Effects of Chemically-Functionalized Single-Walled Carbon Nanotubes on the Morphology and Vitality of D54MG Human Glioblastoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Water-Soluble Single-Walled Carbon Nanotubes
2.2. Human Glioblastoma Multiforme Cell Culture
2.3. Live Cell Imaging
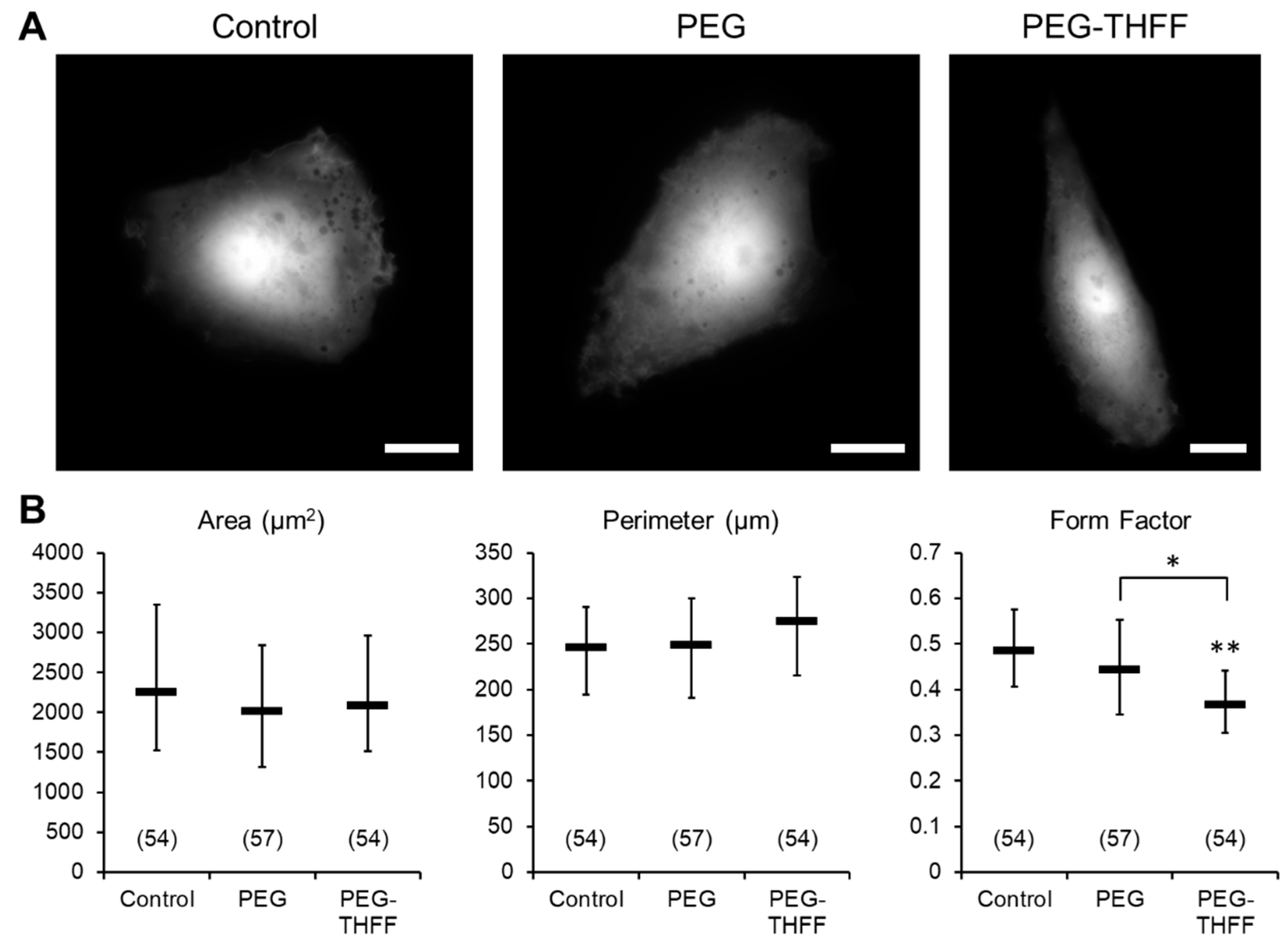
2.4. Morphometric Analysis
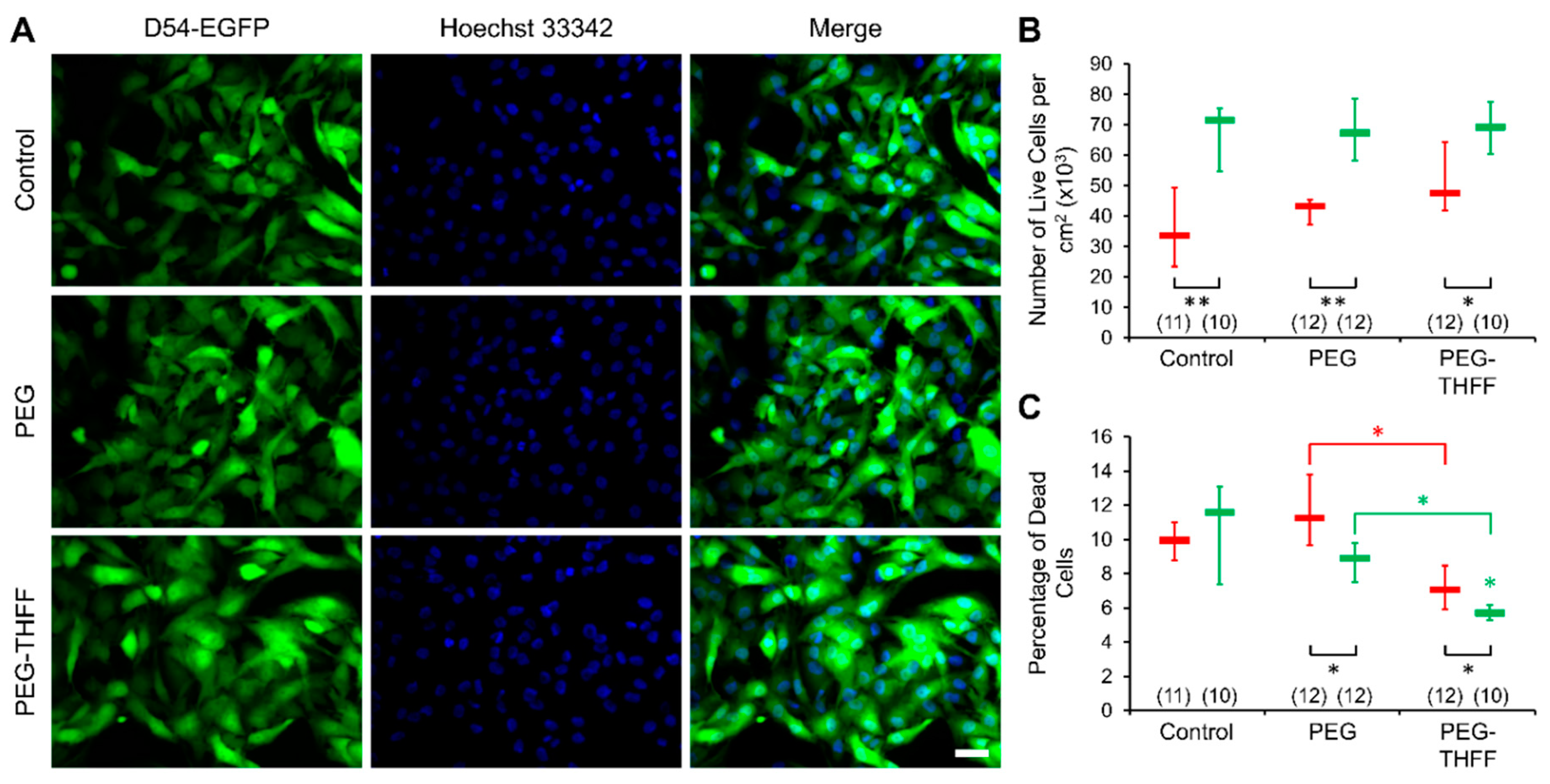
2.5. Vitality Assay
2.6. Statistics
3. Results
3.1. Effect of wsSWCNTs on the Morphology of D54MG-EGFP Glioma Cells
3.2. Effect of wsSWCNTs on the Vitality of D54MG-EGFP Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zagzag, D.; Esencay, M.; Mendez, O.; Yee, H.; Smirnova, I.; Huang, Y.; Chiriboga, L.; Lukyanov, E.; Liu, M.; Newcomb, E.W. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1α/CXCR4 expression in glioblastomas. Am. J. Pathol. 2008, 173, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, H. Role of Ion Channels and Amino-Acid Transporters in the Biology of Astrocytic Tumors. In Astrocytes in (Patho)Physiology of the Nervous System; Parpura, V., Haydon, P.G., Eds.; Springer: New York, NY, USA, 2009; pp. 527–546. [Google Scholar]
- Nakada, M.; Niska, J.A.; Tran, N.L.; McDonough, W.S.; Berens, M.E. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am. J. Pathol. 2005, 167, 565–576. [Google Scholar] [CrossRef]
- Wang, S.D.; Rath, P.; Lal, B.; Richard, J.P.; Li, Y.; Goodwin, C.R.; Laterra, J.; Xia, S. EphB2 receptor controls proliferation/migration dichotomy of glioblastoma by interacting with focal adhesion kinase. Oncogene 2012, 31, 5132–5143. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, E.; Ni, Y.; Malarkey, E.B.; Montana, V.; McWilliams, J.L.; Haddon, R.C.; Parpura, V. Applications of carbon nanotubes in biotechnology and biomedicine. J. Biomed. Nanotechnol. 2005, 1, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, E.; Haddon, R.C.; Parpura, V. Biofunctionalization of Carbon Nanotubes. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Zhao, D.; Alizadeh, D.; Zhang, L.; Liu, W.; Farrukh, O.; Manuel, E.; Diamond, D.J.; Badie, B. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin. Cancer Res. 2010, 17, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, D.; White, E.E.; Sanchez, T.C.; Liu, S.; Zhang, L.; Badie, B.; Berlin, J.M. Immunostimulatory CpG on carbon nanotubes selectively inhibits migration of brain tumor cells. Bioconjug. Chem. 2018, 29, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, W.; He, L.; Nakayama, N.; Chen, K.; Sun, X.; Chen, X.; Dai, H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2006, 2, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.; Fang, X.; Chen, M.-T.; Wang, W.; Ferreira, R.; Jhaveri, N.; Gundersen, M.; Zhou, C.; Pagnini, P.; Hofman, F.M.; et al. Sequential administration of carbon nanotubes and near-infrared radiation for the treatment of gliomas. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Wu, B.; Xing, D.; Zhou, F.; Wang, H.; Tang, Y. Functional single-walled carbon nanotubes based on an integrin αvβ3 monoclonal antibody for highly efficient cancer cell targeting. Nanotechnology 2009, 20, 105102. [Google Scholar] [CrossRef] [PubMed]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Diao, S.; Wang, C.; Gong, H.; Liu, T.; Hong, G.; Shi, X.; Dai, H.; Liu, Z. Tumor metastasis inhibition by imaging-guided photothermal therapy with single-walled carbon nanotubes. Adv. Mater. 2014, 26, 5646–5652. [Google Scholar] [CrossRef] [PubMed]
- Itkis, M.E.; Borondics, F.; Yu, A.; Haddon, R.C. Bolometric infrared photoresponse of suspended single-walled carbon nanotube films. Science 2006, 312, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, M.K.; Verkhratsky, A.; Parpura, V. Probing astroglia with carbon nanotubes: Modulation of form and function. Philos. Trans. R. Soc. B Boil. Sci. 2014, 369, 20130598. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, M.K.; Kalinina, I.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized water-soluble single-walled carbon nanotubes modulate morpho-functional characteristics of astrocytes. Nano Lett. 2012, 12, 4742–4747. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, M.K.; Bekyarova, E.; Brenner, M.; Haddon, R.C.; Parpura, V. Changes in the morphology and proliferation of astrocytes induced by two modalities of chemically functionalized single-walled carbon nanotubes are differentially mediated by glial fibrillary acidic protein. Nano Lett. 2014, 14, 3720–3727. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, I.; Worsley, K.; Lugo, C.; Mandal, S.; Bekyarova, E.; Haddon, R.C. Synthesis, dispersion, and viscosity of Poly(ethylene glycol)-functionalized water-soluble single-walled carbon nanotubes. Chem. Mater. 2011, 23, 1246–1253. [Google Scholar] [CrossRef]
- Gottipati, M.K.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized single-walled carbon nanotubes enhance the glutamate uptake characteristics of mouse cortical astrocytes. Amino Acids 2015, 47, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Montana, V.; Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. 2011, 31, 4858–4867. [Google Scholar] [CrossRef] [PubMed]
- Habela, C.W.; Ernest, N.J.; Swindall, A.F.; Sontheimer, H. Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J. Neurophysiol. 2009, 101, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Parpura, V. Dissociated Cell Culture for Testing Effects of Carbon Nanotubes on Neuronal Growth. In Neurotrophic Factors; Humana Press: Totowa, NJ, USA, 2012; pp. 261–276. [Google Scholar]
- Gottipati, M.K.; Samuelson, J.J.; Kalinina, I.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Chemically functionalized single-walled carbon nanotube films modulate the morpho-functional and proliferative characteristics of astrocytes. Nano Lett. 2013, 13, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Eliasson, C.; Bjerkvig, R.; Pekny, M. Loss of GFAP expression in high-grade astrocytomas does not contribute to tumor development or progression. Oncogene 2003, 22, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, J.H.; Proctor, M.; Haroun, R.I.; Avellino, A.M.; Pindzola, A.A.; Kliot, M. Semipermeable polymer tubes provide a microenvironment for in vivo analysis of dorsal root regeneration. J. Biomech. Eng. 1991, 113, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Haddon, R.C.; Rao, A.M. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J. Mol. Neurosci. 2000, 14, 175–182. [Google Scholar] [CrossRef]
- Guo, W.; Li, A.; Jia, Z.; Yuan, Y.; Dai, H.; Li, H. Transferrin modified PEG-PLA-resveratrol conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharmacol. 2013, 718, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Hu, Q.; Feng, X.; Gao, X.; Menglin, J.; Kang, T.; Jiang, D.; Song, Q.; Chen, H.; Chen, J. PEG-PLA nanoparticles modified with APTEDB peptide for enhanced anti-angiogenic and anti-glioma therapy. Biomaterials 2014, 35, 8215–8226. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zhu, S.; Wang, C.; Zhou, L.; Zhang, L.; Li, Z.; Dai, Y. Glioma homing peptide-modified PEG-PCL nanoparticles for enhanced anti-glioma therapy. J. Drug Target. 2015, 24, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Saiki, M.; Matsui, T.; Soya, M.; Kashibe, T.; Shima, T.; Shimizu, T.; Naruto, T.; Kitayoshi, T.; Akimoto, K.; Ninomiya, S.; et al. Thiamine tetrahydrofurfuryl disulfide promotes voluntary activity through dopaminergic activation in the medial prefrontal cortex. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Huang, H.-Y.; Hsu, Y.-J.; Su, W.-H.; Shen, S.-Y.; Lee, M.-C.; Lin, C.-L.; Huang, C.-C. The effects of thiamine tetrahydrofurfuryl disulfide on physiological adaption and exercise performance improvement. Nutrients 2018, 10, 851. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hopkins, S.; Gottipati, M.K.; Montana, V.; Bekyarova, E.; Haddon, R.C.; Parpura, V. Effects of Chemically-Functionalized Single-Walled Carbon Nanotubes on the Morphology and Vitality of D54MG Human Glioblastoma Cells. Neuroglia 2018, 1, 327-338. https://doi.org/10.3390/neuroglia1020022
Hopkins S, Gottipati MK, Montana V, Bekyarova E, Haddon RC, Parpura V. Effects of Chemically-Functionalized Single-Walled Carbon Nanotubes on the Morphology and Vitality of D54MG Human Glioblastoma Cells. Neuroglia. 2018; 1(2):327-338. https://doi.org/10.3390/neuroglia1020022
Chicago/Turabian StyleHopkins, Seantel, Manoj K. Gottipati, Vedrana Montana, Elena Bekyarova, Robert C. Haddon, and Vladimir Parpura. 2018. "Effects of Chemically-Functionalized Single-Walled Carbon Nanotubes on the Morphology and Vitality of D54MG Human Glioblastoma Cells" Neuroglia 1, no. 2: 327-338. https://doi.org/10.3390/neuroglia1020022
APA StyleHopkins, S., Gottipati, M. K., Montana, V., Bekyarova, E., Haddon, R. C., & Parpura, V. (2018). Effects of Chemically-Functionalized Single-Walled Carbon Nanotubes on the Morphology and Vitality of D54MG Human Glioblastoma Cells. Neuroglia, 1(2), 327-338. https://doi.org/10.3390/neuroglia1020022





