundamental disorder of microtubules from the earliest time of neuronal differentiation. It is noteworthy that in patients with FCD type II, significantly altered cellular elements (DNs and BCs) are intermixed with neurons of a normal phenotype. FCD type IIa and FCD type IIb are epileptogenic lesions composed of DNs with or without BCs. The age of detection and surgery is earlier in patients with FCD type IIb, where the lesions are more epileptogenic. The later onset of epilepsy in cases of FCD type IIa is also due to the smaller size of the lesion or its superficial cortical localization, which correlates with easier implementation of complete lesionectomy. DNs show characteristic immunostaining for Neurofilament protein (Figure 1B and Figure 2B), and all are negative for the biomarker Notch-1. The density of Notch-1-positive BCs is more pronounced in younger patients. The highest density of BCs was found in the autopsy cases with TS (Table 2). Our studies on these two autopsy cases further confirm the stem cell origin of BCs in immature brain tissue. There were no DNs, as we found in the cortical tubers of the 10-year-old patient with TSC. The presence of BCs during intrauterine fetal development suggests that epileptogenesis begins before birth. It is possible that some of the BCs later differentiate into DNs. We cannot completely exclude the possibility of abnormal neuronal differentiation from other precursors. In the developing tubers of histologically immature brain structures, there is also a tendency for the perivascular arrangement of Notch-1-positive BCs (Figure 3D). Such a tendency is not found in the other cases of our study.
Immunoreactivity is demonstrated in the cytoplasm and cell membrane of Notch-1-positive cells. We found that the immunophenotype of Notch-1 positive BCs shows two variants. In some of the cellular elements, cytoplasmic processes are absent, and binucleated cell forms are more commonly found, showing a back-to-back arrangement. Other cells positive for the marker possess numerous cytoplasmic processes, thus resembling spider-like cells. While it is arguable that 2D tissue analysis might incorrectly introduce phenotypic differences based on the section plane and field of view, we are confident that such a difference truly exists since the cells exhibit distinct and mutually exclusive morphology. Spider-like cells are characterized by numerous radially oriented projections while the classical BC are large and smooth. In addition, spider-like cells appear to have more intense immunostaining with Notch-1 compared to BCs. This might suggest that they are a more immature cell form. Moreover, early studies of the well-characterized BCs have demonstrated that they lack synaptic inputs and are unable to generate action potentials, disputing an active role of these cells in epileptogenesis [,]. In contrast, the spider-like cells described herein, with their multiple processes, could be involved in neuronal hyperexcitability, which could be the subject of future electrophysiological studies. BCs typically show positivity for glial (GFAP) and neuronal (NeuN) markers, thus displaying a dual glio-neuronal immunophenotype []. Histologically immature brain tissues from the autopsy cases show exclusively the spider-like model of Notch-1 immunoreactivity. It is possible that this type of cell elements undergoes differentiation, on the one hand, into BCs, and on the other, into DNs. Other authors report an intermediate phenotype of dysmorphic cells (neither DNs nor BCs) in cases of FCD type IIb and TS [,]. We believe that typical BCs present with a round shape, a relatively frequent back-to-back arrangement, and the absence of cytoplasmic processes. Spider-like cells are more likely to represent less mature cellular forms that can differentiate in various directions. It is noteworthy that this novel morphology phenotype of BC became apparent only after immunostaining with Notch-1 marker.
The commonly used marker for visualizing BC, Vimentin [], also showed a positive reaction in the cytoplasm and processes of BC but sometimes stains in a similar manner the reactive astrocytes, which are almost always present in FCD lesions. The immunoreactivity for Notch-1 marker found by us seems to show a “cleaner” background in thi study and does not mark the reactive glial proliferation.
The only previous IHC investigations for Notch-1 in cortical dysplasia showed that the marker was prominent in the cytoplasm of abnormally large neurons (nowadays, so-called DNs), while BCs did not stain with Notch-1 []. Normal-appearing neurons from both normal and abnormal areas showed less prominent cytoplasmic staining. The authors described prominent membranous staining on some of the normal-appearing neurons and the DNs, but not on the BCs or glial cells []. The clone of the antibody they used was different from the one we selected. This could explain the difference between our results and theirs.
Of particular interest to us was the only case of meningioangiomatosis in a 14-year-old patient with long-standing pharmacoresistant epilepsy. It concerns a rare tumor-like lesion of hamartomatous character []. Sporadic cases of meningioangiomatosis are typically symptomatic, presenting with epileptic seizures, while those associated with Neurofibromatosis type 2 are asymptomatic, found as an incidental finding []. In our patient, there were no objective data for neurofibromatosis. The histological picture was illustrated not only by meningiendothelial proliferations (Figure 6D) but also by the presence of cells whose morphology corresponds to DNs, as well as those whose morphology corresponded to spider-like cells, detailed in cases of FCD type IIb and TS. These cells showed positive immunoreactivity for both GFAP and NeuN, but also for the biomarker Notch-1. These IHC findings of dysmorphic and spider-like cells in the only case of meningioangiomatosis in our series, especially with immunoreactivity for the stem cell marker Notch-1, point to an immature glio-neuronal profile. Not surprisingly, according to the last revised classification of FCD, meningioangiomatosis does not represent just a meningeal-vascular hamartomatous lesion but also a dysplastic epileptogenic brain tissue in the context of FCD type IIIc []. The leading factor in epileptogenesis is most likely the presence of dysplastic cellular elements with an identical phenotype to the spider-like cells described in TS and FCD type IIb.
The lack of Notch-1 immunoreactivity in cases of FCD type I, IIIa, mild MCD, and MOGHE can be explained by different pathogenetic mechanisms in these lesions. In cases of FCD type Ia and MOGHE, pathogenic mutations in SLC35A2 have been identified [,]. Currently, cases of FCD type IIIa are associated with infection by Herpes virus type 6 []. Cases of Ib, Ic, and mild MCD have an unclear etiology [,].
Descriptive IHC analysis with the stem cell biomarker Notch-1 can be used to distinguish FCD type IIa from IIb, which would be especially useful in highly fragmented or scarce materials. BCs are positive for the marker, while DNs are not. In cases of TS and FCD type IIb, we found two patterns of immunoreactivity for the same marker, with one group of cells being round in shape, without processes (typical BCs), and another group showing numerous cytoplasmic processes (spider-like cells). Epileptogenesis begins before birth, with spider-like Notch-1 positive cells predominating in histologically immature brain tissue, showing a tendency for perivascular arrangement. The spider-like cell population is found in both meningioangiomatosis and is absent in cases of FCD type IIa. It is possible that FCD type IIa represents a more mature form with more prominent neuronal differentiation than type IIb. The stem cell origin of BCs, and especially their spider-like cellular forms, should be confirmed or refuted after conducting further studies with various surface and intracellular stem cell markers such as Notch-2, Nestin, SOX2, and SOX9.
5. Conclusions
The use of IHC in this study revealed a distinct expression of Notch-1 in specific developmental lesions associated with pharmacoresistant epilepsy, particularly FCD type IIb, TS, and meningioangiomatosis. These lesions demonstrated balloon and spider-like cells that were positive for Notch-1, indicative of a stem-like or glio-neuronal phenotype. The Notch-1 biomarker could provide a valuable diagnostic tool for distinguishing FCD type IIb from other forms of cortical dysplasia and identifying epileptogenic lesions with stem-like cells involved in the pathophysiology of pharmacoresistant epilepsy. Further studies focusing on the role of Notch-1 and other stem cell markers in epilepsy may offer new insights into the mechanisms of abnormal brain activity as well as novel therapeutic strategies targeting these aberrant cell populations.
Author Contributions
Conceptualization, D.M., P.D. and K.M.; methodology, D.M. and R.M.; formal analysis, D.M., M.R. and D.P.; investigation, D.M., R.M. and V.I.; resources, S.N., K.M., P.D., V.I. and G.S.; data curation, D.M., M.R. and R.G.; writing—original draft preparation, D.M. and M.R.; writing—review and editing, D.M., M.R. and D.P.; visualization, D.M., M.R. and R.G.; supervision, K.M., P.D. and G.S.; project administration, K.M. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Nadezhda Women’s Health Hospital (protocol code 9A/01.04.2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
The authors would like to thank all participating patients for contributing specimens for science.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sultana, B.; Panzini, M.A.; Carpentier, A.V.; Comtois, J.; Rioux, B.; Gore, G.; Bauer, P.R.; Kwon, C.-S.; Jetté, N.; Josephson, C.B.; et al. Incidence and Prevalence of Drug-Resistant Epilepsy. Neurology 2021, 96, 805–817. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Dobyns, W.B.; Guerrini, R. Malformations of cortical development and epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022392. [Google Scholar] [CrossRef] [PubMed]
- Wirrell, E.C.; Wong-Kisiel, L.C.L.; Mandrekar, J.; Nickels, K.C. What predicts enduring intractability in children who appear medically intractable in the first 2 years after diagnosis? Epilepsia 2013, 54, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, B.; Péoc’H, M.; Fabre-Bocquentin, B.; Bensaadi, L.; Pasquier, D.; Hoffmann, D.; Kahane, P.; Tassi, L.; Le Bas, J.-F.; Benabid, A.L. Surgical pathology of drug-resistant partial epilepsy. A 10-year-experience with a series of 327 consecutive resections. Epileptic Disord. 2002, 4, 99–119. [Google Scholar] [CrossRef]
- Severino, M.; Geraldo, A.F.; Utz, N.; Tortora, D.; Pogledic, I.; Klonowski, W.; Triulzi, F.; Arrigoni, F.; Mankad, K.; Leventer, R.J.; et al. Definitions and classification of malformations of cortical development: Practical guidelines. Brain 2020, 143, 2874–2894. [Google Scholar] [CrossRef]
- Northrup, H.; Arronow, M.E.; Bebin, M.; Bissler, J.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Gosnell, E.S.; Gupta, N.; Jansen, A.C.; et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef]
- Najm, I.; Lal, D.; Vanegas, M.A.; Cendes, F.; Lopes-Cendes, I.; Palmini, A.; Paglioli, E.; Sarnat, H.B.; Walsh, C.A.; Wiebe, S.; et al. The ILAE consensus classification of focal cortical dysplasia: An update proposed by an ad hoc task force of the ILAE diagnostic methods commission. Epilepsia 2022, 63, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- Rossini, L.; Villani, F.; Granata, T.; Tassi, L.; Tringali, G.; Cardinale, F.; Aronica, E.; Spreafico, R.; Garbelli, R. FCD Type II and mTOR pathway: Evidence for different mechanisms involved in the pathogenesis of dysmorphic neurons. Epilepsy Res. 2017, 129, 146–156. [Google Scholar] [CrossRef]
- Arruda, I.L.; Arruda, R.F.; da Silveira, R.M.B.; Duarte, J.T.C.; Guaranha, M.S.B.; Guilhoto, L.M.; Júnior, H.C.; Stavale, J.N.; Centeno, R.S.; Yacubian, E.M.T.; et al. A controversial question: Can morphometry and clinical history be enough to diagnose hippocampal dysplasia? Epileptic Disord. 2024, 26, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Ruppe, V.; Dilsiz, P.; Reiss, C.S.; Carlson, C.; Devinsky, O.; Zagzag, D.; Weiner, H.L.; Talos, D.M. Developmental brain abnormalities in tuberous sclerosis complex: A comparative tissue analysis of cortical tubers and perituberal cortex. Epilepsia 2014, 55, 539–550. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Takashima, S. Neuropathology of tuberous sclerosis. Brain Dev. 2001, 23, 508–515. [Google Scholar] [CrossRef]
- Grajkowska, W.; Kotulska, K.; Jurkiewicz, E.; Matyja, E. Brain lesions in tuberous sclerosis complex. Review. Folia Neuropathol. 2010, 48, 139–149. [Google Scholar] [PubMed]
- Talos, D.M.; Kwiatkowski, D.J.; Cordero, K.; Black, P.M.; Jensen, F.E. Cell-specific alterations of glutamate receptor expression in tuberous sclerosis complex cortical tubers. Ann. Neurol. 2008, 63, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Orlova, K.A.; Crino, P.B. The tuberous sclerosis complex. Ann. New York Acad. Sci. 2010, 1184, 87–105. [Google Scholar] [CrossRef]
- Lampada, A.; Taylor, V. Notch signaling as a master regulator of adult neurogenesis. Front. Neurosci. 2023, 17, 1179011. [Google Scholar] [CrossRef]
- Zhao, N.; Guo, Y.; Zhang, M.; Lin, L.; Zheng, Z. Akt-mTOR signaling is involved in Notch-1-mediated glioma cell survival and proliferation. Oncol. Rep. 2010, 23, 1443–1447. [Google Scholar] [CrossRef]
- Xu, P.; Qiu, M.; Zhang, Z.; Kang, C.; Jiang, R.; Jia, Z.; Wang, G.; Jiang, H.; Pu, P. The oncogenic roles of Notch1 in astrocytic gliomas in vitro and in vivo. J. Neurooncol. 2010, 97, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hales, E.C.; Taub, J.W.; Matherly, L.H. New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: Targeted therapy of gamma-secretase inhibitor resistant T-cell acute lymphoblastic leukemia. Cell Signal. 2014, 26, 149–161. [Google Scholar] [CrossRef]
- Yi, L.; Zhou, X.; Li, T.; Liu, P.; Hai, L.; Tong, L.; Ma, H.; Tao, Z.; Xie, Y.; Zhang, C.; et al. Notch1 signaling pathway promotes invasion, self-renewal and growth of glioma initiating cells via modulating chemokine system CXCL12/CXCR4. J. Exp. Clin. Cancer Res. 2019, 38, 339. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, M.; Chen, H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 2007, 12, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wu, S.; Wu, H.; Liu, L.; Zhou, J. Effect of the Notch1-mediated PI3K-Akt-mTOR pathway in human osteosarcoma. Aging 2021, 13, 21090–21101. [Google Scholar] [CrossRef]
- Sibbe, M.; Häussler, U.; Dieni, S.; Althof, D.; Haas, C.A.; Frotscher, M. Experimental epilepsy affects Notch1 signalling and the stem cell pool in the dentate gyrus. Eur. J. Neurosci. 2012, 36, 3643–3652. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Yin, Y.; Deng, X. Increased expression of Notch1 in temporal lobe epilepsy: Animal models and clinical evidence. Neural Regen. Res. 2014, 9, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, F.; Modarres Mousavi, S.M.; Lotfinia, A.A.; Alipour, F.; Hosseini Ravandi, H.; Karimzadeh, F. Discrepancies of Notch 1 receptor during development of chronic seizures. J. Cell Physiol. 2019, 234, 13773–13780. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Wu, X.; Yao, Y.; Wen, B.; Feng, J.; Sha, Z.; Wang, X.; Xing, X.; Dou, W.; Jin, L.; et al. Notch signaling activation promotes seizure activity in temporal lobe epilepsy. Mol. Neurobiol. 2014, 49, 633–644. [Google Scholar] [CrossRef]
- Sun, C.; Fu, J.; Qu, Z.; Li, D.; Si, P.; Qiao, Q.; Zhang, W.; Xue, Y.; Zhen, J.; Wang, W. Chronic mild hypoxia promotes hippocampal neurogenesis involving Notch1 signaling in epileptic rats. Brain Res. 2019, 1714, 88–98. [Google Scholar] [CrossRef]
- Ganeva, R.; Parvanov, D.; Vidolova, N.; Ruseva, M.; Handzhiyska, M.; Arsov, K.; Decheva, I.; Metodiev, D.; Moskova-Doumanova, V.; Stamenov, G. Endometrial immune cell ratios and implantation success in patients with recurrent implantation failure. J. Reprod. Immunol. 2023, 156, 103816. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P.; Knyihar-Csillik, E.; Csillik, B. Polarity of microtubule assemblies during neuronal cell migration. Proc. Natl. Acad. Sci. USA 1996, 93, 9218–9222. [Google Scholar] [CrossRef] [PubMed]
- Mathern, G.W.; Cepeda, C.; Hurst, R.S.; Flores-Hernandez, J.; Mendoza, D.; Levine, M.S. Neurons recorded from pediatric epilepsy surgery patients with cortical dysplasia. Epilepsia 2000, 41, S162–S167. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, C.; Hurst, R.S.; Flores-Hernández, J.; Hernández-Echeagaray, E.; Klapstein, G.J.; Boylan, M.K.; Calvert, C.R.; Jocoy, E.L.; Nguyen, O.K.; André, V.M.; et al. Morphological and electrophysiological characterization of abnormal cell types in pediatric cortical dysplasia. J. Neurosci. Res. 2003, 72, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Aronica, E.; Miyata, H.; Sarnat, H.B.; Thom, M.; Roessler, K.; Rydenhag, B.; Jehi, L.; Krsek, P.; Wiebe, S.; et al. International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue: A consensus Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 2016, 57, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Vinters, H.V.; Palmini, A.; Jacques, T.S.; Avanzini, G.; Barkovich, A.J.; Battaglia, G.; et al. The clinicopathologic spectrum of focal cortical dysplasias: A consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011, 52, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Cotter, D.; Honavar, M.; Lovestone, S.; Raymond, L.; Kerwin, R.; Anderton, B.; Everall, I. Disturbance of Notch-1 and Wnt signalling proteins in neuroglial balloon cells and abnormal large neurons in focal cortical dysplasia in human cortex. Acta Neuropathol. 1999, 98, 465–472. [Google Scholar] [CrossRef]
- Aizpuru, R.N.; Quencer, R.M.; Norenberg, M.; Altman, N.; Smirniotopoulos, J. Meningioangiomatosis: Clinical, radiologic, and histopathologic correlation. Radiology 1991, 179, 819–821. [Google Scholar] [CrossRef]
- Kim, N.R.; Cho, S.J.; Suh, Y.L. Allelic loss on chromosomes 1p32, 9p21, 13q14, 16q22, 17p, and 22q12 in meningiomas associated with meningioangiomatosis and pure meningioangiomatosis. J. Neurooncol. 2009, 94, 425–430. [Google Scholar] [CrossRef]
- Bonduelle, T.; Hartlieb, T.; Baldassari, S.; Sim, N.S.; Kim, S.H.; Kang, H.C.; Kobow, K.; Coras, R.; Chipaux, M.; Dorfmüller, G.; et al. Frequent SLC35A2 brain mosaicism in mild malformation of cortical development with oligodendroglial hyperplasia in epilepsy (MOGHE). Acta Neuropathol. Commun. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Elziny, S.; Crino, P.B.; Winawer, M. SLC35A2 somatic variants in drug resistant epilepsy: FCD and MOGHE. Neurobiol. Dis. 2023, 187, 106299. [Google Scholar] [CrossRef]
- Kobow, K.; Blümcke, I. Epigenetics in epilepsy. Neurosci. Lett. 2018, 667, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Akinsoji, E.O.; Leibovitch, E.; Billioux, B.J.; Abath Neto, O.L.; Ray-Chaudhury, A.; Inati, S.K.; Zaghloul, K.; Heiss, J.; Jacobson, S.; Theodore, W.H. HHV-6 and hippocampal volume in patients with mesial temporal sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 1674–1680. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).