Abstract
The human central nervous system is convolutedly connected to the gut microbiome, a diverse community of microorganisms residing in the gastrointestinal tract. Recent research has highlighted the bidirectional communication between the gut microbiome and neuroglial cells, which include astrocytes, microglia, oligodendrocytes, and ependymal cells. These neuroglial cells are essential for maintaining CNS homeostasis, supporting neuronal function, and responding to pathological conditions. This review examines the interactions between the gut microbiome and neuroglia, emphasizing their critical roles in brain health and the development of neurological disorders. Dysbiosis, or imbalance in the gut microbiome, has been associated with various neurological and psychiatric conditions, such as autism spectrum disorder, anxiety, depression, and neurodegenerative diseases like Alzheimer’s and Parkinson’s. The microbiome influences brain function through microbial metabolites, immune modulation, and neuroinflammatory responses. Understanding these interactions paves the way for new therapeutic targets and strategies for preventing and treating CNS disorders. This scoping review aims to highlight the mechanisms of the microbiome-neuroglia axis in maintaining brain health and its potential as a therapeutic target.
1. Introduction
The human central nervous system (CNS) is a complex network controlling various physiological processes and behaviors. Recent research has uncovered the significant influence of the gut microbiome, a diverse community of microorganisms in the gastrointestinal tract, on CNS function and overall brain health. Neuroglial cells, including astrocytes, microglia, oligodendrocytes, and ependymal cells, play critical roles in maintaining CNS homeostasis, supporting neuronal function, and responding to pathological conditions. Emerging evidence of a bidirectional communication pathway between the gut microbiome and neuroglia highlights the need for a comprehensive understanding of their interactions and implications for brain health and neurological disorders [1,2].
The gut microbiome consists of trillions of microorganisms, including bacteria, viruses, fungi, and protozoa, that coexist symbiotically with their human host. These microbes perform functions such as digesting complex carbohydrates, synthesizing vitamins, and modulating the immune system. The gut–brain axis refers to the biochemical signaling between the gastrointestinal tract and the CNS, involving neural, hormonal, and immunological mechanisms [3,4,5,6,7,8].
Neuroglia, or glial cells, are non-neuronal cells in the CNS that support and protect neurons. Astrocytes regulate blood–brain barrier (BBB) permeability and maintain the extracellular environment. Microglia are the primary immune cells in the CNS, responding to injury and infection. Oligodendrocytes form and maintain myelin sheaths around neuronal axons, crucial for rapid signal transmission. Ependymal cells line the brain’s ventricular system, playing a role in cerebrospinal fluid (CSF) production and circulation [9].
Understanding the interaction between the gut microbiome and neuroglia is pivotal. Dysbiosis, or the imbalance of the gut microbiome, has been linked to neurological and psychiatric conditions, including autism spectrum disorder (ASD), anxiety, depression, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s. The microbiome influences brain function through microbial metabolites, immune system modulation, and the alteration of neuroinflammatory responses [10,11,12].
Neuroglial cells mediate neuroinflammation and immune responses within the CNS and are affected by signals from the gut microbiome, leading to functional changes and potentially contributing to neurological disorders. By elucidating the pathways and mechanisms of microbiome–neuroglia interactions, we can identify novel therapeutic targets and develop strategies for preventing and treating CNS disorders [9,11,12].
The primary objective of this review is to provide a comprehensive analysis of the microbiome–neuroglia axis and its implications for brain health and neurological disorders. We aim to highlight the critical role of this axis in maintaining brain health and its potential as a therapeutic target for various neurological disorders.
2. The Gut Microbiome
The human gut microbiome, a vast and complex ecosystem of microorganisms, is crucial for maintaining overall health and influencing brain function. It comprises trillions of microorganisms, including bacteria, archaea, viruses, fungi, and protozoa. Among these, bacteria are the most studied, with Firmicutes and Bacteroidetes being the predominant phyla, followed by Proteobacteria, Actinobacteria, Verrucomicrobia, and Fusobacteria [11,13].
Firmicutes, including genera such as Lactobacillus, Clostridium, and Faecalibacterium, ferment dietary fibers to produce short-chain fatty acids (SCFAs) like butyrate, which serves as an energy source for colonocytes and has anti-inflammatory properties. Bacteroidetes, represented by genera such as Bacteroides and Prevotella, also participate in the carbohydrate metabolism. Proteobacteria, though less abundant, include genera like Escherichia and Salmonella, some of which can become pathogenic, contributing to dysbiosis. Actinobacteria, particularly the genus Bifidobacterium, are important for early-life gut colonization, and have health-promoting effects such as inhibiting pathogenic bacteria and modulating the immune system [13,14].
The gut mycobiome, though less studied, includes fungi such as Candida and Saccharomyces, which interact with the bacterial microbiome and the host immune system [15,16]. The gut virome consists predominantly of bacteriophages, which influence bacterial population dynamics and genetic diversity [17,18]. Archaea, primarily methanogens like Methanobrevibacter smithii, play a role in reducing hydrogen gas and enhancing fermentation efficiency [19,20].
The gut microbiome performs several essential functions critical to overall health. It aids in the digestion of complex carbohydrates, proteins, and lipids, producing SCFAs that provide energy (ATP) to host cells and which have anti-inflammatory effects (Figure 1). The microbiome modulates the immune system by educating immune cells, promoting the development of gut-associated lymphoid tissue (GALT), and maintaining immune homeostasis. Additionally, it enhances gut barrier integrity by promoting mucus production, producing antimicrobial peptides, and influencing tight junction protein expression, preventing the translocation of pathogens and toxins into the systemic circulation. The microbiome is also involved in the metabolism of bile acids, vitamins (such as vitamin K and B vitamins), and other bioactive compounds, regulating host metabolism and energy balance. Notably, certain gut microbes produce neurotransmitters such as serotonin, gamma-aminobutyric acid (GABA), and dopamine, influencing the host’s nervous system [21,22,23] (Figure 1).
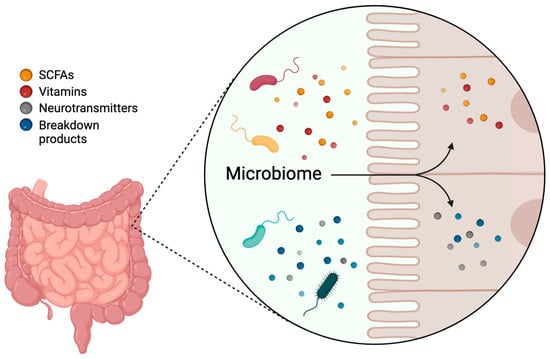
Figure 1.
Functions of the gut microbiome. The gut microbiome aids in the digestion of complex carbohydrates, proteins, and lipids, producing short-chain fatty acids (SCFAs), vitamins, neurotransmitters, and other bioactive compounds. This Figure was made in BioRender.
3. Overview of Neuroglial Cells
Glial cells are non-neuronal cells in the CNS that play crucial roles in supporting and maintaining neuronal function. Neuroglial cells are diverse and can be broadly categorized into four main types: astrocytes, microglia, oligodendrocytes, and ependymal cells. Each type has distinct functions and contributes uniquely to CNS homeostasis and brain health. Glial cells are highly responsive to changes in their environment, adjusting their functions to maintain CNS homeostasis and respond to pathological conditions. This dynamic responsiveness is critical for protecting the brain and supporting neuronal health [24,25,26].
Astrocytes are the most abundant type of glial cells in the CNS, characterized by their star-shaped morphology. They interact extensively with neurons, blood vessels, and other glial cells. Astrocytes are crucial for maintaining the BBB, regulating cerebral blood flow, providing metabolic support to neurons, and maintaining the extracellular ion balance. They also modulate synaptic activity by regulating neurotransmitter levels in the synaptic cleft, thus influencing neuronal signaling and synaptic plasticity [27,28,29]. Under normal conditions, astrocytes support neuronal activity and synaptic function. In response to CNS injury or disease, astrocytes can become reactive, characterized by changes in morphology, proliferation, and specific protein expression, such as glial fibrillary acidic protein (GFAP). Reactive astrocytes can form a glial scar, which isolates the injured area and limits damage spread, but can also inhibit axon regeneration and repair. Astrocytes also regulate the flow of CSF through the glymphatic system via their aquaporin-4 (AQP4) water channels, which facilitate the rapid movement of water, and thus CSF, across the BBB and into the brain’s interstitial spaces. The glymphatic system removes metabolic waste products such as amyloid-beta (Aβ) and tau proteins, linked to neurodegenerative diseases. Efficient waste removal helps prevent the accumulation of harmful substances that can lead to neuronal damage and cognitive decline [26,29,30].
Microglia are the resident immune cells of the CNS, serving as the first line of defense against pathogens and injury. They continuously survey the brain environment for signs of infection, damage, or disease. Upon detecting such signals, microglia become activated, changing their morphology and behavior. Activated microglia can phagocytose cellular debris, dead cells, and pathogens, and release cytokines and chemokines to coordinate the immune response. They play a dual role in the CNS: promoting inflammation to defend against threats, and facilitating tissue repair and resolution of inflammation once the threat has passed. Chronic microglial activation is associated with neuroinflammatory conditions and has been implicated in the pathogenesis of neurodegenerative diseases such as Alzheimer’s and Parkinson’s [31,32,33,34].
Oligodendrocytes form and maintain myelin sheaths around neuronal axons in the CNS. Myelin is a fatty insulating layer that enhances the speed and efficiency of electrical signal transmission along axons. Each oligodendrocyte extends multiple processes to wrap around segments of multiple axons, forming the myelin sheath. Proper myelination is essential for rapid signal conduction and overall neural circuit function [35,36]. Oligodendrocyte dysfunction or damage can lead to demyelinating diseases such as multiple sclerosis, impairing neural communication and resulting in neurological deficits [35]. Oligodendrocytes and their precursor cells (OPCs) are influenced by neuronal activity and extracellular signals. During development and in response to injury, OPCs proliferate and differentiate into mature oligodendrocytes to repair and remyelinate axons. Various signaling pathways, including those mediated by growth factors and extracellular matrix components, regulate oligodendrocyte differentiation and myelination. Disruptions in these signaling pathways can impair myelin repair and contribute to demyelinating diseases [37,38].
Ependymal cells line the ventricles of the brain and the central canal of the spinal cord, forming a barrier between the CSF and the brain parenchyma. These ciliated cells play a role in the production and circulation of CSF, providing mechanical protection, nutrient delivery, and waste removal for the CNS. The movement of their cilia helps circulate the CSF, ensuring it bathes the brain and spinal cord adequately. Ependymal cells respond to changes in the brain’s environment, particularly in conditions affecting CSF dynamics. In response to injury or disease, ependymal cells can alter their function and morphology, potentially contributing to repair processes or the pathology of conditions such as hydrocephalus [39,40,41].
4. Gut Brain Axis
The gut–brain axis refers to the bidirectional communication network connecting the gastrointestinal tract and the CNS. This complex system involves neural, hormonal, immunological, and metabolic pathways.
Neural pathways are a primary component of the gut–brain axis. The vagus nerve, the main neural conduit between the gut and the brain, transmits sensory information from the gut to the brain and carries efferent signals from the brain to the gut, influencing gut motility, secretion, and immune responses [42]. Vagus nerve stimulation has been shown to modulate mood and is used in treating conditions such as depression and epilepsy [43]. The enteric nervous system (ENS), often referred to as the “second brain”, operates autonomously but communicates with the CNS via the vagus nerve and spinal cord, regulating gut motility, secretion, and blood flow. Hormonal pathways also play a significant role. Gut hormones such as ghrelin, leptin, and peptide tyrosine tyrosine (PYY), produced by enteroendocrine cells, influence appetite, satiety, and energy balance. These hormones can cross the BBB or act on vagal afferents to affect CNS function [44,45].
The hypothalamic–pituitary–adrenal (HPA) axis, which regulates the body’s stress response, is also modulated by the microbiome. Dysbiosis has been linked to altered HPA axis function, contributing to stress-related disorders. The gut microbiome influences both systemic and local immune responses by modulating cytokine production and immune cell activity. These immune mediators can affect brain function and behavior; for example, increased levels of pro-inflammatory cytokines have been associated with depression and anxiety [46,47,48].
Metabolic pathways are crucial in the gut–brain axis. The gut microbiome produces various metabolites, including SCFAs, tryptophan metabolites, and secondary bile acids, which can cross the BBB and influence brain function [49,50]. SCFAs, such as butyrate, have neuroprotective effects and can modulate brain inflammation and neurogenesis [51,52]. Neurotransmitter production by the gut microbiome is another vital aspect. Approximately 90% of the body’s serotonin is produced in the gut. Certain gut bacteria can influence serotonin production by enterochromaffin cells, affecting mood and gastrointestinal motility. These microbial-derived neurotransmitters can influence the CNS either directly by crossing the BBB, or indirectly by modulating peripheral nervous system activity. Serotonin, a key neurotransmitter involved in mood regulation, is synthesized in large quantities in the gut, primarily by enterochromaffin cells (Figure 2). Gut microbiota can influence serotonin production by modulating the availability of its precursor, tryptophan. Increased gut-derived serotonin can affect CNS function by activating serotonin receptors on neuroglial cells, influencing neuroglial signaling and neuroinflammation [53,54,55,56].
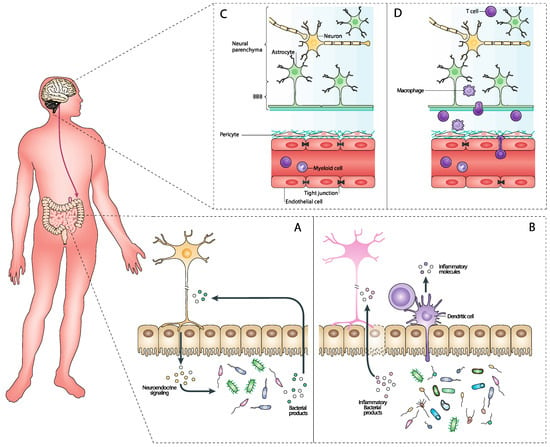
Figure 2.
Reciprocal interactions between the CNS and the gut microbiome. (A) The gut microbiome produces various neuroactive compounds, such as neurotransmitters (e.g., serotonin and GABA) and neuromodulators (e.g., short-chain fatty acids [SCFAs]), that can cross the blood–brain barrier (BBB) and influence CNS function. These microbial metabolites play a significant role in regulating systemic inflammation, which impacts neuroinflammation and neuroglial activation within the CNS. Additionally, microbiome-derived metabolites affect CNS metabolism and energy balance, thereby influencing neuronal health and function. On the other hand, the CNS exerts its influence on the gut microbiome through neuroendocrine signaling, primarily via the hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous system. These pathways regulate gut motility, secretion, and microbial composition. CNS neurotransmitters and peptides, such as acetylcholine, also affect gut motility and microbial growth. (B) When the gut microbiome is dysregulated, it can produce an increased amount of pro-inflammatory metabolites such as lipopolysaccharides (LPS) and other endotoxins. These compounds can permeate the gut barrier, entering the bloodstream and triggering systemic inflammation. Additionally, the pro-inflammatory environment affects dendritic cells, which are crucial for the immune system’s adaptive responses. The systemic inflammation caused by these microbial products influences the CNS by crossing the BBB, leading to neuroinflammation. This neuroinflammatory response involves the activation of microglia and astrocytes, which release cytokines and other inflammatory mediators that can damage neuronal cells and disrupt normal brain function (D). The altered microbiome and its inflammatory products enhance dendritic cell activation and maturation, leading to increased antigen presentation and a heightened immune response. This can exacerbate the inflammatory state, contributing further to the CNS inflammation and potentially leading to or worsening neurodegenerative conditions. (C) In a balanced state, the gut microbiome produces various metabolites such as SCFAs, neurotransmitters, and anti-inflammatory compounds. These beneficial metabolites help maintain the integrity of the gut barrier, preventing harmful substances from entering the bloodstream. This intact gut barrier ensures that only beneficial microbial products reach the systemic circulation. Once in the bloodstream, these compounds cross the BBB and positively influence CNS function by supporting neuronal health, enhancing synaptic plasticity, and reducing neuroinflammation. This Figure was made in BioRender.
Conversely, the CNS significantly influences the gut microbiome through a complex network of neural, hormonal, and immunological pathways. The autonomic nervous system (ANS) plays a crucial role in brain–gut communication, primarily through its sympathetic and parasympathetic branches. The sympathetic nervous system (SNS) is activated during stress responses, significantly altering gut motility and secretion. For example, stress-induced SNS activation can change gastrointestinal transit times, creating an environment that may favor the growth of certain bacterial populations over others, altering the overall composition of the gut microbiome. The parasympathetic nervous system (PNS), particularly through the vagus nerve, provides a direct neural route for the brain to influence the gut. Vagal activity is known to affect gut permeability, modulate inflammatory responses, and alter the microbial composition [57,58,59]. The vagus nerve acts as a conduit for sending signals from the brain to the gut, affecting various gastrointestinal functions that can subsequently influence microbial habitats. Also, the ENS governs gut motility, fluid exchange, and blood flow, all of which are critical for maintaining a stable environment for gut microbes [60]. The ENS’s regulatory functions ensure that gut conditions such as pH, nutrient availability, and oxygen levels are maintained within ranges that support a healthy microbiome [61]. The CNS also influences the gut microbiome through hormonal signaling. The HPA axis is particularly important in this regard. Stress-induced activation of the HPA axis leads to the release of cortisol and other glucocorticoids, which can alter gut permeability and immune function [46] (Figure 2). Increased gut permeability, often referred to as “leaky gut”, allows for the translocation of microbial products such as lipopolysaccharides (LPS) into the bloodstream, potentially leading to systemic inflammation and further impacting the microbiome. Additionally, various neuropeptides and neurotransmitters released by the brain, such as norepinephrine, serotonin, and dopamine, can influence gut motility, secretion, and blood flow. These substances can create microenvironments within the gut that are either conducive or detrimental to the growth of specific microbial populations [62,63,64] (Figure 2). The CNS can also modulate the immune system, which in turn affects the gut microbiome. Neuroimmune interactions involve the release of cytokines and other immune mediators in response to neural signals. For instance, stress can lead to increased production of pro-inflammatory cytokines, which can disrupt the gut barrier and alter the microbial ecosystem [65,66,67]. The gut-associated lymphoid tissue (GALT) plays a critical role in mediating immune responses to gut microbiota. Neural signals from the brain can influence GALT activity, modulating immune responses to maintain or alter the composition of the gut microbiome. For example, stress-induced changes in immune function can lead to an imbalance in gut microbial communities, favoring pathogenic bacteria over commensal microbes [68,69].
5. Immune Interactions and Inflammation
Understanding the mechanisms by which the gut microbiome interacts with neuroglial cells is crucial for elucidating the pathways that connect gut health with brain function. These interactions occur through several key mechanisms, including immune system modulation, metabolic pathways and neurotransmitter production, and the regulation of inflammation and neuroglial activation.
The gut microbiome plays a pivotal role in regulating the host’s immune system. Microbial communities in the gut interact with the mucosal immune system, influencing both local and systemic immune responses. Commensal bacteria contribute to the development and function of GALT and help maintain immune homeostasis by promoting the differentiation of regulatory T cells (Tregs) and the production of anti-inflammatory cytokines such as interleukin-10 (IL-10) [68,70,71,72].
The microbiome helps train the immune system to distinguish between pathogenic and non-pathogenic antigens, essential for preventing inappropriate immune responses that could lead to chronic inflammation or autoimmunity. Dysbiosis, an imbalance in the microbial community, can disrupt this regulatory environment, leading to increased permeability of the gut barrier (leaky gut) and the translocation of microbial products such as LPS into the bloodstream. This can trigger systemic inflammation and impact distant organs, including the brain [73,74,75].
Neuroglial cells, particularly microglia and astrocytes, are directly influenced by the immune-modulating effects of the gut microbiome. Microbial metabolites and immune signaling molecules can cross the BBB or signal through the vagus nerve to modulate neuroglial function [49]. For example, SCFAs like butyrate and propionate, produced by the fermentation of dietary fibers by gut bacteria, promote the maturation and function of microglia, enhancing their ability to maintain CNS homeostasis and respond to injury. Butyrate, for instance, enhances the integrity of the BBB and exerts anti-inflammatory effects on microglial cells. These SCFAs can enter the bloodstream and cross the BBB, directly affecting the CNS environment. Additionally, tryptophan metabolites produced by gut bacteria, such as indole and its derivatives, modulate neuroglial activity. Indole derivatives can cross the BBB and interact with receptors on neuroglial cells, influencing their function and contributing to the regulation of CNS inflammation and homeostasis [76,77,78]. Furthermore, systemic immune responses modulated by the microbiome can influence the activation state of microglia. Anti-inflammatory cytokines promoted by a healthy microbiome help maintain microglia in a surveillant state, while pro-inflammatory cytokines resulting from dysbiosis can lead to microglial activation, contributing to neuroinflammation and potentially neurodegeneration [77,79,80].
The gut microbiome plays a significant role in modulating inflammatory processes within the host. A balanced microbiome promotes anti-inflammatory pathways, while dysbiosis can lead to the production of pro-inflammatory cytokines and mediators. For instance, LPS from Gram-negative bacteria can trigger systemic inflammation if it translocates into the bloodstream, activating Toll-like receptor 4 (TLR4) on immune cells and leading to the release of pro-inflammatory cytokines [81,82,83]. Inflammatory signals originating from the gut can profoundly impact neuroglial activation and function. Microglia are particularly sensitive to systemic inflammatory signals. Elevated levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), can activate microglia, shifting them from a resting to an activated state. Activated microglia can produce additional pro-inflammatory cytokines, ROS, and nitric oxide, contributing to a neuroinflammatory environment that can damage neurons and disrupt CNS homeostasis [84,85,86]. Astrocytes also respond to inflammatory signals from the gut microbiome. In response to pro-inflammatory cytokines, astrocytes can become reactive, characterized by hypertrophy, increased GFAP expression, and the release of inflammatory mediators. Reactive astrocytes can support the inflammatory response by producing cytokines and chemokines that recruit peripheral immune cells to the CNS, further amplifying neuroinflammation [87,88,89].
Gut microbiota modulates the immune system’s cytokine profile, affecting CNS inflammation and neuronal waste clearance. The glymphatic system facilitates the clearance of interstitial waste products, including Aβ and tau proteins, from the CNS. This process primarily occurs during sleep, when CSF flows through perivascular channels formed by astrocytic endfeet, flushing out waste. Efficient glymphatic clearance is crucial for preventing neurodegenerative diseases such as AD, characterized by the accumulation of these toxic proteins. Pro-inflammatory cytokines, such as IL-6 and TNF-α, can cross the BBB and disrupt glymphatic function. Elevated systemic inflammation from gut-derived cytokines can impair glymphatic clearance, leading to the accumulation of neurotoxic waste in the brain [26,30]. Several mechanisms underpin the interaction between the gut microbiome, sleep, and glymphatic function. Gut bacteria produce neurotransmitters such as GABA and serotonin, which regulate sleep architecture [90,91,92]. Imbalances in these neurotransmitters due to dysbiosis can lead to sleep disturbances and reduced glymphatic activity. Microbial metabolites, including SCFAs, influence the host’s circadian system and sleep patterns. SCFAs like butyrate can modulate the expression of genes involved in the circadian clock and enhance BBB integrity, crucial for efficient glymphatic function [93,94,95]. The gut microbiome modulates systemic inflammation through the production of anti-inflammatory metabolites [77,83]. Chronic low-grade inflammation, often resulting from dysbiosis, can impair glymphatic function by promoting a pro-inflammatory state in the CNS, leading to microglial activation and reduced efficiency of waste clearance. Gut-derived serotonin is a precursor to melatonin, which regulates sleep–wake cycles and circadian rhythms. Melatonin also has direct effects on the glymphatic system, enhancing its activity during sleep. Dysbiosis can disrupt serotonin production, leading to decreased melatonin levels and impaired glymphatic function [96,97].
6. Implications for Brain Health
The interactions between the microbiome and neuroglia have profound implications for brain health, influencing cognitive function, mental health, and neurodevelopmental disorders.
Research has shown that alterations in the gut microbiome can influence mood, anxiety, and cognitive performance. One key mechanism involves the production of microbial metabolites, such as SCFAs, which can cross the blood–brain barrier and modulate brain function [76]. SCFAs like butyrate have neuroprotective effects, enhancing brain plasticity, reducing neuroinflammation, and supporting the integrity of the blood–brain barrier [52,98,99,100]. These effects collectively contribute to improved cognitive function and mental health. The gut microbiome also influences the production and availability of neurotransmitters, such as serotonin, dopamine, and GABA. Serotonin, for example, is predominantly produced in the gut, and its levels are modulated by gut bacteria. Alterations in serotonin levels can affect mood and anxiety, as serotonin is a critical regulator of these states [54,55]. The gut microbiome can affect the synthesis and modulation of other neurotransmitters, impacting neural signaling and brain function [56,57,98,99,100].
Microglia and astrocytes respond to signals from the gut microbiome, influencing brain health. Microglia, in particular, are involved in synaptic pruning and the removal of damaged cells and debris. Proper functioning of microglia is essential for maintaining cognitive health, as dysregulated microglial activity is associated with cognitive impairments and neurodegenerative diseases. Astrocytes support synaptic function and neurotransmission, and their interaction with gut-derived metabolites and immune signals underscores the importance of the microbiome–neuroglia axis in cognitive processes [98,99,100].
Microbiome–neuroglia interactions also have significant implications for neurodevelopmental disorders such as ASD and attention-deficit/hyperactivity disorder (ADHD). Emerging evidence suggests that dysbiosis during critical periods of brain development can impact the risk and severity of these disorders.
In ASD, altered gut microbiota composition has been linked to gastrointestinal symptoms and behavioral changes commonly observed in affected individuals. Studies have shown that children with ASD often have different gut microbiota profiles compared to neurotypical children, with a higher prevalence of certain bacterial species and a reduction in microbial diversity [101,102,103,104]. These microbial alterations can affect neuroglial function and brain development through immune modulation, increased gut permeability, and the production of neuroactive metabolites. Microglia play a crucial role in brain development, including the formation and refinement of neural circuits. Dysregulation of microglial function due to altered microbial signals can contribute to the neurodevelopmental abnormalities seen in ASD. Excessive microglial activation and inflammation can disrupt synaptic pruning, leading to the synaptic connectivity abnormalities characteristic of ASD [105,106,107].
Similarly, in ADHD, the gut microbiome’s composition and activity may influence the disorder’s development and manifestation. Research indicates that gut-derived metabolites and immune signals can impact neuroglial function and neurotransmitter systems implicated in ADHD, such as the dopaminergic and noradrenergic pathways. Disruptions in these pathways can affect attention, impulse control, and hyperactivity, core symptoms of ADHD [108,109,110].
Overall, microbiome–neuroglia interactions play a crucial role in brain health, affecting cognitive function, mental health, and the risk and progression of neurodevelopmental disorders. Understanding these interactions opens new avenues for therapeutic interventions aimed at modulating the gut microbiome to promote brain health and treat neurological and psychiatric conditions.
7. Implications for Neurological Disorders
The relationship between the microbiome and neuroglia significantly impacts the pathophysiology of various neurological disorders. Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) are characterized by progressive loss of neuronal function and structure. The gut microbiome and neuroglia are increasingly recognized as critical players in these diseases’ progression.
AD, the most common cause of dementia, is marked by Aβ plaques, neurofibrillary tangles, and neuroinflammation. Recent studies have shown that dysbiosis in the gut microbiome can exacerbate AD pathology. For instance, altered gut microbiota composition has been linked to increased production of pro-inflammatory cytokines and systemic inflammation, which can cross the BBB and activate microglia. Activated microglia can enhance Aβ plaque deposition and tau phosphorylation, furthering neurodegenerative processes. Additionally, SCFAs produced by gut bacteria can modulate microglial activation states, influencing their neuroprotective versus neurotoxic roles [111,112,113,114,115].
PD is characterized by the degeneration of dopaminergic neurons in the substantia nigra, leading to motor and non-motor symptoms. Gastrointestinal dysfunction is a common early symptom of PD, and the gut microbiome is thought to play a crucial role in disease pathogenesis. Research has demonstrated that certain gut bacteria can produce neuroactive metabolites that influence the aggregation of alpha-synuclein, a hallmark of PD. Moreover, gut-derived inflammation can activate microglia and astrocytes, contributing to the neuroinflammatory milieu that exacerbates neuronal loss [116,117,118]. Interestingly, it has been determined that there is an increased expression of pro-inflammatory cytokines (TNF-α, IFN-γ, IL-6, and IL-1β) in PD patients using real-time PCR analysis of mRNA expression of pro-inflammatory cytokines in the ascending colon biopsies of PD patients and controls [119]. In addition, nicotinic and muscarinic acetylcholine receptors (nAChRs and mAChRs) on glial cells have gained attention for their potential role in managing PD. Glial cells, including astrocytes and microglia, express nAChRs and mAChRs, which are involved in regulating neuroinflammation, synaptic transmission, and neuronal survival. These receptors mediate the effects of acetylcholine (ACh), a neurotransmitter that modulates numerous central nervous system functions. Recent studies suggest that targeting nAChRs on glial cells may help in reducing neuroinflammation and enhancing neuronal survival in PD. Activation of nAChRs on microglia has been shown to reduce the release of pro-inflammatory cytokines, thus potentially mitigating the chronic inflammation associated with PD. Additionally, nAChR activation on astrocytes can enhance the release of neurotrophic factors, supporting neuronal health and function. On the other hand, mAChRs, which are G protein-coupled receptors, also play significant roles in glial cell function. Activation of mAChRs on astrocytes can influence calcium signaling and the release of gliotransmitters, which are crucial for maintaining synaptic homeostasis and neuronal communication [120,121]. In PD, where dopaminergic neurons are progressively lost, modulating mAChRs on astrocytes may help in preserving synaptic integrity and improving motor function. The potential for targeting glial nAChRs and mAChRs in PD lies in their ability to regulate the inflammatory environment and provide neuroprotective support. By reducing neuroinflammation and promoting neuronal survival, interventions aimed at these receptors could slow disease progression and improve patient outcomes.
MS is an autoimmune disorder characterized by demyelination and neuroinflammation. The gut microbiome has been implicated in modulating the immune responses involved in MS. Dysbiosis can lead to a pro-inflammatory immune environment, promoting the differentiation of Th17 cells and reducing Treg populations, which are crucial for maintaining immune tolerance [122,123]. These immune alterations can enhance the activation of microglia and astrocytes, leading to the production of pro-inflammatory cytokines and ROS, which damage myelin and oligodendrocytes [124,125]. Studies in animal models have shown that modifying the gut microbiota can ameliorate MS symptoms, highlighting the therapeutic potential of targeting the microbiome. MS serves as a prime example of how microbiome-induced inflammation interacts with neuroglial cells to drive disease pathology [126]. In MS, the disruption of the gut microbiome can lead to increased intestinal permeability, allowing for microbial products like LPS to enter the circulation and trigger systemic inflammation. This systemic inflammation can then influence the CNS, where it activates microglia and astrocytes, perpetuating a cycle of neuroinflammation and neurodegeneration [127,128]. Microglia in MS can adopt a pro-inflammatory phenotype, producing cytokines such as IL-1β, TNF-α, and IL-6, which further damage myelin and exacerbate neuronal injury. Astrocytes, in their reactive state, can also contribute to the inflammatory environment by releasing chemokines that recruit peripheral immune cells to the CNS [127,128,129,130]. The gut microbiome’s role in modulating these immune responses is critical, as it can either promote a pro-inflammatory environment or support regulatory pathways that protect against excessive inflammation.
The gut microbiome also affects sleep quality and patterns by influencing the production of sleep-regulating hormones and neurotransmitters such as melatonin and serotonin. Dysbiosis, associated with poor sleep quality, can lead to reduced glymphatic clearance, as this system is most active during sleep. Disruptions in sleep directly impact the efficiency of glymphatic waste removal. The gut microbiome helps regulate circadian rhythms, crucial for synchronizing the timing of glymphatic clearance. Disruptions in circadian rhythms, influenced by microbiome composition, can alter the timing and efficiency of glymphatic activity and clearance of neurotoxic waste [91,131,132,133].
8. Use of Microbiome-Targeted Therapies in Modulating Neuroglial Function
Understanding the interactions between the microbiome and neuroglia opens new therapeutic avenues for managing brain health and neurological disorders. This section explores the potential of probiotics and prebiotics, diet and lifestyle modifications, and pharmacological interventions, and identifies research gaps and future directions in this promising field.
Probiotics, which are live microorganisms that confer health benefits when consumed, and prebiotics, which are non-digestible food ingredients that promote the growth of beneficial bacteria, have shown potential in modulating neuroglial function and improving brain health [134,135,136,137,138]. Probiotic strains such as Lactobacillus and Bifidobacterium have been studied for their effects on the gut–brain axis. For example, Lactobacillus rhamnosus has been shown to reduce anxiety and depression-like behavior in animal models, potentially through its impact on GABA receptor expression in the brain [138,139]. Similarly, Bifidobacterium longum has demonstrated the ability to reduce stress-induced corticosterone levels and modulate hippocampal neurogenesis [140,141,142]. Prebiotics, such as fructooligosaccharides (FOS) and galactooligosaccharides (GOS), can enhance the production of SCFAs, which have anti-inflammatory properties and can cross the blood–brain barrier to influence neuroglial function. Clinical trials have shown that prebiotic supplementation can improve cognitive performance and reduce anxiety in healthy adults, highlighting their therapeutic potential [143,144,145,146,147,148].
Diet and lifestyle can significantly influence the composition and function of the gut microbiome, which in turn affects neuroglial health. Diets rich in fiber, polyphenols, and omega-3 fatty acids have been shown to promote a healthy microbiome and exert neuroprotective effects [149,150]. A high-fiber diet increases the production of SCFAs, which support the integrity of the BBB and reduce neuroinflammation. Polyphenols, found in fruits, vegetables, and tea, can modulate gut microbiota composition and have antioxidant and anti-inflammatory effects on the brain. Omega-3 fatty acids, present in fish oil, reduce neuroinflammation and support neuronal function by modulating microglial activity [151,152,153,154]. Lifestyle factors such as exercise and sleep also play crucial roles. Regular physical activity is associated with increased microbial diversity and the production of beneficial metabolites [155,156,157], while adequate sleep is essential for maintaining a balanced gut microbiome and reducing systemic inflammation. Alignment of microbiome health with regular sleep patterns and circadian rhythms is increasingly recognized as crucial for human health [90,91,92]. The gut microbiome exhibits its own circadian rhythms, with microbial populations and their metabolic activities fluctuating throughout the day. These fluctuations are influenced by host circadian clocks, which regulate various physiological processes, including digestion, metabolism, and immune function [158,159,160]. Disruptions in host circadian rhythms, such as those caused by irregular sleep patterns, shift work, or jet lag, can lead to dysbiosis, characterized by an imbalance in microbial communities [161,162]. The gut microbiome also influences sleep quality through the production of neurotransmitters and metabolites that affect CNS function. Certain gut bacteria are involved in the synthesis of serotonin, a precursor to melatonin, a key regulator of sleep–wake cycles. Melatonin facilitates the initiation and maintenance of sleep and regulates circadian rhythms, ensuring the synchronization of various physiological processes, including glymphatic clearance [163,164]. Studies have demonstrated that dysbiosis can lead to sleep disturbances, which in turn impair glymphatic function. The glymphatic system, responsible for clearing metabolic waste from the brain, operates most effectively during the deeper stages of non-rapid eye movement (NREM) sleep, when the interstitial space in the brain expands, allowing for more efficient clearance of waste products. Poor sleep quality or sleep fragmentation, often associated with dysbiosis, reduces the time spent in these restorative sleep stages, compromising glymphatic clearance. Aligning microbiome health with regular sleep patterns and circadian rhythms is essential for enhancing glymphatic function [92,131,133]. By maintaining a balanced microbiome, optimal sleep quality and circadian synchronization can be achieved, promoting the efficient glymphatic clearance of metabolic waste from the brain. This alignment is particularly important for preventing the accumulation of neurotoxic substances and reducing the risk of neurodegenerative diseases.
Emerging pharmacological interventions targeting the microbiome–neuroglia axis offer promising therapeutic strategies for neurological disorders. These interventions aim to modulate the gut microbiome to influence neuroglial function, potentially mitigating neuroinflammation, enhancing neuroprotection, and improving cognitive and mental health outcomes. One area of interest is the development of microbial-derived metabolites or their analogs as drugs. SCFA supplements, for example, are being explored for their potential to modulate neuroinflammation and improve cognitive function in disorders like AD. SCFAs are produced by the fermentation of dietary fibers by gut bacteria and have been shown to exert anti-inflammatory and neuroprotective effects. For instance, butyrate can enhance the integrity of the BBB, reduce neuroinflammation, and promote the maturation and function of microglia, which are crucial for maintaining CNS homeostasis and responding to injury. Supplementation with SCFAs or their analogs is being explored as a potential therapeutic approach to modulate neuroinflammatory responses and support neuronal health in disorders such as AD and PD [165,166,167]. Another promising approach is the use of antibiotics and microbial ecosystem therapeutics to selectively modulate the gut microbiome. Rifaximin, a non-absorbable antibiotic, has shown potential in altering the gut microbiome to reduce systemic inflammation and improve neuropsychiatric symptoms by selectively targeting pathogenic bacteria and preserving beneficial microbes. These therapies aim to restore a healthy gut microbiome and its beneficial effects on neuroglial function [168,169,170]. Additionally, fecal microbiota transplantation (FMT), which involves the transfer of gut microbiota from a healthy donor to a recipient, has shown potential in restoring a healthy microbiome and improving symptoms in conditions like MS and ASD. FMT can re-establish a balanced gut microbiome, which can influence neuroglial function by modulating immune responses, reducing gut permeability, and enhancing the production of beneficial metabolites [171,172,173].
Despite the promising therapeutic potential, several limitations exist in the current research on the microbiome–neuroglia axis. One major challenge is the complexity and variability of the human microbiome, influenced by numerous factors including genetics, diet, and environment. This variability makes it difficult to standardize interventions and predict outcomes. Another limitation is the lack of longitudinal studies and large-scale clinical trials that can provide robust evidence on the long-term effects of microbiome-targeted therapies. Most studies to date have been preclinical or small-scale clinical trials, which limit the generalizability of the findings.
Future research should focus on elucidating the precise mechanisms by which the microbiome influences neuroglial function. Advanced techniques such as metagenomics, metabolomics, and neuroimaging can provide deeper insights into these interactions. Longitudinal studies and large-scale clinical trials are needed to assess the efficacy and safety of microbiome-targeted therapies over time. Additionally, personalized approaches that consider individual variations in the microbiome and host response should be explored to optimize therapeutic outcomes. Research should also investigate the potential synergistic effects of combining multiple therapeutic strategies, such as probiotics, diet modifications, and pharmacological agents, to enhance brain health and treat neurological disorders more effectively.
Targeting the microbiome–neuroglia axis holds significant promise for improving brain health and treating neurological disorders. Continued research and clinical advancements in this field have the potential to revolutionize therapeutic approaches and enhance our understanding of the gut–brain connection.
9. Conclusions
The active rapport between the microbiome and neuroglia represents a fundamental frontier in understanding brain health and the pathophysiology of neurological disorders.
The gut microbiome, through its production of metabolites, modulation of neurotransmitter systems, and influence on immune responses, significantly impacts neuroglial function and, consequently, brain health. Microglia and astrocytes, essential for maintaining neural homeostasis and responding to brain injury, are highly responsive to signals from the gut microbiota. This bidirectional communication forms the basis of the microbiome–neuroglia axis, crucial for understanding how alterations in the gut microbiome can lead to neuroinflammation, synaptic dysfunction, and neurodegeneration.
In neurodegenerative diseases such as AD, PD, and MS, dysbiosis of the gut microbiome has been linked to disease progression through mechanisms involving systemic inflammation, microglial activation, and neuroinflammatory cascades. Similarly, neurodevelopmental disorders like ASD and ADHD are influenced by early-life microbiome composition, which can affect brain development and function via immune and neuroglial pathways.
Therapeutic strategies aimed at the microbiome–neuroglia axis hold considerable promise. Probiotics, prebiotics, diet and lifestyle modifications, and emerging pharmacological interventions offer novel approaches to modulate the gut microbiome and, in turn, influence neuroglial function. These strategies have the potential to mitigate neuroinflammation, enhance neuroprotection, and improve cognitive and mental health outcomes.
However, current research faces several limitations, including the complexity of the microbiome, individual variability, and the need for large-scale longitudinal clinical trials. Future studies should focus on elucidating the precise mechanisms underlying microbiome–neuroglia interactions, exploring personalized therapeutic approaches, and investigating the synergistic effects of combined interventions.
Advancing our understanding of this complex interplay opens new avenues for therapeutic interventions that can transform the management and treatment of neurological disorders, ultimately improving patient outcomes and quality of life. Continued research in this field is essential for translating these scientific insights into clinical practice, heralding a new era in neurotherapeutics.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahman, M.M.; Islam, M.R.; Yamin, M.; Islam, M.M.; Sarker, M.T.; Meem, A.F.K.; Akter, A.; Emran, T.B.; Cavalu, S.; Sharma, R. Emerging Role of Neuron-Glia in Neurological Disorders: At a Glance. Oxid. Med. Cell. Longev. 2022, 2022, 3201644. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota–brain axis in neurological disorder. Front. Neurosci. 2023, 17, 1225875. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Dorrestein, P.C.; Turnbaugh, P.J.; Knight, R. The intestinal metabolome: An intersection between microbiota and host. Gastroenterology 2014, 146, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Leviatan, S.; Shoer, S.; Rothschild, D.; Gorodetski, M.; Segal, E. An expanded reference map of the human gut microbiome reveals hundreds of previously unknown species. Nat. Commun. 2022, 13, 3863. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What is the Healthy Gut Microbiota composition? A Changing Ecosystem across age, environment, diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 235525. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J. Microbiome and Gut Dysbiosis. Exp. Suppl. 2018, 109, 459–476. [Google Scholar] [CrossRef]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef] [PubMed]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3426293/ (accessed on 18 June 2024). [CrossRef] [PubMed]
- Huffnagle, G.B.; Noverr, M.C. The emerging world of the fungal microbiome. Trends Microbiol. 2013, 21, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.C. Fungi of the human gut microbiota: Roles and significance. Int. J. Med. Microbiol. 2021, 311, 151490. [Google Scholar] [CrossRef] [PubMed]
- Pargin, E.; Roach, M.J.; Skye, A.; Papudeshi, B.; Inglis, L.K.; Mallawaarachchi, V.; Grigson, S.R.; Harker, C.; Edwards, R.A.; Giles, S.K. The human gut virome: Composition, colonization, interactions, and impacts on human health. Front. Microbiol. 2023, 14, 963173. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. eBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef] [PubMed]
- Chibani, C.M.; Mahnert, A.; Borrel, G.; Almeida, A.; Werner, A.; Brugère, J.-F.; Gribaldo, S.; Finn, R.D.; Schmitz, R.A.; Moissl-Eichinger, C. A catalogue of 1,167 genomes from the human gut archaeome. Nat. Microbiol. 2022, 7, 48–61. [Google Scholar] [CrossRef]
- Kim, J.Y.; Whon, T.W.; Lim, M.Y.; Kim, Y.B.; Kim, N.; Kwon, M.-S.; Kim, J.; Lee, S.H.; Choi, H.-J.; Nam, I.-H.; et al. The human gut archaeome: Identification of diverse haloarchaea in Korean subjects. Microbiome 2020, 8, 114. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Ogunrinola, G.A.; Oyewale, J.O.; Oshamika, O.O.; Olasehinde, G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020, 2020, 8045646. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Milichko, V.; Dyachuk, V. Novel Glial Cell Functions: Extensive Potency, Stem Cell-Like Properties, and Participation in Regeneration and Transdifferentiation. Front. Cell Dev. Biol. 2020, 8, 809. [Google Scholar] [CrossRef]
- Camberos-Barraza, J.; Camacho-Zamora, A.; Bátiz-Beltrán, J.C.; Osuna-Ramos, J.F.; Rábago-Monzón, Á.R.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; Guadrón-Llanos, A.M.; Picos-Cárdenas, V.J.; Calderón-Zamora, L.; et al. Sleep, Glial Function, and the Endocannabinoid System: Implications for Neuroinflammation and Sleep Disorders. Int. J. Mol. Sci. 2024, 25, 3160. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Lee, H.-G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef]
- Valles, S.L.; Singh, S.K.; Campos-Campos, J.; Colmena, C.; Campo-Palacio, I.; Alvarez-Gamez, K.; Caballero, O.; Jorda, A. Functions of Astrocytes under Normal Conditions and after a Brain Disease. Int. J. Mol. Sci. 2023, 24, 8434. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Ramos, J.F.; Camberos-Barraza, J.; Torres-Mondragón, L.E.; Rábago-Monzón, Á.R.; Camacho-Zamora, A.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; Guadrón-Llanos, A.M.; Picos-Cárdenas, V.J.; Calderón-Zamora, L.; et al. Interplay between the Glymphatic System and the Endocannabinoid System: Implications for Brain Health and Disease. Int. J. Mol. Sci. 2023, 24, 17458. [Google Scholar] [CrossRef]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Tsirka, S. The Diverse Roles of Microglia in the Neurodegenerative Aspects of Central Nervous System (CNS) Autoimmunity. Int. J. Mol. Sci. 2017, 18, 504. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Chun, W.; Lee, H.J.; Kim, S.-M.; Min, J.-H.; Kim, D.-Y.; Kim, M.-O.; Ryu, H.W.; Lee, S.U. The Role of Microglia in the Development of Neurodegenerative Diseases. Biomedicines 2021, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2009, 119, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.-P.; Kothary, R. Oligodendrocytes in a Nutshell. Front. Cell. Neurosci. 2015, 9, 340. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed]
- Munyeshyaka, M.; Fields, R.D. Oligodendroglia are emerging players in several forms of learning and memory. Commun. Biol. 2022, 5, 1148. [Google Scholar] [CrossRef]
- Deng, S.; Gan, L.; Liu, C.; Xu, T.; Zhou, S.; Guo, Y.; Zhang, Z.; Yang, G.-Y.; Tian, H.; Tang, Y. Roles of Ependymal Cells in the Physiology and Pathology of the Central Nervous System. Aging Dis. 2022, 14, 468. [Google Scholar] [CrossRef]
- Jiménez, A.J.; Domínguez-Pinos, M.-D.; Guerra, M.M.; Fernández-Llebrez, P.; Pérez-Fígares, J.-M. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2014, 2, e28426. [Google Scholar] [CrossRef]
- Nelles, D.G.; Hazrati, L.-N. Ependymal cells and neurodegenerative disease: Outcomes of compromised ependymal barrier function. Brain Commun. 2022, 4, fcac288. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, Y. Significance of vagus nerve function in terms of pathogenesis of psychosocial disorders. Neurochem. Int. 2021, 143, 104934. [Google Scholar] [CrossRef] [PubMed]
- Howland, R.H. Vagus Nerve Stimulation. Curr. Behav. Neurosci. Rep. 2014, 1, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Date, Y. Ghrelin and the vagus nerve. Methods Enzymol. 2012, 514, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Perelló, M.; Cornejo, M.P.; De Francesco, P.N.; Fernandez, G.; Gautron, L.; Valdivia, L.S. The controversial role of the vagus nerve in mediating ghrelin’s actions: Gut feelings and beyond. IBRO Neurosci. Rep. 2022, 12, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mondragón, L.E.; León-Pimentel, L.C.; Pérez-Tamayo, D.E.; Alberto, K. The endocannabinoid system: A new frontier in addressing psychosomatic challenges. J. Clin. Basic Psychosom. 2024, 2, 2288. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.-L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Frankiensztajn, L.M.; Elliott, E.; Koren, O. The microbiota and the hypothalamus-pituitary-adrenocortical (HPA) axis, implications for anxiety and stress disorders. Curr. Opin. Neurobiol. 2020, 62, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef]
- Guo, T.-T.; Zhang, Z.; Sun, Y.; Zhu, R.-Y.; Wang, F.-X.; Ma, L.-J.; Jiang, L.; Liu, H.-D. Neuroprotective Effects of Sodium Butyrate by Restoring Gut Microbiota and Inhibiting TLR4 Signaling in Mice with MPTP-Induced Parkinson’s Disease. Nutrients 2023, 15, 930. [Google Scholar] [CrossRef]
- Wen, J.; Xu, Q.; Li, J.; Shen, X.; Zhou, X.; Huang, J.; Liu, S. Sodium butyrate exerts a neuroprotective effect in rats with acute carbon monoxide poisoning by activating autophagy through the mTOR signaling pathway. Sci. Rep. 2024, 14, 4610. [Google Scholar] [CrossRef]
- Terry, N.; Margolis, K.G. Serotonergic Mechanisms Regulating the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb. Exp. Pharmacol. 2017, 239, 319–342. [Google Scholar] [CrossRef]
- Shajib, M.S.; Baranov, A.; Khan, W.I. Diverse Effects of Gut-Derived Serotonin in Intestinal Inflammation. ACS Chem. Neurosci. 2017, 8, 920–931. [Google Scholar] [CrossRef]
- Israelyan, N.; Del Colle, A.; Li, Z.; Park, Y.; Xing, A.; Jacobsen, J.P.R.; Luna, R.A.; Jensen, D.D.; Madra, M.; Saurman, V.; et al. Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 2019, 157, 507–521.e4. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Lee, J.; del Carmen Rosas, M.; Chen, J.; Henderson, W.A.; Starkweather, A.R.; Cong, X.S. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci. Rep. 2022, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Cai, X.; Luan, Y.; Yang, S.; Yang, J.; Dong, H.; Zeng, H.; Shao, L. Regulation of the Autonomic Nervous System on Intestine. Front. Physiol. 2021, 12, 700129. [Google Scholar] [CrossRef] [PubMed]
- Iovino, P.; Azpiroz, F.; Domingo, E.; Malagelada, J.R. The sympathetic nervous system modulates perception and reflex responses to gut distention in humans. Gastroenterology 1995, 108, 680–686. [Google Scholar] [CrossRef]
- Zubcevic, J.; Richards, E.M.; Yang, T.; Kim, S.; Sumners, C.; Pepine, C.J.; Raizada, M.K. Impaired Autonomic Nervous System-Microbiome Circuit in Hypertension. Circ. Res. 2019, 125, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef]
- Hamilton, M.K.; Wall, E.S.; Robinson, C.D.; Guillemin, K.; Eisen, J.S. Enteric nervous system modulation of luminal pH modifies the microbial environment to promote intestinal health. PLoS Pathog. 2022, 18, e1009989. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. The Bowel and beyond: The Enteric Nervous System in Neurological Disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Zheng, Z.; Tang, J.; Hu, Y.; Zhang, W. Role of gut microbiota-derived signals in the regulation of gastrointestinal motility. Front. Med. 2022, 9, 961703. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; Codutti, A.; Alim, K.; El Aidy, S. Gut microbiota-motility interregulation: Insights from in vivo, ex vivo and in silico studies. Gut Microbes 2022, 14, 1997296. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Ramayo-Caldas, Y.; Estellé, J.; Tambosco, K.; Chadi, S.; Maillard, F.; Gallopin, M.; Planchais, J.; Chain, F.; Kropp, C.; et al. Gut barrier-microbiota imbalances in early life lead to higher sensitivity to inflammation in a murine model of C-section delivery. Microbiome 2023, 11, 140. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2023, 19, 275–293. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Bemark, M.; Pitcher, M.J.; Dionisi, C.; Spencer, J. Gut-associated lymphoid tissue: A microbiota-driven hub of B cell immunity. Trends Immunol. 2024, 45, 211–223. [Google Scholar] [CrossRef]
- Arrazuria, R.; Pérez, V.; Molina, E.; Juste, R.A.; Khafipour, E.; Elguezabal, N. Diet induced changes in the microbiota and cell composition of rabbit gut associated lymphoid tissue (GALT). Sci. Rep. 2018, 8, 14103. [Google Scholar] [CrossRef]
- Jacobse, J.; Li, J.; Rings, E.H.; Samsom, J.N.; Goettel, J.A. Intestinal Regulatory T Cells as Specialized Tissue-Restricted Immune Cells in Intestinal Immune Homeostasis and Disease. Front. Immunol. 2021, 12, 716499. [Google Scholar] [CrossRef]
- de Moreno de LeBlanc, A.; del Carmen, S.; Zurita-Turk, M.; Santos Rocha, C.; van de Guchte, M.; Azevedo, V.; Miyoshi, A.; LeBlanc, J.G. Importance of IL-10 Modulation by Probiotic Microorganisms in Gastrointestinal Inflammatory Diseases. ISRN Gastroenterol. 2011, 2011, 892971. [Google Scholar] [CrossRef]
- Zeng, H.; Chi, H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015, 36, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Martín, F.; Blanco-Suárez, M.; Zambrano, P.; Cáceres, O.; Almirall, M.; Alegre, J.; Lobo, B.; González-Castro, A.M.; Santos, J.; Joan Carles Domingo Jurek, J.; et al. Increased gut permeability and bacterial translocation are associated with fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome: Implications for disease-related biomarker discovery. Front. Immunol. 2023, 14, 1253121. [Google Scholar] [CrossRef]
- Chae, Y.-R.; Lee, Y.R.; Kim, Y.-S.; Park, H.-Y. Diet-Induced Gut Dysbiosis and Leaky Gut Syndrome. J. Microbiol. Biotechnol. 2024, 34, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Rund, L.A.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Colombo, A.V.; Sadler, R.K.; Llovera, G.; Singh, V.; Roth, S.; Heindl, S.; Sebastian Monasor, L.; Verhoeven, A.; Peters, F.; Parhizkar, S.; et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. ELife 2021, 10, e59826. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haq, R.; Schlachetzk, J.C.M.; Glass, C.K.; Mazmanian, S.K. Microbiome–microglia connections via the gut–brain axis. J. Exp. Med. 2019, 216, 41–59. [Google Scholar] [CrossRef]
- Chunchai, T.; Thunapong, W.; Yasom, S.; Wanchai, K.; Eaimworawuthikul, S.; Metzler, G.; Lungkaphin, A.; Pongchaidecha, A.; Sirilun, S.; Chaiyasut, C.; et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflamm. 2018, 15, 11. [Google Scholar] [CrossRef]
- Wang, M.; Feng, J.; Zhou, D.; Wang, J. Bacterial lipopolysaccharide-induced endothelial activation and dysfunction: A new predictive and therapeutic paradigm for sepsis. Eur. J. Med. Res. 2023, 28, 339. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; Gasbarrini, A.; Franceschi, F. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.-S.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Fragas, M.G.; May, D.; Hiyane, M.I.; Braga, T.T.; Olsen, N. The dual effect of acetate on microglial TNF-α production. Clinics 2022, 77, 100062. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.D.; Sparkman, N.L.; Johnson, R.W. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J. Neuroinflamm. 2011, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Zißler, J.; Rothhammer, V.; Linnerbauer, M. Gut–Brain Interactions and Their Impact on Astrocytes in the Context of Multiple Sclerosis and Beyond. Cells 2024, 13, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Wei, D.-N.; Tang, Y. Gut microbiota regulate astrocytic functions in the brain: Possible therapeutic consequences. Curr. Neuropharmacol. 2021, 19, 1354. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Y.; Long, C.; Peng, Q.; Yue, R. The gut microbiota-astrocyte axis: Implications for type 2 diabetic cognitive dysfunction. CNS Neurosci. Ther. 2023, 29, 59–73. [Google Scholar] [CrossRef]
- Yue, M.; Jin, C.; Jiang, X.; Xue, X.; Wu, N.; Li, Z.; Zhang, L. Causal Effects of Gut Microbiota on Sleep-Related Phenotypes: A Two-Sample Mendelian Randomization Study. Clocks Sleep 2023, 5, 566–580. [Google Scholar] [CrossRef]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef]
- Neroni, B.; Evangelisti, M.; Radocchia, G.; Di Nardo, G.; Pantanella, F.; Villa, M.P.; Schippa, S. Relationship between sleep disorders and gut dysbiosis: What affects what? Sleep Med. 2021, 87, 1–7. [Google Scholar] [CrossRef]
- Tahara, Y.; Yamazaki, M.; Sukigara, H.; Motohashi, H.; Sasaki, H.; Miyakawa, H.; Haraguchi, A.; Ikeda, Y.; Fukuda, S.; Shibata, S. Gut Microbiota-Derived Short Chain Fatty Acids Induce Circadian Clock Entrainment in Mouse Peripheral Tissue. Sci. Rep. 2018, 8, 1395. [Google Scholar] [CrossRef]
- Segers, A.; Desmet, L.; Thijs, T.; Verbeke, K.; Tack, J.; Depoortere, I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol. 2018, 225, e13193. [Google Scholar] [CrossRef]
- Swanson, G.R.; Siskin, J.; Gorenz, A.; Shaikh, M.; Raeisi, S.; Fogg, L.; Forsyth, C.; Keshavarzian, A. Disrupted diurnal oscillation of gut-derived Short chain fatty acids in shift workers drinking alcohol: Possible mechanism for loss of resiliency of intestinal barrier in disrupted circadian host. Transl. Res. 2020, 221, 97–109. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, T.; Cao, M.; Yuan, C.; Reiter, R.J.; Zhao, Z.; Zhao, Y.; Chen, L.; Fan, W.; Wang, X.; et al. Gut Microbiota Dysbiosis Induced by Decreasing Endogenous Melatonin Mediates the Pathogenesis of Alzheimer’s Disease and Obesity. Front. Immunol. 2022, 13, 900132. [Google Scholar] [CrossRef]
- Iesanu, M.I.; Zahiu, C.D.M.; Dogaru, I.-A.; Chitimus, D.M.; Pircalabioru, G.G.; Voiculescu, S.E.; Isac, S.; Galos, F.; Pavel, B.; O’Mahony, S.M.; et al. Melatonin–Microbiome two-sided interaction in dysbiosis-associated conditions. Antioxidants 2022, 11, 2244. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Mansuy-Aubert, V.; Ravussin, Y. Short chain fatty acids: The messengers from down below. Front. Neurosci. 2023, 17, 1197759. [Google Scholar] [CrossRef] [PubMed]
- Davoli-Ferreira, M.; Thomson, C.A.; McCoy, K.D. Microbiota and Microglia Interactions in ASD. Front. Immunol. 2021, 12, 676255. [Google Scholar] [CrossRef]
- De Sales-Millán, A.; Aguirre-Garrido, J.F.; González-Cervantes, R.M.; Velázquez-Aragón, J.A. Microbiome–Gut–Mucosal–Immune–Brain Axis and Autism Spectrum Disorder (ASD): A Novel Proposal of the Role of the Gut Microbiome in ASD Aetiology. Behav. Sci. 2023, 13, 548. [Google Scholar] [CrossRef]
- Fowlie, G.; Cohen, N.; Ming, X. The Perturbance of Microbiome and Gut-Brain Axis in Autism Spectrum Disorders. Int. J. Mol. Sci. 2018, 19, 2251. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Dy AB, C.; Hagerman, R.J. The Gut Microbiota and Autism Spectrum Disorders. Front. Cell. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.-W.; Gandal, M.J.; Wang, B.; Kim, Y.-M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef]
- Yousefi, B.; Kokhaei, P.; Mehranfar, F.; Bahar, A.; Abdolshahi, A.; Emadi, A.; Eslami, M. The role of the host microbiome in autism and neurodegenerative disorders and effect of epigenetic procedures in the brain functions. Neurosci. Biobehav. Rev. 2022, 132, 998–1009. [Google Scholar] [CrossRef]
- Liao, X.; Chen, M.; Li, Y. The glial perspective of autism spectrum disorder convergent evidence from postmortem brain and PET studies. Front. Neuroendocrinol. 2023, 70, 101064. [Google Scholar] [CrossRef]
- Taş, E.; Ulgen, K.O. Understanding the ADHD-Gut Axis by Metabolic Network Analysis. Metabolites 2023, 13, 592. [Google Scholar] [CrossRef]
- Wang, L.-J.; Li, S.-C.; Li, S.-W.; Kuo, H.-C.; Lee, S.-Y.; Huang, L.-H.; Chin, C.-Y.; Yang, C.-Y. Gut microbiota and plasma cytokine levels in patients with attention-deficit/hyperactivity disorder. Transl. Psychiatry 2022, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Payen, A.; Chen, M.J.; Carter, T.G.; Kilmer, R.P.; Bennett, J.M. Childhood ADHD, Going Beyond the Brain: A Meta-Analysis on Peripheral Physiological Markers of the Heart and the Gut. Front. Endocrinol. 2022, 13, 738065. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.O.; Holtzman, D.M. Current understanding of the Alzheimer’s disease-associated microbiome and therapeutic strategies. Exp. Mol. Med. 2024, 56, 86–94. [Google Scholar] [CrossRef]
- Ferreiro, A.L.; Choi, J.; Ryou, J.; Newcomer, E.P.; Thompson, R.; Bollinger, R.M.; Hall-Moore, C.; Ndao, I.M.; Sax, L.; Benzinger, T.L.; et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med. 2023, 15, eabo2984. [Google Scholar] [CrossRef]
- Cammann, D.; Lu, Y.; Cummings, M.J.; Zhang, M.L.; Cue, J.M.; Do, J.; Ebersole, J.; Chen, X.; Oh, E.C.; Cummings, J.L.; et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci. Rep. 2023, 13, 5258. [Google Scholar] [CrossRef]
- Dissanayaka, D.S.; Jayasena, V.; Rainey-Smith, S.R.; Martins, R.N.; Fernando, W.B. The Role of Diet and Gut Microbiota in Alzheimer’s Disease. Nutrients 2024, 16, 412. [Google Scholar] [CrossRef]
- Grabrucker, S.; Marizzoni, M.; Silajdžić, E.; Lopizzo, N.; Mombelli, E.; Nicolas, S.; Dohm-Hansen, S.; Scassellati, C.; Moretti, D.V.; Rosa, M.; et al. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain 2023, 146, 4916–4934. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Hu, Y.; Lu, L.; Zheng, C.-Y.; Fan, Y.; Wu, B.; Zou, T.; Luo, X.; Zhang, X.; et al. Gut bacterial profiles in Parkinson’s disease: A systematic review. CNS Neurosci. Ther. 2022, 29, 140–157. [Google Scholar] [CrossRef]
- Romano, S.; Savva, G.M.; Bedarf, J.R.; Charles, I.G.; Hildebrand, F.; Narbad, A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Parkinson’s Dis. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Hey, G.; Nair, N.; Klann, E.; Gurrala, A.; Safarpour, D.; Mai, V.; Ramirez-Zamora, A.; Vedam-Mai, V. Therapies for Parkinson’s disease and the gut microbiome: Evidence for bidirectional connection. Front. Aging Neurosci. 2023, 15, 1151850. [Google Scholar] [CrossRef]
- Devos, D.; Lebouvier, T.; Lardeux, B.; Biraud, M.; Rouaud, T.; Pouclet, H.; Coron, E.; Bruley des Varannes, S.; Naveilhan, P.; Nguyen, J.-M.; et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013, 50, 42–48. [Google Scholar] [CrossRef]
- Tizabi, Y.; Getachew, B.; Hauser, S.R.; Tsytsarev, V.; Manhães, A.C.; Da Silva, V.D.A. Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction. Brain Sci. 2024, 14, 558. [Google Scholar] [CrossRef]
- Hanslik, K.L.; Marino, K.M.; Ulland, T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell. Neurosci. 2021, 15, 718324. [Google Scholar] [CrossRef]
- Su, X.; Yin, X.; Liu, Y.; Yan, X.; Zhang, S.; Wang, X.; Lin, Z.; Zhou, X.; Gao, J.; Wang, Z.; et al. Gut Dysbiosis Contributes to the Imbalance of Treg and Th17 Cells in Graves’ Disease Patients by Propionic Acid. J. Clin. Endocrinol. Metab. 2020, 105, 3526–3547. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Li, M.; Zhang, B.; Chang, P. Bacterial dysbiosis incites Th17 cell revolt in irradiated gut. Biomed. Pharmacother. 2020, 131, 110674. [Google Scholar] [CrossRef]
- Cosorich, I.; Dalla-Costa, G.; Sorini, C.; Ferrarese, R.; Messina, M.J.; Dolpady, J.; Radice, E.; Mariani, A.; Testoni, P.A.; Canducci, F.; et al. High frequency of intestinal T H 17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017, 3, e1700492. [Google Scholar] [CrossRef]
- Sauma, S.; Casaccia, P. Does the gut microbiota contribute to the oligodendrocyte progenitor niche? Neurosci. Lett. 2020, 715, 134574. [Google Scholar] [CrossRef]
- Bronzini, M.; Maglione, A.; Rosso, R.; Matta, M.; Masuzzo, F.; Rolla, S.; Clerico, M. Feeding the gut microbiome: Impact on multiple sclerosis. Front. Immunol. 2023, 14, 1176016. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Thirion, F.; Sellebjerg, F.; Fan, Y.; Lyu, L.; Hansen, T.H.; Pons, N.; Levenez, F.; Quinquis, B.; Stankevic, E.; Søndergaard, H.B.; et al. The gut microbiota in multiple sclerosis varies with disease activity. Genome Med. 2023, 15, 1. [Google Scholar] [CrossRef]
- Ordoñez-Rodriguez, A.; Roman, P.; Rueda-Ruzafa, L.; Campos-Rios, A.; Cardona, D. Changes in Gut Microbiota and Multiple Sclerosis: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 4624. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Hohlfeld, R.; Baranzini, S.E. The role of the gut microbiota in multiple sclerosis. Nat. Rev. Neurol. 2022, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, B.; Zhou, S.; Huang, Z.; Xu, Y.; Lu, X.; Zheng, X.; Ouyang, D. Associations between gut microbiota and sleep: A two-sample, bidirectional Mendelian randomization study. Front. Microbiol. 2023, 14, 1236847. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Karl, J.P.; Whitney, C.C.; Wilson, M.A.; Fagnant, H.S.; Radcliffe, P.N.; Chakraborty, N.; Campbell, R.; Hoke, A.; Gautam, A.; Hammamieh, R.; et al. Severe, short-term sleep restriction reduces gut microbiota community richness but does not alter intestinal permeability in healthy young men. Sci. Rep. 2023, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Durante-Mangoni, E. Unraveling the Puzzle: Health Benefits of Probiotics—A Comprehensive Review. J. Clin. Med. 2024, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.P.; Kuruvalli, G.; Syed, K.; Reddy, V.D. An Updated Review on Probiotic Production and Applications. Gastroenterol. Insights 2024, 15, 221–236. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S. “Chillax” with probiotics. Nat. Rev. Neurosci. 2011, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Tette, F.-M.; Kwofie, S.K.; Wilson, M.D. Therapeutic Anti-Depressant Potential of Microbial GABA Produced by Lactobacillus rhamnosus Strains for GABAergic Signaling Restoration and Inhibition of Addiction-Induced HPA Axis Hyperactivity. Curr. Issues Mol. Biol. 2022, 44, 1434–1451. [Google Scholar] [CrossRef]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef]
- Allen, A.P.; Hutch, W.; Borre, Y.E.; Kennedy, P.J.; Temko, A.; Boylan, G.; Murphy, E.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Bifidobacterium longum 1714 as a translational psychobiotic: Modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 2016, 6, e939. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, Ł.S.; Socała, K.; Michalak, K.; Wiater, A.; Ciszewski, A.; Majewska, M.; Marek, A.; Grądzki, Z.; Wlaź, P. The effect of psychoactive bacteria, Bifidobacterium longum Rosell®-175 and Lactobacillus rhamnosus JB-1, on brain proteome profiles in mice. Psychopharmacology 2023, 241, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Kanakupt, K.; Vester Boler, B.M.; Dunsford, B.R.; Fahey, G.C. Effects of short-chain fructooligosaccharides and galactooligosaccharides, individually and in combination, on nutrient digestibility, fecal fermentative metabolite concentrations, and large bowel microbial ecology of healthy adults cats. J. Anim. Sci. 2011, 89, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tan, D.; Yang, Z.; Tang, J.; Bai, W.; Tian, L. Fermentation patterns of prebiotics fructooligosaccharides-SCFA esters inoculated with fecal microbiota from ulcerative colitis patients. Food Chem. Toxicol. 2023, 180, 114009. [Google Scholar] [CrossRef] [PubMed]
- Mahalak, K.K.; Firrman, J.; Narrowe, A.B.; Hu, W.; Jones, S.M.; Bittinger, K.; Moustafa, A.M.; Liu, L. Fructooligosaccharides (FOS) differentially modifies the in vitro gut microbiota in an age-dependent manner. Front. Nutr. 2023, 9, 1058910. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Galacto-oligosaccharides and colorectal cancer: Feeding our intestinal probiome. J. Funct. Foods 2015, 12, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Arthur, J.C.; Jobin, C.; Keku, T.; Bruno-Barcena, J.M.; Azcarate-Peril, M.A. High purity galacto-oligosaccharides enhance specific Bifidobacterium species and their metabolic activity in the mouse gut microbiome. Benef. Microbes 2016, 7, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101828. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Church, J.S.; Bannish, J.A.; Adrian, L.A.; Rojas Martinez, K.; Henshaw, A.; Schwartzer, J.J. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.; Davies, M.; Bateman, E.; Crame, E.; Joyce, P.; Wignall, A.; Ariaee, A.; Gladman, M.A.; Wardill, H.; Bowen, J. Fibre-rich diet attenuates chemotherapy-related neuroinflammation in mice. Brain Behav. Immun. 2024, 115, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fan, D.; Huang, J.; Zuo, T. The gut microbiome: Linking dietary fiber to inflammatory diseases. Med. Microecol. 2022, 14, 100070. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated with Aging in Mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. Physical activity induced alterations of gut microbiota in humans: A systematic review. BMC Sports Sci. Med. Rehabil. 2022, 14, 122. [Google Scholar] [CrossRef] [PubMed]
- Boytar, A.N.; Skinner, T.L.; Wallen, R.E.; Jenkins, D.G.; Dekker Nitert, M. The Effect of Exercise Prescription on the Human Gut Microbiota and Comparison between Clinical and Apparently Healthy Populations: A Systematic Review. Nutrients 2023, 15, 1534. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid. Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Barber, A.F.; Noya, S.B.; Williams, J.A.; Li, F.; Daniel, S.G.; Bittinger, K.; Fang, J.; Sehgal, A. The microbiome stabilizes circadian rhythms in the gut. Proc. Natl. Acad. Sci. USA 2023, 120, e2217532120. [Google Scholar] [CrossRef] [PubMed]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Engen, P.A.; Keshavarzian, A. Circadian Rhythm and the Gut Microbiome. Int. Rev. Neurobiol. 2016, 131, 193–205. [Google Scholar] [CrossRef]
- Heddes, M.; Altaha, B.; Niu, Y.; Reitmeier, S.; Kleigrewe, K.; Haller, D.; Kiessling, S. The intestinal clock drives the microbiome to maintain gastrointestinal homeostasis. Nat. Commun. 2022, 13, 6068. [Google Scholar] [CrossRef]
- Amara, J.; Itani, T.; Hajal, J.; Bakhos, J.J.; Saliba, Y.; Aboushanab, S.A.; Kovaleva, E.G.; Fares, N.; Mondragon, A.C.; Miranda, J.M. Circadian Rhythm Perturbation Aggravates Gut Microbiota Dysbiosis in Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2024, 16, 247. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.; Bowers, S.J.; Depner, C.M.; González, A.; Reynolds, A.C.; Wright, K.P. Sleep and circadian disruption and the gut microbiome-possible links to dysregulated metabolism. Curr. Opin. Endocr. Metab. Res. 2021, 17, 26–37. [Google Scholar] [CrossRef]
- Vasey, C.; McBride, J.; Penta, K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients 2021, 13, 3480. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xie, R.; Liu, X.; Chen, S.; Tang, H. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, B.; He, Y.; Huang, P.; Du, J.; He, G.; Zhang, P.; Tang, H.; Chen, S. Early changes of fecal short-chain fatty acid levels in patients with mild cognitive impairments. CNS Neurosci. Ther. 2023, 29, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, M.; Fang, R.; Tang, C.; Wang, Q. Diet and physical activity influence the composition of gut microbiota, benefit on Alzheimer’s Disease. Food Sci. Hum. Wellness 2023, 13, 541–555. [Google Scholar] [CrossRef]
- Hong, C.T.; Chan, L.; Chen, K.Y.; Lee, H.H.; Huang, L.K.; Yang, Y.C.S.; Liu, Y.-R.; Hu, C.-J. Rifaximin Modifies Gut Microbiota and Attenuates Inflammation in Parkinson’s Disease: Preclinical and Clinical Studies. Cells 2022, 11, 3468. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.C.; Lee, S.; McPhail, M.; Da Silva, K.; Guilly, S.; Zamalloa, A.; Witherden, E.; Støy, S.; Manakkat Vijay, G.K.; Pons, N.; et al. Rifaximin reduces gut-derived inflammation and mucin degradation in cirrhosis and encephalopathy: RIFSYS randomised controlled trial. J. Hepatol. 2021, 76, 332–342. [Google Scholar] [CrossRef]
- Kimer, N.; Meldgaard, M.; Hamberg, O.; Kronborg, T.M.; Lund, A.M.; Møller, H.J.; Bendtsen, F.; Ytting, H. The impact of rifaximin on inflammation and metabolism in alcoholic hepatitis: A randomized clinical trial. PLoS ONE 2022, 17, e0264278. [Google Scholar] [CrossRef]
- Engen, P.A.; Zaferiou, A.; Rasmussen, H.; Naqib, A.; Green, S.J.; Fogg, L.F.; Forsyth, C.B.; Raeisi, S.; Hamaker, B.; Keshavarzian, A. Single-Arm, Non-randomized, Time Series, Single-Subject Study of Fecal Microbiota Transplantation in Multiple Sclerosis. Front. Neurol. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Al, K.F.; Craven, L.J.; Gibbons, S.; Parvathy, S.N.; Wing, A.C.; Graf, C.; Parham, K.A.; Kerfoot, S.M.; Wilcox, H.; Burton, J.P.; et al. Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial. Mult. Scler. J.-Exp. Transl. Clin. 2022, 8, 205521732210866. [Google Scholar] [CrossRef] [PubMed]
- Matheson, J.-A.T.; Holsinger, R.M.D. The Role of Fecal Microbiota Transplantation in the Treatment of Neurodegenerative Diseases: A Review. Int. J. Mol. Sci. 2023, 24, 1001. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).