Clinical Management in Multiple Sclerosis
Abstract
1. Introduction
Atypical Subtypes of MS
- 1
- Clinically Isolated Syndrome (CIS)
- 2
- Radiologically Isolated Syndrome (RIS)
- 3
- Balo’s Concentric Sclerosis (BCS)
- 4
- Schilder’s Disease (SD)
- 5
- Progressive-Relapsing MS (PRMS).
2. Possible Origin of MS
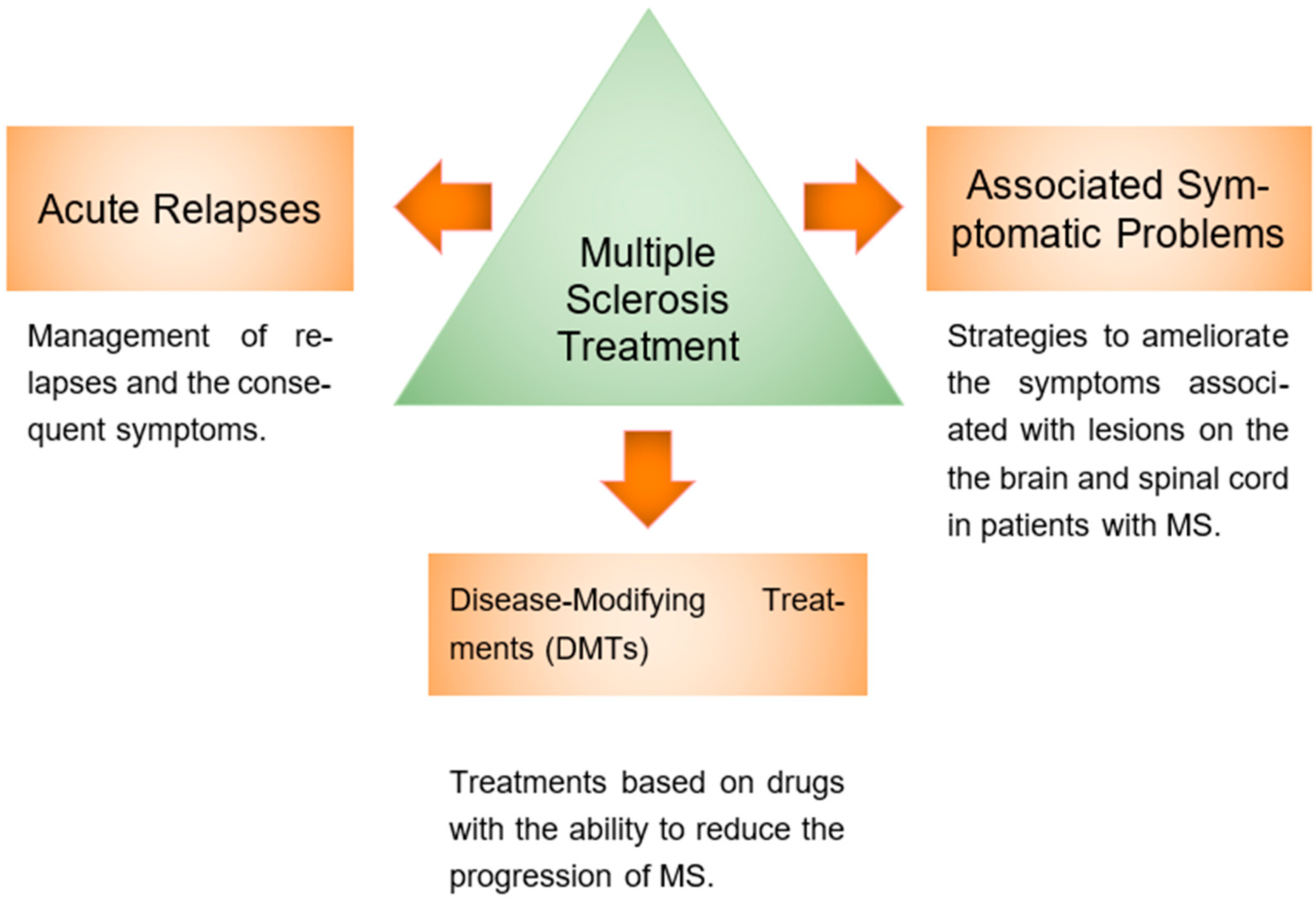
3. Multiple Sclerosis Treatment
3.1. MS Acute Relapses Treatment
| Category | Treatment | Mechanism of Action | Common Side Effects | References |
|---|---|---|---|---|
| Acute Relapse Management | Corticosteroids | Restores the blood–brain barrier and reduces metalloproteinase activity and mononuclear trafficking. | Gastrointestinal intolerance, insomnia, weight gain, osteoporosis. | [29,30,31,32] |
| Plasma Exchange (PE) and Intravenous Immunoglobulin G (IVIG) | Removes circulating antibodies; counteracts antibodies against myelin proteins. | Limited clinical efficacy. | [27,33,34,35,36] | |
| Symptomatic Treatments | Fatigue | Amantadine, modafinil, L-carnitine. | Insomnia, dizziness, nausea. | [37,38,39,40,41] |
| Depression | Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs). | Nausea, dry mouth, insomnia. | [42,43,44] | |
| Bladder dysfunction | Oxybutynin and tolterodine. | Dry mouth, blurred vision. | [44,45] | |
| Spasticity | Intrathecal baclofen, tizanidine, botulinum toxin type A, dantrolene, benzodiazepines. | Drowsiness, muscle weakness, hepatotoxicity (dantrolene). | [46,47,48,49,50,51,52] | |
| Disease-Modifying Therapies (DMTs) | Interferon beta | Modulates T- and B-cell functions; reduces inflammatory cytokines. | Flu-like symptoms and injection site reactions. | [53,54,55,56] |
| Glatiramer acetate | Stimulates specific suppressor T cells. | Local reactions and injection site pain. | [57,58,59] | |
| Fingolimod | Sphingosine-1-phosphate receptor agonist; inhibits lymphocyte exit from lymph nodes. | Infections and bradycardia. | [60,61] | |
| Teriflunomide | Dihydroorotate dehydrogenase inhibitor; blocks lymphocyte proliferation. | Alopecia, diarrhea, hypertension. | [61,62] | |
| Natalizumab | Inhibits leukocyte adhesion to endothelium; prevents immune cell migration into the CNS. | Risk of progressive multifocal leukoencephalopathy (PML). | [55,63,64,65,66] | |
| Dimethyl fumarate | Modulates proinflammatory T cells; activates antioxidant pathways. | Mild infections and gastrointestinal discomfort. | [61,67] | |
| Emerging Therapies | Alemtuzumab | Monoclonal antibody against CD52; induces cell lysis and prolonged lymphopenia. | Autoimmune reactions and infections. | [57,68,69] |
| Ocrelizumab | Monoclonal antibody against CD20; depletes mature B cells. | Respiratory infections and potential increased risk of neoplasms. | [70,71,72,73] |
3.1.1. Corticosteroids
3.1.2. Plasma Exchange (PE) and Intravenous Immunoglobulin G (IVIG)
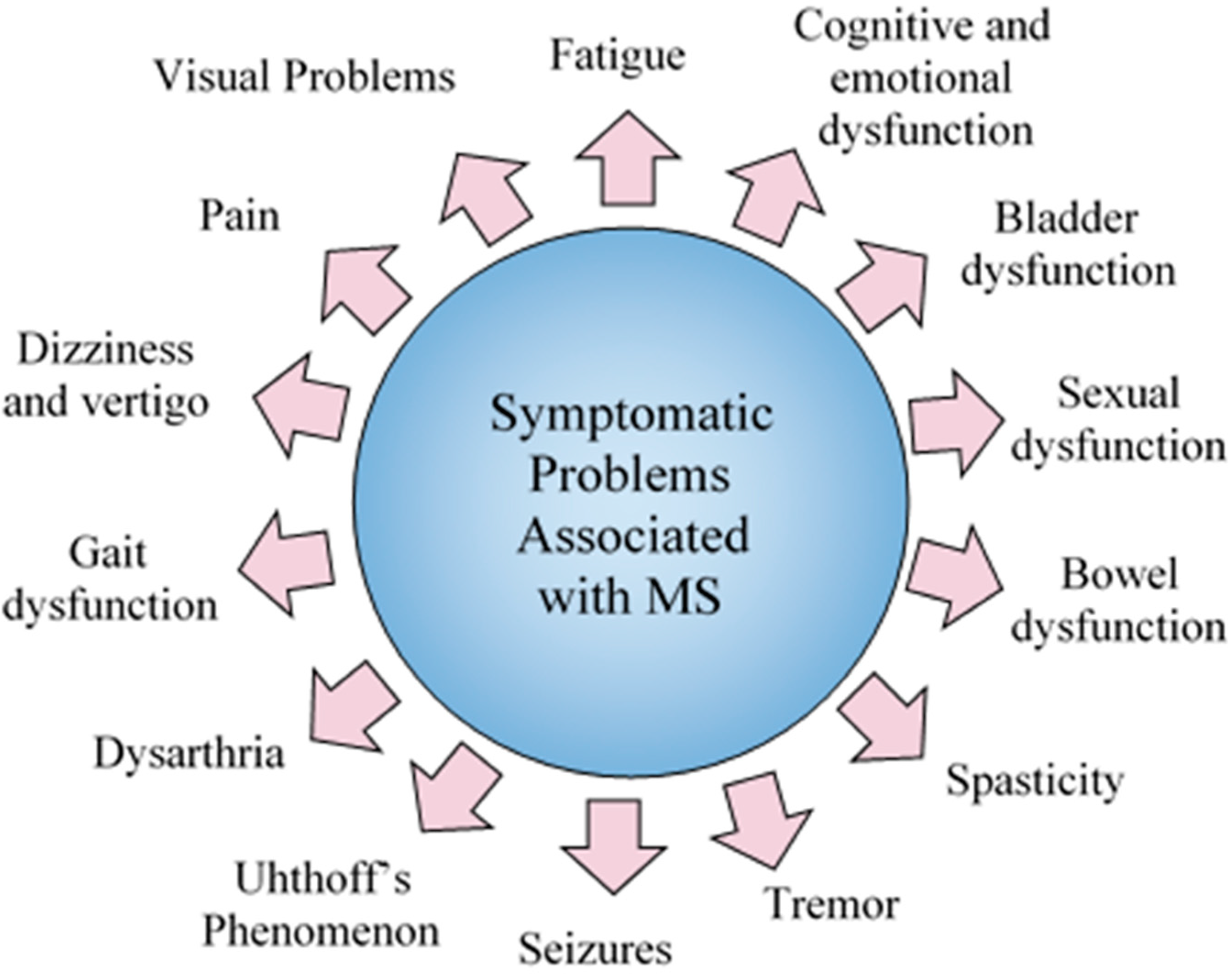
3.2. Symptomatic Problems
3.2.1. Fatigue
3.2.2. Cognitive and Emotional Dysfunction (Social Functioning)
3.2.3. Bladder Dysfunction
3.2.4. Male Sexual Dysfunction
3.2.5. Female Sexual Dysfunction
3.2.6. Bowel Dysfunction
3.2.7. Spasticity
3.2.8. Seizures
3.2.9. Uhthoff’s Phenomenon
3.2.10. Dysarthria
3.2.11. Gait Dysfunction
3.2.12. Dizziness and Vertigo
3.2.13. Pain
3.2.14. Visual Problems
3.2.15. Neurorehabilitation
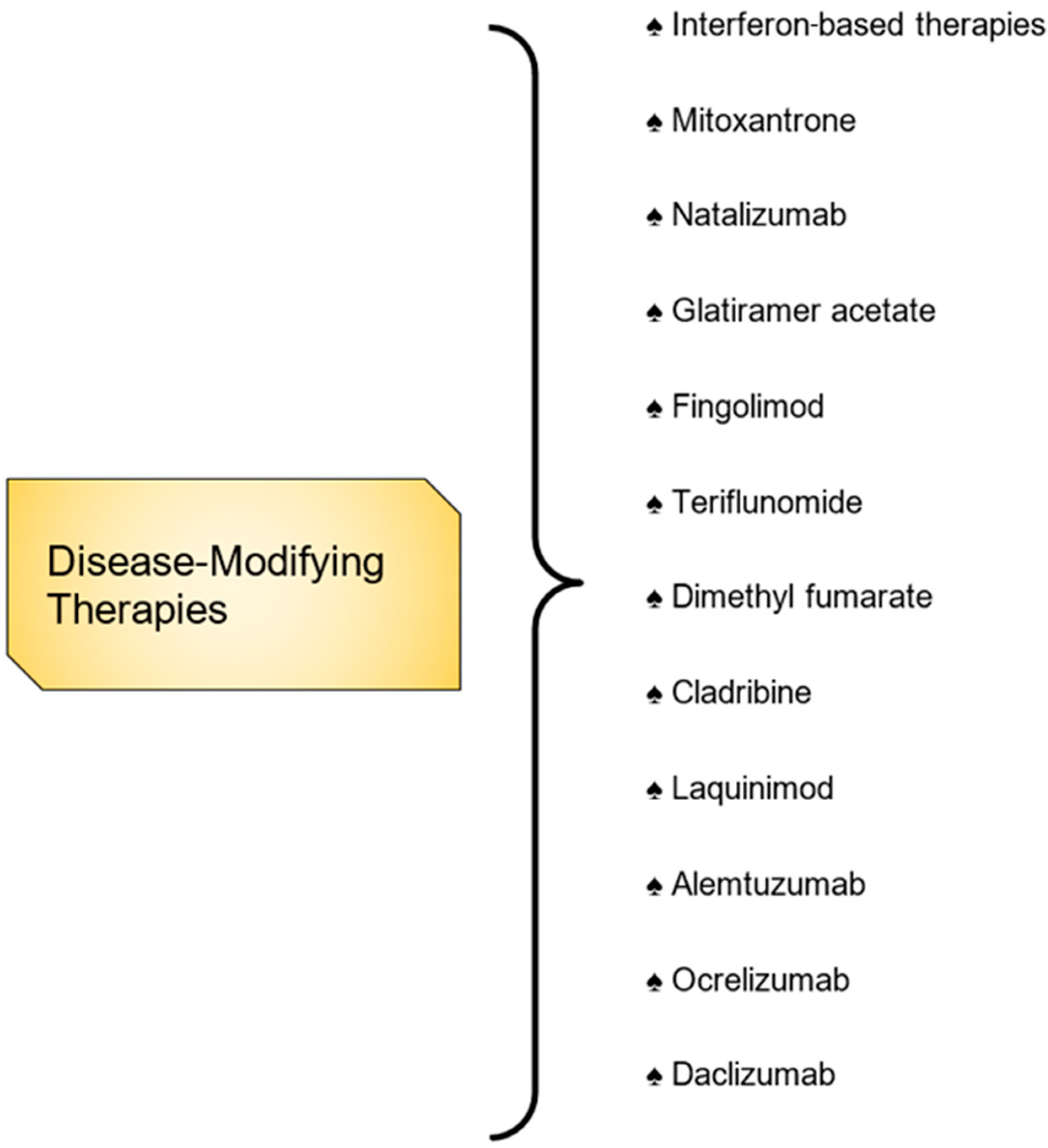
3.3. Disease-Modifying Therapies
3.3.1. Self-Injectables Therapies
3.3.2. Interferon-Based Therapies
3.3.3. General Immunosuppression
3.3.4. Oral DMTs
3.4. Emerging Therapies
3.4.1. Alemtuzumab
3.4.2. Ocrelizumab
3.4.3. Daclizumab
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mörk, S.; Bö, L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Ransohoff, R.M.; Fisher, E.; Rudick, R.A. Neurodegeneration in multiple sclerosis: Relationship to neurological disability. Neuroscientist 1999, 5, 48–57. [Google Scholar] [CrossRef]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. N. Engl. J. Med. 2006, 354, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Prescott, J.; Sr Director, C.P. Communications Naomi Musaji, AJMC ® Perspectives MARCH 2018 3 PERSPECTIVES IN MS PERSPECTIVES IN MS Senior Vice President, Managed Markets. 2018. Available online: www.ajmc.com (accessed on 10 September 2021).
- Whitacre, C.C. Sex differences in autoimmune disease. Nat. Immunol. 2001, 2, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Bove, R.; Chitnis, T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult. Scler. J. 2014, 20, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Zeydan, B.; Kantarci, O.H. Impact of age on multiple sclerosis disease activity and progression. Curr. Neurol. Neurosci. Rep. 2020, 20, 24. [Google Scholar] [CrossRef]
- Habbestad, A.; Willumsen, J.S.; Aarseth, J.H.; Grytten, N.; Midgard, R.; Wergeland, S.; Myhr, K.M.; Torkildsen, Ø. Increasing age of multiple sclerosis onset from 1920 to 2022: A population-based study. J. Neurol. 2024, 271, 1610–1617. [Google Scholar] [CrossRef]
- Prosperini, L.; Lucchini, M.; Ruggieri, S.; Tortorella, C.; Haggiag, S.; Mirabella, M.; Pozzilli, C.; Gasperini, C. Shift of multiple sclerosis onset towards older age. J. Neurol. Neurosurg. Psychiatry 2022, 93, 1137–1139. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Thygesen, L.C.; Stenager, E.; Laursen, B.; Magyari, M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology 2018, 90, e1954–e1963. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Miller, D.H. Clinically isolated syndromes and the relationship to multiple sclerosis. J. Clin. Neurosci. 2014, 21, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Frenay, C.; Kantarci, O.; Siva, A.; Azevedo, C.J.; Makhani, N.; Pelletier, D.; Okuda, D.T. Radiologically isolated syndrome. Lancet Neurol. 2023, 22, 1075–1086. [Google Scholar] [CrossRef]
- Hardy, T.A.; Miller, D.H. Baló’s concentric sclerosis. Lancet Neurol. 2014, 13, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Kraus, D.; Konen, O.; Straussberg, R. Schilder’s disease: Non-invasive diagnosis and successful treatment with human immunoglobulins. Eur. J. Paediatr. Neurol. 2012, 16, 206–208. [Google Scholar] [CrossRef] [PubMed]
- McKay, K.A.; Kwan, V.; Duggan, T.; Tremlett, H. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: A systematic review. BioMed Res. Int. 2015, 2015, 817238. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M. Have we finally identified an autoimmune demyelinating disease? Ann. Neurol. 2009, 66, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Wootla, B.; Eriguchi, M.; Rodriguez, M. Is multiple sclerosis an autoimmune disease? Autoimmune Dis. 2012, 2012, 1–12. [Google Scholar] [CrossRef]
- Miller, D.; Barkhof, F.; Montalban, X.; Thompson, A.; Filippi, M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: Natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005, 4, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Behan, P.O. Multiple Sclerosis Is Not an Autoimmune Disease. Arch Neurol. 2004, 61, 1610. [Google Scholar] [CrossRef]
- Kasper, L.H.; Shoemaker, J. Multiple sclerosis immunology: The healthy immune system vs the MS immune system. Neurology 2010, 74 (Suppl. S1), S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, B.W.; Lai, M.; Hodgkinson, S.; Barkhof, F.; Miller, D.H.; Moseley, I.F.; Ader, H.J. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: Results of a randomized, double-blind, placebo-controlled MR-monitored phase II trial. Neurology 1997, 49, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH 17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef]
- Johnson, A.J.; Suidan, G.L.; McDole, J.; Pirko, I. The CD8 T cell in multiple sclerosis: Suppressor cell or mediator of neuropathology? Int. Rev. Neurobiol. 2007, 79, 73–97. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Miller, S.D.; Jenkins, M.K. Neuroantigen-specific Th2 cells are inefficient suppressors of experimental autoimmune encephalomyelitis induced by effector Th1 cells. J. Immunol. 1995, 155, 5011–5017. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16, s53–s59. [Google Scholar] [CrossRef]
- Perry, M.; Swain, S.; Kemmis-Betty, S.; Cooper, P. Multiple sclerosis: Summary of NICE guidance. BMJ 2014, 349, g5701. [Google Scholar] [CrossRef]
- Frohman, E.M.; Shah, A.; Eggenberger, E.; Metz, L.; Zivadinov, R.; Stüve, O. Corticosteroids for multiple sclerosis: I. application for treating exacerbations. Neurotherapeutics 2007, 4, 618–626. [Google Scholar] [CrossRef]
- Ciccone, A.; Beretta, S.; Brusaferri, F.; Galea, I.; Protti, A.; Spreafico, C. Corticosteroids for the long-term treatment in multiple sclerosis. Cochrane Database Syst. Rev. 2008, CD006264. [Google Scholar] [CrossRef] [PubMed]
- Ontaneda, D.; Rae-Grant, A.D. Management of acute exacerbations in multiple sclerosis. Ann. Indian Acad. Neurol. 2009, 12, 264. [Google Scholar] [CrossRef]
- Tumani, H. Corticosteroids and plasma exchange in multiple sclerosis. J. Neurol. 2008, 255, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Khatri, B.O.; McQuillen, M.P.; Hoffmann, R.G.; Harrington, G.J.; Schmoll, D. Plasma exchange in chronic progressive multiple sclerosis. Neurology 1991, 41, 409. [Google Scholar] [CrossRef]
- Linker, R.A.; Chan, A.; Sommer, M.; Koziolek, M.; Müller, G.-A.; Paulus, W.; Gold, R. Plasma exchange therapy for steroid-refractory superimposed relapses in secondary progressive multiple sclerosis. J. Neurol. 2007, 254, 1288–1289. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, P.S. The role of intravenous immunoglobulin in the treatment of multiple sclerosis. J. Neurol. Sci. 2003, 206, 123–130. [Google Scholar] [CrossRef]
- Elovaara, I.; Apostolski, S.; Van Doorn, P.; Gilhus, N.E.; Hietaharju, A.; Honkaniemi, J.; Udd, B. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur. J. Neurol. 2008, 15, 893–908. [Google Scholar] [CrossRef] [PubMed]
- Glanz, B.I.; Dégano, I.; Rintell, D.; Chitnis, T.; Weiner, H.L.; Healy, B. Work Productivity in Relapsing Multiple Sclerosis: Associations with Disability, Depression, Fatigue, Anxiety, Cognition, and Health-Related Quality of Life. Value Health 2012, 15, 1029–1035. [Google Scholar] [CrossRef]
- Pucci, E.; Brañas Tato, P.; D’Amico, R.; Giuliani, G.; Solari, A.; Taus, C. Amantadine for fatigue in multiple sclerosis. Cochrane Database Syst. Rev. 2007, CD002818. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Pasqualetti, P.; Pozzilli, C.; Grasso, M.G.; Millefiorini, E.; Graceffa, A.; Caltagirone, C. Fatigue in progressive multiple sclerosis: Results of a randomized, double-blind, placebo-controlled, crossover trial of oral 4-aminopyridine. Mult. Scler. J. 2001, 7, 354–358. [Google Scholar] [CrossRef]
- Brenner, P.; Piehl, F. Fatigue and depression in multiple sclerosis: Pharmacological and non-pharmacological interventions. Acta Neurol. Scand. 2016, 134, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, V.; Pozzilli, C.; Onesti, E.; Pasqualetti, P.; Marinelli, F.; Pisani, A.; Fieshchi, C. Comparison of the effects of acetyl L-carnitine and amantadine for the treatment of fatigue in multiple sclerosis: Results of a pilot, randomized, double-blind, crossover trial. J. Neurol. Sci. 2004, 218, 103–108. [Google Scholar] [CrossRef]
- Marrie, R.A.; Zhang, L.; Lix, L.M.; Graff, L.A.; Walker, J.R.; Fisk, J.D.; Patten, S.B.; Hitchon, C.A.; Bolton, J.M.; Sareen, J.; et al. The validity and reliability of screening measures for depression and anxiety disorders in multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 20, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Brassington, J.C.; Marsh, N.V. Neuropsychological Aspects of Multiple Sclerosis. Neuropsychol. Rev. 1998, 8, 43–77. [Google Scholar] [CrossRef] [PubMed]
- Kesselring, J.; Beer, S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol. 2005, 4, 643–652. [Google Scholar] [CrossRef] [PubMed]
- DasGupta, R.; Fowler, C.J. Bladder, Bowel and Sexual Dysfunction in Multiple Sclerosis. Ther. Pract. 2003, 63, 153–166. [Google Scholar] [CrossRef]
- Kesselring, J. Chapter 14 Complications of Multiple Sclerosis: Fatigue; Spasticity; Ataxia; Pain; and Bowel, Bladder, and Sexual Dysfunction. In Blue Books of Practical Neurology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 27, pp. 217–227. [Google Scholar] [CrossRef]
- Krach, L.E. Pharmacotherapy of spasticity: Oral medications and intrathecal baclofen. J. Child Neurol. 2001, 16, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, S.A.; Wang, G.C. Long–term intrathecal baclofen therapy in ambulatory patients with spasticity. J. Neurol. 2006, 253, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Rekand, T.; Grønning, M. Treatment of spasticity related to multiple sclerosis with intrathecal baclofen: A long-term follow-up. J. Rehabil. Med. 2011, 43, 511–514. [Google Scholar] [CrossRef]
- Coward, D.M. Tizanidine: Neuropharmacology and mechanism of action. Neurology 1994, 44 (Suppl. S9), S6–S10, discussion S10–S11. [Google Scholar] [PubMed]
- Ghanavatian, S.; Derian, A. Tizanidine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kamen, L.; Henney, H.R., III; Runyan, J.D. A practical overview of tizanidine use for spasticity secondary to multiple sclerosis, stroke, and spinal cord injury. Curr. Med. Res. Opin. 2008, 24, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; O’Connor, P.W. Established disease-modifying treatments in relapsing-remitting multiple sclerosis. Curr. Opin. Neurol. 2015, 28, 220–229. [Google Scholar] [CrossRef]
- Yong, K.P.; Kim, H.J. Disease modifying therapies and infection risks in multiple sclerosis-a decision-making conundrum. Ann. Transl. Med. 2020, 8, 722. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Carter, J.L. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 2014, 89, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, A.; Loebermann, M.; Reisinger, E.C.; Hartung, H.P.; Zettl, U.K. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat. Rev. Neurol. 2016, 12, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Weinstock-Guttman, B.; Nair, K.V.; Glajch, J.L.; Ganguly, T.C.; Kantor, D. Two decades of glatiramer acetate: From initial discovery to the current development of generics. J. Neurol. Sci. 2017, 376, 255–259. [Google Scholar] [CrossRef]
- Rocco, P.; Eberini, I.; Musazzi, U.M.; Franzè, S.; Minghetti, P. Glatiramer acetate: A complex drug beyond biologics. Eur. J. Pharm. Sci. 2019, 133, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Hall, S.; Grabner, M.; Balu, S.; Zhang, X.; Kantor, D. Multiple sclerosis relapse rates and healthcare costs of two versions of glatiramer acetate. Curr. Med. Res. Opin. 2020, 36, 1167–1175. [Google Scholar] [CrossRef]
- Scott, L.J. Fingolimod: A review of its use in the management of relapsing-remitting multiple sclerosis. CNS Drugs 2011, 25, 673–698. [Google Scholar] [CrossRef]
- Pardo, G.; Jones, D.E. The sequence of disease-modifying therapies in relapsing multiple sclerosis: Safety and immunologic considerations. J. Neurol. 2017, 264, 2351–2374. [Google Scholar] [CrossRef]
- He, D.; Zhang, C.; Zhao, X.; Zhang, Y.; Dai, Q.; Li, Y.; Chu, L. Teriflunomide for multiple sclerosis. Cochrane Database Syst. Rev. 2016, 3, CD009882. [Google Scholar] [CrossRef]
- Filippini, G.; Del Giovane, C.; Clerico, M.; Beiki, O.; Mattoscio, M.; Piazza, F.; Fredrikson, S.; Tramacere, I.; Scalfari, A.; Salanti, G. Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis. Cochrane Database Syst. Rev. 2017, 4, CD012200. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, L.Z.; Foley, J.; Chang, I.; Kister, I.; Cutter, G.; Metzger, R.R.; Goldberg, J.D.; Li, X.; Riddle, E.; Smirnakis, K.; et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology 2019, 93, 1452–1462. [Google Scholar] [CrossRef]
- D’Amico, E.; Zanghì, A.; Leone, C.; Tumani, H.; Patti, F. Treatment-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: A Comprehensive Review of Current Evidence and Future Needs. Drug Saf. 2016, 39, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Ho, P.R.; Campbell, N.; Chang, I.; Deykin, A.; Forrestal, F.; Lucas, N.; Yu, B.; Arnold, D.L.; Freedman, M.S.; et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018, 17, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Montes Diaz, G.; Fraussen, J.; Van Wijmeersch, B.; Hupperts, R.; Somers, V. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci. Rep. 2018, 8, 8194. [Google Scholar] [CrossRef]
- Ruck, T.; Bittner, S.; Wiendl, H.; Meuth, S.G. Alemtuzumab in Multiple Sclerosis: Mechanism of Action and Beyond. Int. J. Mol. Sci. 2015, 16, 16414–16439. [Google Scholar] [CrossRef] [PubMed]
- Riera, R.; Porfírio, G.J.; Torloni, M.R. Alemtuzumab for multiple sclerosis. Cochrane Database Syst. Rev. 2016, 4, CD011203. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Sorensen, P.S.; Blinkenberg, M. The potential role for ocrelizumab in the treatment of multiple sclerosis: Current evidence and future prospects. Ther. Adv. Neurol. Disord. 2016, 9, 44–52. [Google Scholar] [CrossRef]
- Barkhof, F.; Kappos, L.; Wolinsky, J.S.; Li, D.; Bar-Or, A.; Hartung, H.P.; Belachew, S.; Han, J.; Julian, L.; Sauter, A.; et al. Onset of clinical and MRI efficacy of ocrelizumab in relapsing multiple sclerosis. Neurology 2019, 93, 1778–1786. [Google Scholar] [CrossRef]
- Mulero, P.; Midaglia, L.; Montalban, X. Ocrelizumab: A new milestone in multiple sclerosis therapy. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418773025. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, J.; Luo, H. Sildenafil citrate for erectile dysfunction in patients with multiple sclerosis. Cochrane Database Syst. Rev. 2012, CD009427. [Google Scholar] [CrossRef] [PubMed]
- Nortvedt, M.W.; Riise, T.; Myhr, K.-M.; Landtblom, A.-M.; Bakke, A.; Nyland, H.I. Reduced quality of life among multiple sclerosis patients with sexual disturbance and bladder dysfunction. Mult. Scler. J. 2001, 7, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zorzon, M.; Zivadinov, R.; Bosco, A.; Bragadin, L.M.; Moretti, R.; Bonfigli, L.; Morassi, P.; Iona, L.G.; Cazzato, G. Sexual dysfunction in multiple sderosis: A case-control study. 1. Frequency and comparison of groups. Mult. Scler. J. 1999, 5, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Hyman, N.; Barnes, M.; Bhakta, B.; Cozens, A.; Bakheit, M.; Kreczy-Kleedorfer, B.; Poewe, W.; Wissel, J.; Bain, P.; Glickman, S.; et al. Botulinum toxin (Dysport®) treatment of hip adductor spasticity in multiple sclerosis: A prospective, randomised, double blind, placebo controlled, dose ranging study. J. Neurol. Neurosurg. Psychiatry 2000, 68, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Moccia, M.; Frau, J.; Carotenuto, A.; Butera, C.; Coghe, G.; Barbero, P.; Frontoni, M.; Groppo, E.; Giovannelli, M.; Del Carro, U.; et al. Botulinum toxin for the management of spasticity in multiple sclerosis: The Italian botulinum toxin network study. Neurol. Sci. 2020, 41, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Muehlschlegel, S.; Sims, J.R. Dantrolene: Mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocritical Care 2009, 10, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ratto, D.; Joyner, R.W. Dantrolene. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Haselkorn, J.K.; Balsdon Richer, C.; Fry Welch, D.; Herndon, R.M.; Johnson, B.; Little, J.W.; Miller, J.R.; Rosenberg, J.H.; Seidle, M.E. Multiple Sclerosis Council for Clinical Practice Guidelines, Overview of spasticity management in multiple sclerosis. Evidence-based management strategies for spasticity treatment in multiple sclerosis. J. Spinal Cord Med. 2005, 28, 167–199. [Google Scholar] [CrossRef] [PubMed]
- Bateson, A.N. The benzodiazepine site of the GABAA receptor: An old target with new potential? Sleep Med. 2004, 5, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Markota, M.; Rummans, T.A.; Bostwick, J.M.; Lapid, M.I. Benzodiazepine use in older adults: Dangers, management, and alternative therapies. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2016; Volume 91, pp. 1632–1639. [Google Scholar] [CrossRef]
- Barnes, M.P. Sativex®: Clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin. Pharmacother. 2006, 7, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Patti, F.; Messina, S.; Solaro, C.; Amato, M.P.; Bergamaschi, R.; Bonavita, S.; Bossio, R.B.; Morra, V.B.; Costantino, G.F.; Cavalla, P.; et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J. Neurol. Neurosurg. Psychiatry 2016, 87, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Patti, F.; Chisari, C.G.; Solaro, C.; Benedetti, M.D.; Berra, E.; Bianco, A.; Bossio, R.B.; Buttari, F.; Castelli, L.; Cavalla, P.; et al. Effects of THC/CBD oromucosal spray on spasticity-related symptoms in people with multiple sclerosis: Results from a retrospective multicenter study. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 2905–2913. [Google Scholar] [CrossRef]
- Alusi, S.H. Evaluation of three different ways of assessing tremor in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Meador, W.; Salter, A.R.; Rinker, J.R. Symptomatic management of multiple sclerosis–associated tremor among participants in the NARCOMS registry. Int. J. MS Care 2016, 18, 147–153. [Google Scholar] [CrossRef]
- Tzellos, T.G.; Papazisis, G.; Toulis, K.A.; Sardeli, C.; Kouvelas, D. A2δ ligands gabapentin and pregabalin: Future implications in daily clinical practice. Hippokratia 2010, 14, 71. [Google Scholar] [PubMed]
- Raju, S.S.; Niranjan, A.; Monaco, E.A.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery for medically refractory multiple sclerosis–related tremor. J. Neurosurg. 2018, 128, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Spatt, J.; Chaix, R.; Mamoli, B. Epileptic and non-epileptic seizures in multiple sclerosis. J. Neurol. 2001, 248, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B.J.; Rodriguez, M. Seizures in patients with multiple sclerosis. CNS Drugs 2009, 23, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Flesch, G.; Czendlik, C.; Renard, D.; Lloyd, P. Pharmacokinetics of the monohydroxy derivative of oxcarbazepine and its enantiomers after a single intravenous dose given as racemate compared with a single oral dose of oxcarbazepine. Drug Metab. Dispos. 2011, 39, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Farooque, P.; Detyniecki, K.; Mattson, R.H. Epilepsy; Antiepileptic drug profiles. In Encyclopedia of the Neurological Sciences; Elsevier: Amsterdam, The Netherlands, 2014; pp. 81–92. [Google Scholar] [CrossRef]
- Dagiasi, I.; Vall, V.; Kumlien, E.; Burman, J.; Zelano, J. Treatment of epilepsy in multiple sclerosis. Seizure 2018, 58, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; Petrou, S. Network-specific mechanisms may explain the paradoxical effects of carbamazepine and phenytoin. Epilepsia 2013, 54, 1195–1202. [Google Scholar] [CrossRef]
- Frohman, T.C.; Davis, S.L.; Beh, S.; Greenberg, B.M.; Remington, G.; Frohman, E.M. Uhthoff’s phenomena in MS—Clinical features and pathophysiology. Nat. Rev. Neurol. 2013, 9, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, H.K.; Sorensen, P.S. Prolonged-release fampridine improves walking in a proportion of patients with multiple sclerosis. Expert Rev. Neurother. 2013, 13, 1309–1317. [Google Scholar] [CrossRef]
- Piacentini, V.; Mauri, I.; Cattaneo, D.; Gilardone, M.; Montesano, A.; Schindler, A. Relationship between quality of life and dysarthria in patients with multiple sclerosis. Arch. Phys. Med. Rehabil. 2014, 95, 2047–2054. [Google Scholar] [CrossRef]
- Hartelius, L.; Runmarker, B.; Andersen, O. Prevalence and characteristics of dysarthria in a multiple-sclerosis incidence cohort: Relation to neurological data. Folia Phoniatr. Logop. 2000, 52, 160–177. [Google Scholar] [CrossRef]
- Rusz, J.; Vaneckova, M.; Benova, B.; Tykalova, T.; Novotny, M.; Ruzickova, H.; Uher, T.; Andelova, M.; Novotna, K.; Friedova, L.; et al. Brain volumetric correlates of dysarthria in multiple sclerosis. Brain Lang. 2019, 194, 58–64. [Google Scholar] [CrossRef]
- Goodwin, S.J.; Carpenter, A.F. Successful treatment of paroxysmal ataxia and dysarthria in multiple sclerosis with levetiracetam. Mult. Scler. Relat. Disord. 2016, 10, 79–81. [Google Scholar] [CrossRef]
- Stevens, V.; Goodman, K.; Rough, K.; Kraft, G.H. Gait impairment and optimizing mobility in multiple sclerosis. Phys. Med. Rehabil. Clin. 2013, 24, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 159, pp. 237–250. [Google Scholar] [CrossRef]
- Egeberg, M.D.; Oh, C.Y.; Bainbridge, J.L. Clinical overview of dalfampridine: An agent with a novel mechanism of action to help with gait disturbances. Clin. Ther. 2012, 34, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.G.; Neri SG, R.; Motl, R.W.; Tauil, C.B.; von Glehn Silva, F.; Corrêa, É.C.; de David, A.C. Effect of hippotherapy on walking performance and gait parameters in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 43, 102203. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Cutter, G.R.; Tyry, T. Substantial burden of dizziness in multiple sclerosis. Mult. Scler. Relat. Disord. 2013, 2, 21–28. [Google Scholar] [CrossRef]
- Tjernström, F.; Zur, O.; Jahn, K. Current concepts and future approaches to vestibular rehabilitation. J. Neurol. 2016, 263, 65–70. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, C.; Cortés-Vega, M.D.; Heredia-Rizo, A.M.; Martín-Valero, R.; García-Bernal, M.I.; Casuso-Holgado, M.J. Effectiveness of Vestibular Training for Balance and Dizziness Rehabilitation in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, F.; Centonze, D.; Galgani, S.; Grasso, M.G.; Haggiag, S.; Strano, S. Painful and involuntary multiple sclerosis. Expert Opin. Pharmacother. 2011, 12, 763–777. [Google Scholar] [CrossRef]
- Scherder, R.J.; Kant, N.; Wolf, E.T.; Pijnenburg, B.; Scherder, E. Sensory Function and Chronic Pain in Multiple Sclerosis. Pain Res. Manag. 2018, 2018, 1924174. [Google Scholar] [CrossRef] [PubMed]
- Scherder, R.; Kant, N.; Wolf, E.; Pijnenburg, A.; Scherder, E. Pain and Cognition in Multiple Sclerosis. Pain Med. 2017, 18, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Kesselring, J. Neurorehabilitation in multiple sclerosis—What is the evidence-base? J. Neurol. 2004, 251 (Suppl. S4), IV25–IV29. [Google Scholar] [CrossRef]
- Costello, F. Vision Disturbances in Multiple Sclerosis. Semin. Neurol. 2016, 36, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Noval, S.; Contreras, I.; Muñoz, S.; Oreja-Guevara, C.; Manzano, B.; Rebolleda, G. Optical coherence tomography in multiple sclerosis and neuromyelitis optica: An update. Mult. Scler. Int. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Dalmau, B.; Martinez-Lapiscina, E.H.; Pulido-Valdeolivas, I.; Zubizarreta, I.; Llufriu, S.; Blanco, Y.; Sola-Valls, N.; Sepulveda, M.; Guerrero, A.; Alba, S.; et al. Predictors of vision impairment in Multiple Sclerosis. PLoS ONE 2018, 13, e0195856. [Google Scholar] [CrossRef] [PubMed]
- Nij Bijvank, J.A.; Petzold, A.; Coric, D.; Tan, H.S.; Uitdehaag, B.; Balk, L.J.; van Rijn, L.J. Quantification of Visual Fixation in Multiple Sclerosis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Balk, L.J.; Coric, D.; Nij Bijvank, J.A.; Killestein, J.; Uitdehaag, B.M.; Petzold, A. Retinal atrophy in relation to visual functioning and vision-related quality of life in patients with multiple sclerosis. Mult. Scler. 2018, 24, 767–776. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Neurological Disorders [OP]: Public Health Challenges (1.a ed.). 2006. Available online: https://www.who.int/publications/i/item/9789241563369 (accessed on 10 September 2021).
- Calabresi, P.A. Diagnosis and management of multiple sclerosis. Am. Fam. Physician 2004, 70, 1935–1944. [Google Scholar] [PubMed]
- Miller, E.; Morel, A.; Redlicka, J.; Miller, I.; Saluk, J. Pharmacological and Non-pharmacological Therapies of Cognitive Impairment in Multiple Sclerosis. Curr. Neuropharmacol. 2018, 16, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kubsik-Gidlewska, A.M.; Klimkiewicz, P.; Klimkiewicz, R.; Janczewska, K.; Woldańska-Okońska, M. Rehabilitation in multiple sclerosis. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2017, 26, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Mohammad, A.; Rastogi, Y.R.; Saini, R.V.; Saini, A.K. Yoga as an intervention to manage multiple sclerosis symptoms. J. Ayurveda Integr. Med. 2020, 11, 114–117. [Google Scholar] [CrossRef] [PubMed]
- de Padilla, C.M.L.; Niewold, T.B. The type I interferons: Basic concepts and clinical relevance in immune-mediated inflammatory diseases. Gene 2016, 576, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Reder, A.T. Therapeutic role of beta-interferons in multiple sclerosis. Pharmacol. Ther. 2006, 110, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Kieseier, B.C.; Calabresi, P.A. PEGylation of Interferon-β-1a. CNS Drugs 2012, 26, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Newsome, S.D.; Kieseier, B.C.; Liu, S.; You, X.; Kinter, E.; Hung, S.; Sperling, B. Peginterferon beta-1a reduces disability worsening in relapsing–remitting multiple sclerosis: 2-year results from ADVANCE. Ther. Adv. Neurol. Disord. 2017, 10, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G. Disease-modifying treatments for early and advanced multiple sclerosis: A new treatment paradigm. Curr. Opin. Neurol. 2018, 31, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Zandoná, M.E.; Kim, S.H.; Kim, H.J. Oral disease-modifying therapies for multiple sclerosis. J. Clin. Neurol. 2015, 11, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Pachner, A.; Menguy-Vacheron, F.; Kaplan, J.; Wiendl, H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014, 74, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Leist, T.; Comi, G.; Montalban, X.; Giovannoni, G.; Nolting, A.; Hicking, C.; Galazka, A.; Sylvester, E. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Mult. Scler. Relat. Disord. 2019, 29, 157–167. [Google Scholar] [CrossRef]
- Giovannoni, G.; Comi, G.; Cook, S.; Rammohan, K.; Rieckmann, P.; Soelberg Sørensen, P.; Vermersch, P.; Chang, P.; Hamlett, A.; Musch, B.; et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.M.; Ammoscato, F.; Giovannoni, G.; Baker, D.; Schmierer, K. Cladribine: Mechanisms and mysteries in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Cladribine Tablets: A Review in Relapsing MS. CNS Drugs 2018, 32, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Thöne, J.; Linker, R.A. Laquinimod in the treatment of multiple sclerosis: A review of the data so far. Drug Des. Dev. Ther. 2016, 10, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Preiningerova, J. Oral laquinimod therapy in relapsing multiple sclerosis. Expert Opin. Investig. Drugs 2009, 18, 985–989. [Google Scholar] [CrossRef]
- Kieseier, B.C. Defining a role for laquinimod in multiple sclerosis. Ther. Adv. Neurol. Disord. 2014, 7, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Varrin-Doyer, M.; Zamvil, S.S.; Schulze-Topphoff, U. Laquinimod, an up-and-coming immunomodulatory agent fortreatment of multiple sclerosis. Exp. Neurol. 2014, 262 Pt A, 66–71. [Google Scholar] [CrossRef]
- Baldassari, L.E.; Rose, J.W. Daclizumab: Development, Clinical Trials, and Practical Aspects of Use in Multiple Sclerosis. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2017, 14, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Bielekova, B. Daclizumab Therapy for Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a034470. [Google Scholar] [CrossRef] [PubMed]
- Milo, R. The efficacy and safety of daclizumab and its potential role in the treatment of multiple sclerosis. Ther. Adv. Neurol. Disord. 2014, 7, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Lugaresi, A.; di Ioia, M.; Travaglini, D.; Pietrolongo, E.; Pucci, E.; Onofrj, M. Risk-benefit considerations in the treatment of relapsing-remitting multiple sclerosis. Neuropsychiatr. Dis. Treat. 2013, 9, 893–914. [Google Scholar] [CrossRef]
| Clinical Presentation | Additional Criteria |
|---|---|
| In patients with an attack * at onset | |
| ≥2 attacks and clinical evidence of ≥2 lesions or ≥2 attacks and clinical evidence of 1 lesion + evidence of previous attacks with lesions in different anatomical areas implicated. | No additional tests to demonstrate dissemination across space and time. |
| ≥2 attacks and clinical evidence of 1 lesion. | Dissemination across space demonstrated by an additional clinical attack implicating a different CNS site or by ≥1 MS-typical T2 lesions in ≥2 areas of CNS: periventricular, cortical, juxtacortical, infratentorial, or spinal cord. |
| 1 attack and clinical evidence of ≥2 lesions. | Dissemination across time demonstrated by an additional clinical attack, or concurrent enhancing and non-enhancing MS-typical MRI lesions, or hyperintense lesions on T2-weighted MRI, or enhancing MRI lesion compared to baseline scan, or cerebrospinal fluid oligoclonal bands. |
| 1 attack and clinical evidence of 1 lesion. | Dissemination across space demonstrated by an additional clinical attack involving a different CNS site or by ≥1 MS-typical T2 lesions in ≥2 CNS locations: periventricular, cortical, juxtacortical, infratentorial or spinal cord; and dissemination across time determined by an additional clinical attack or concurrent enhancing and non-enhancing MS-typical MRI lesions, or hyperintense lesions on T2-weighted MRI, or enhancing MRI lesion compared to baseline scan or cerebrospinal fluid oligoclonal bands. |
| Patients with steady progression of disease since onset | |
| 1 year of disease progression (retrospective or prospective) | Dissemination across space demonstrated by two of the following: 1 or more MS-typical hyperintense lesions on T2-weighted MRI (periventricular, cortical, juxtacortical or infratentorial), 2 or more T2 spinal cord lesions, or cerebrospinal fluid oligoclonal bands. No distinction between symptomatic and asymptomatic MRI lesions is required. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arredondo-Robles, A.V.; Rodríguez-López, K.P.; Ávila-Avilés, R.D. Clinical Management in Multiple Sclerosis. Neuroglia 2025, 6, 6. https://doi.org/10.3390/neuroglia6010006
Arredondo-Robles AV, Rodríguez-López KP, Ávila-Avilés RD. Clinical Management in Multiple Sclerosis. Neuroglia. 2025; 6(1):6. https://doi.org/10.3390/neuroglia6010006
Chicago/Turabian StyleArredondo-Robles, Ana Victoria, Karen Paola Rodríguez-López, and Rodolfo Daniel Ávila-Avilés. 2025. "Clinical Management in Multiple Sclerosis" Neuroglia 6, no. 1: 6. https://doi.org/10.3390/neuroglia6010006
APA StyleArredondo-Robles, A. V., Rodríguez-López, K. P., & Ávila-Avilés, R. D. (2025). Clinical Management in Multiple Sclerosis. Neuroglia, 6(1), 6. https://doi.org/10.3390/neuroglia6010006





