Interlaminar Glia and Other Glial Themes Revisited: Pending Answers Following Three Decades of Glial Research
Abstract
:1. Introduction
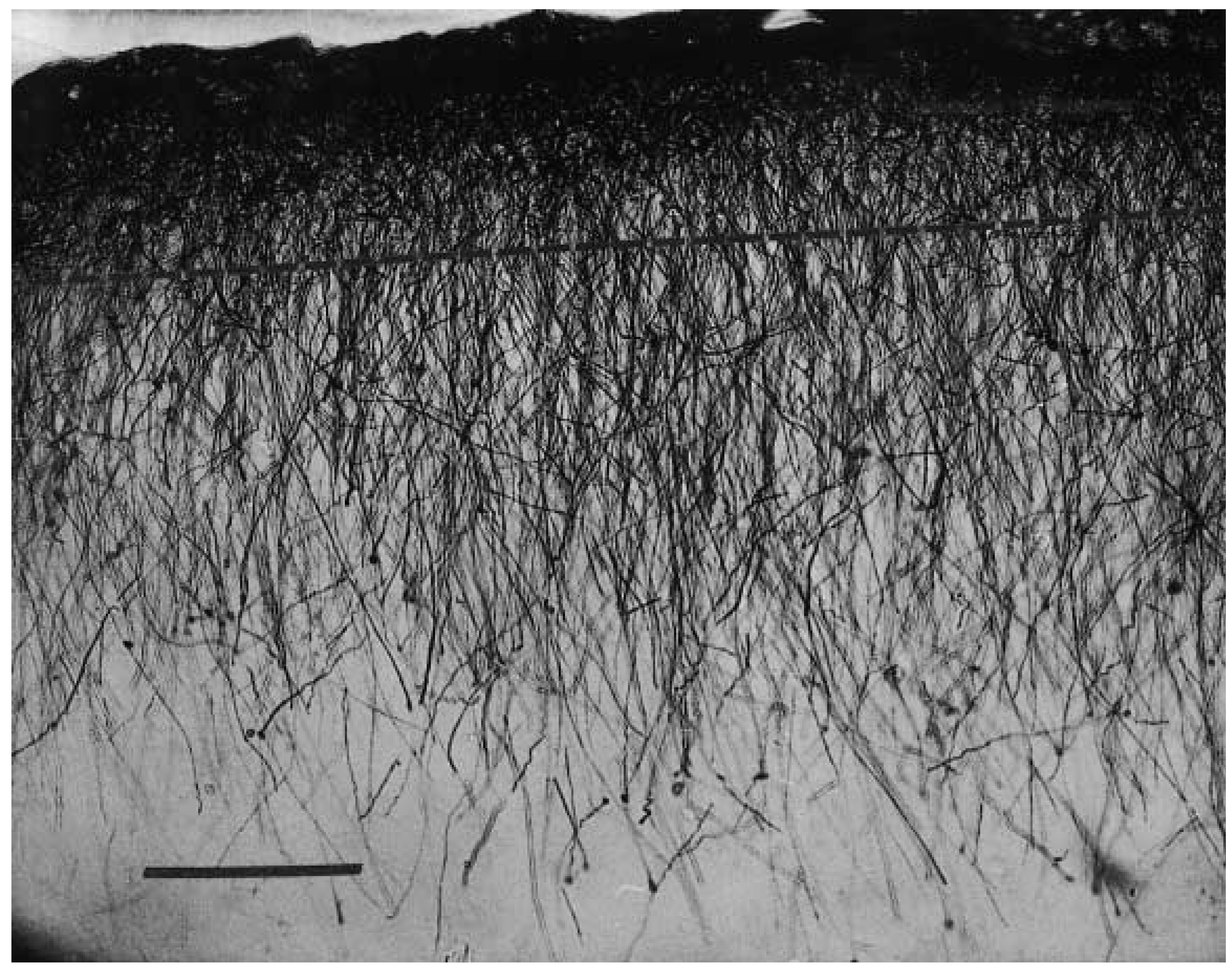
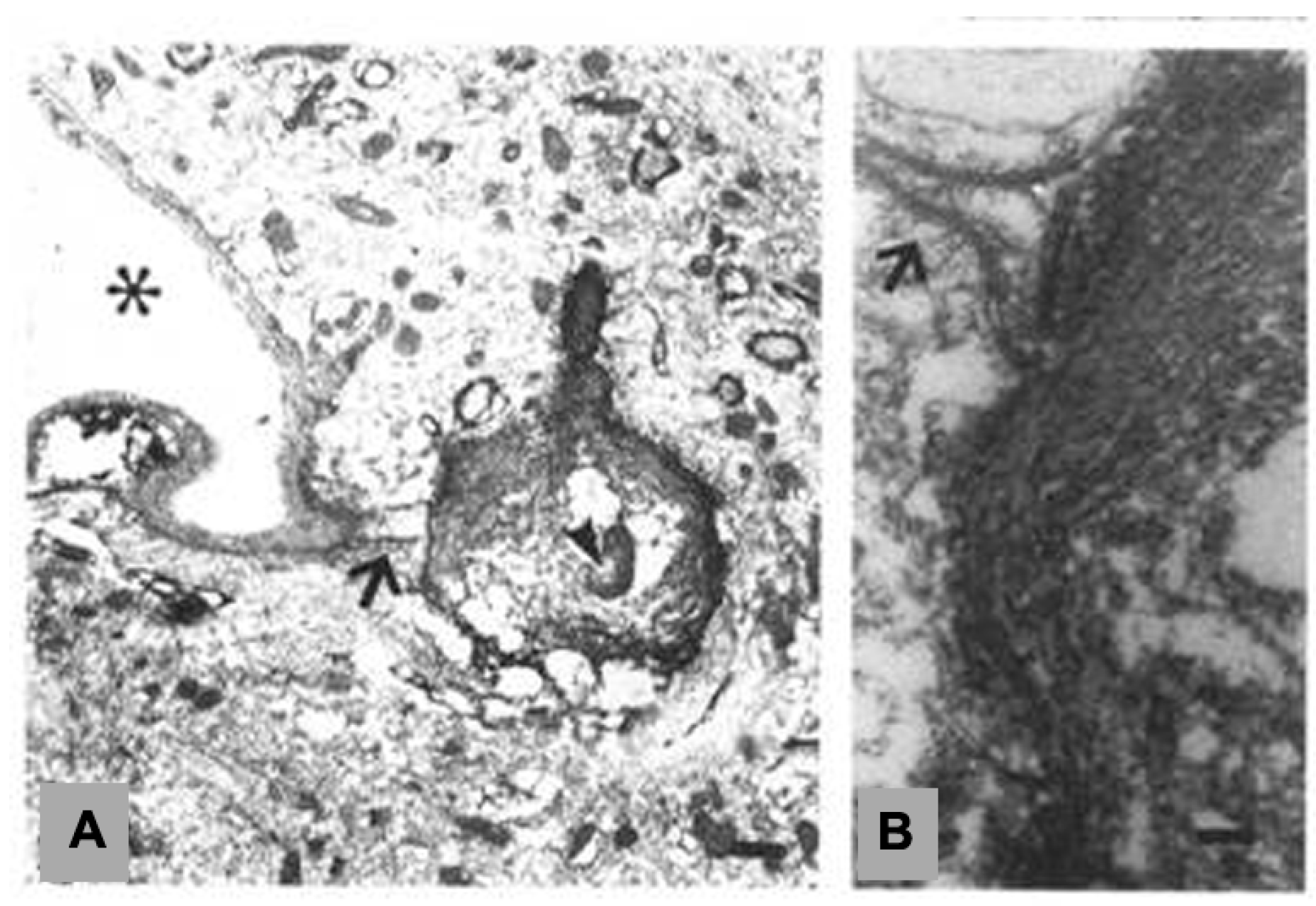
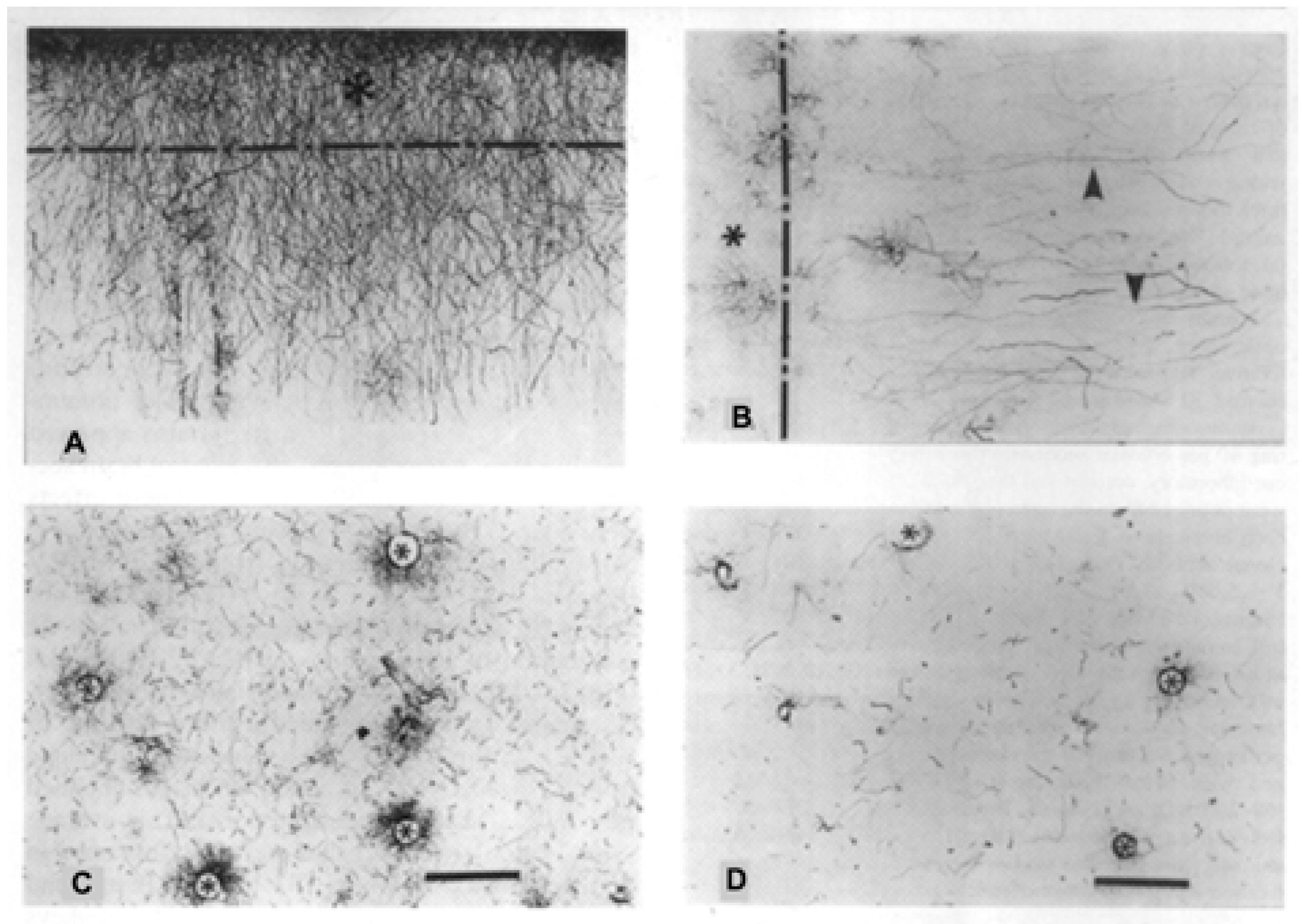
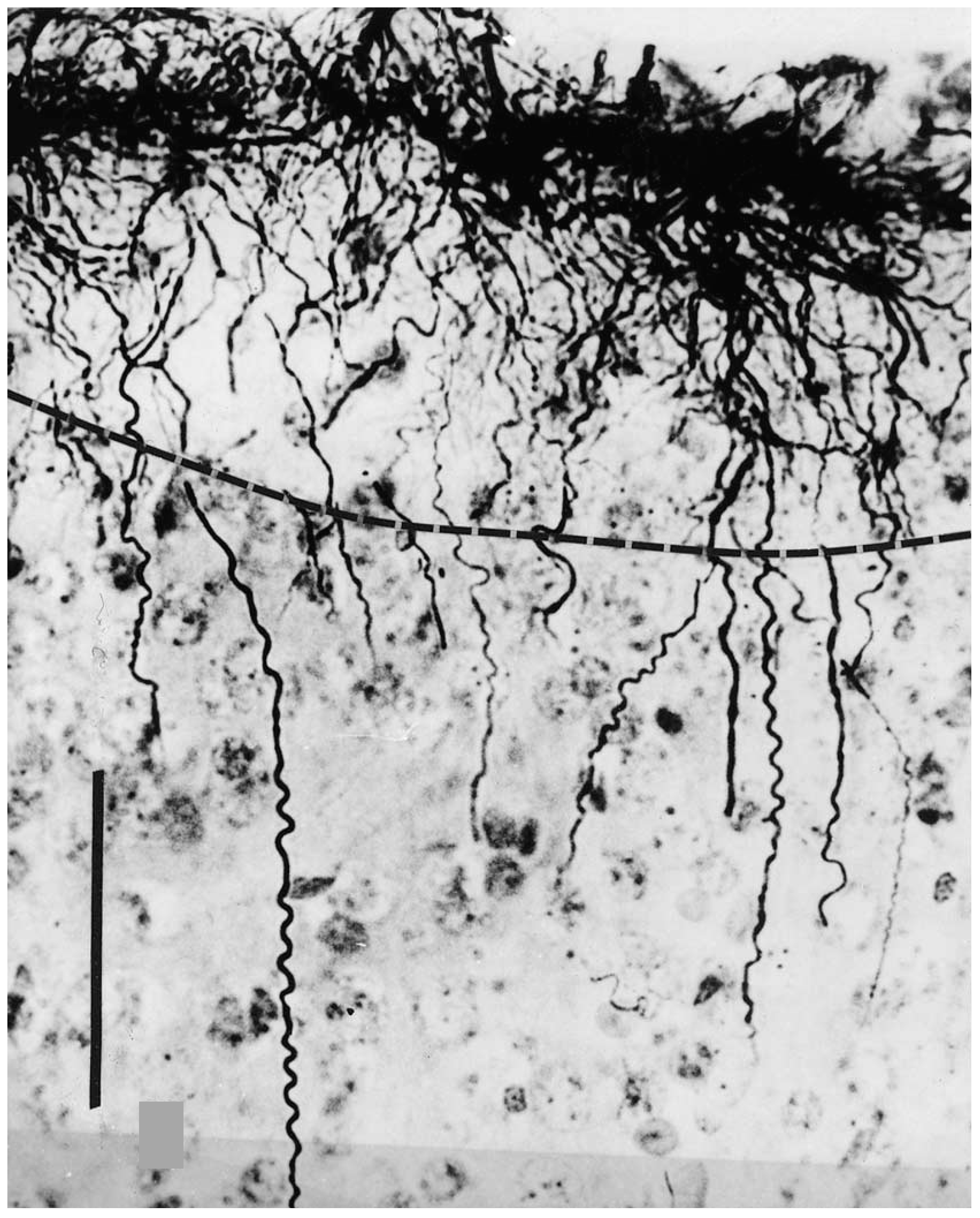
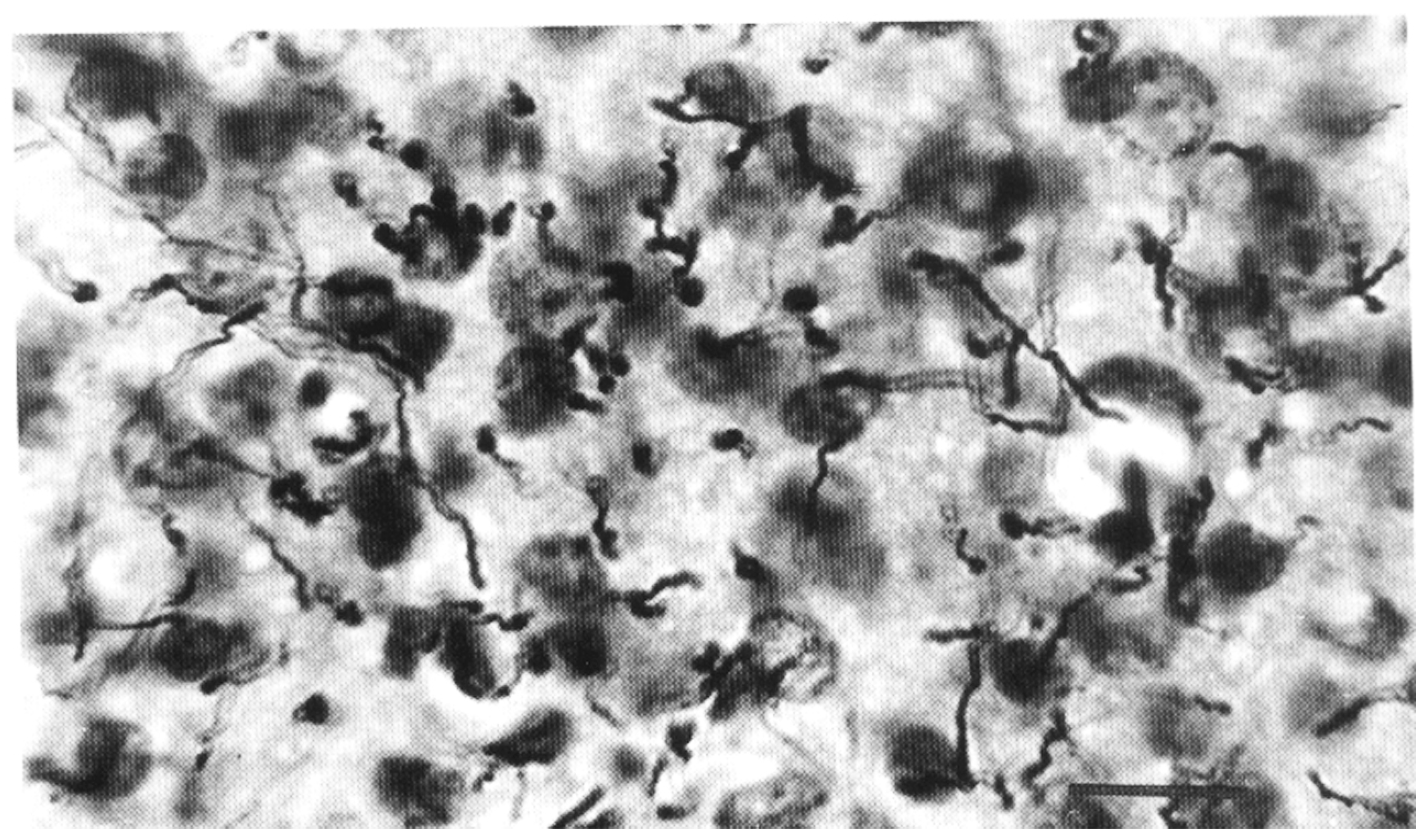
2. Interlaminar Astrocytes and Primate Brain Evolution
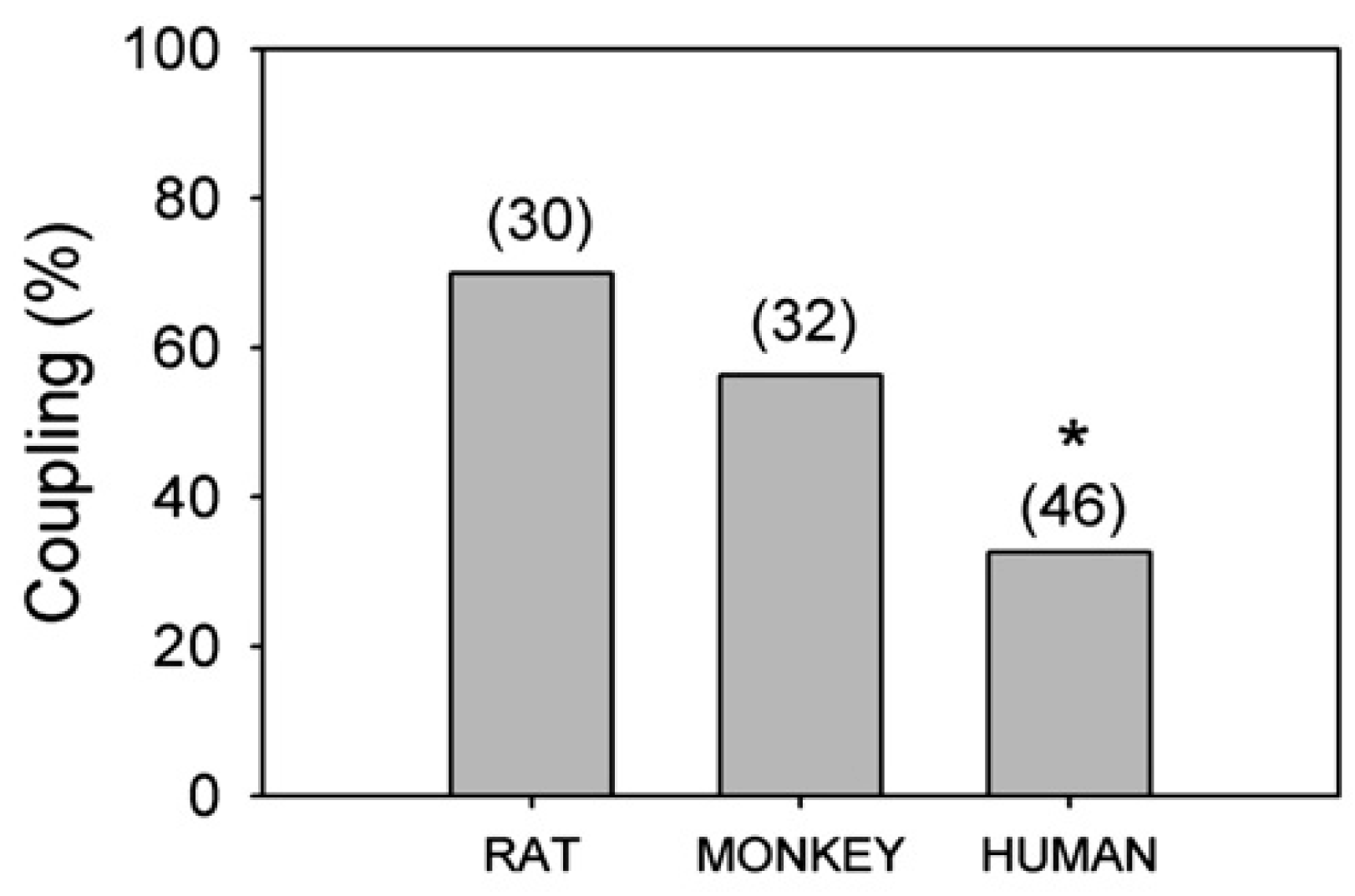
3. Analysis of Astroglia in the In Vitro Conditions
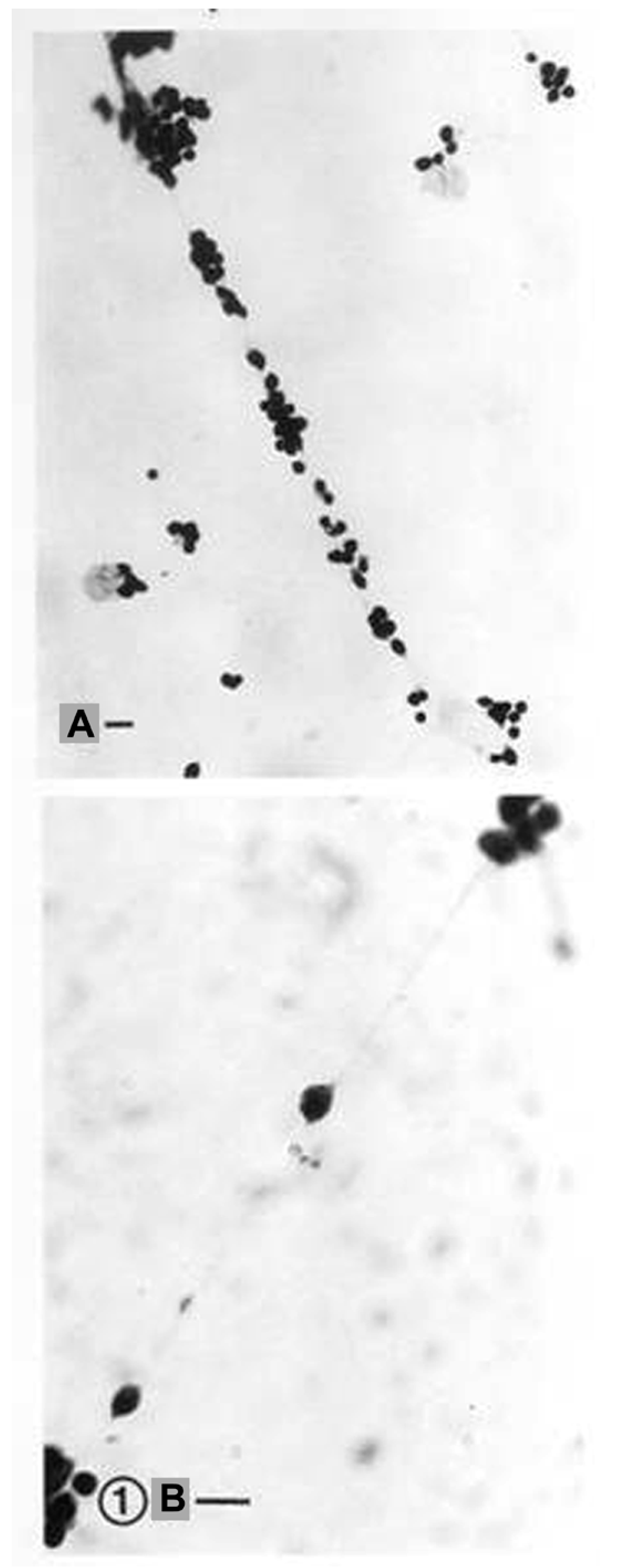
4. Glial Cells in Subcortical White Matter: Elements of a Subcortical, Distributed Information Neural Control Circuit?
5. Diffusible Factors Released by Astroglia
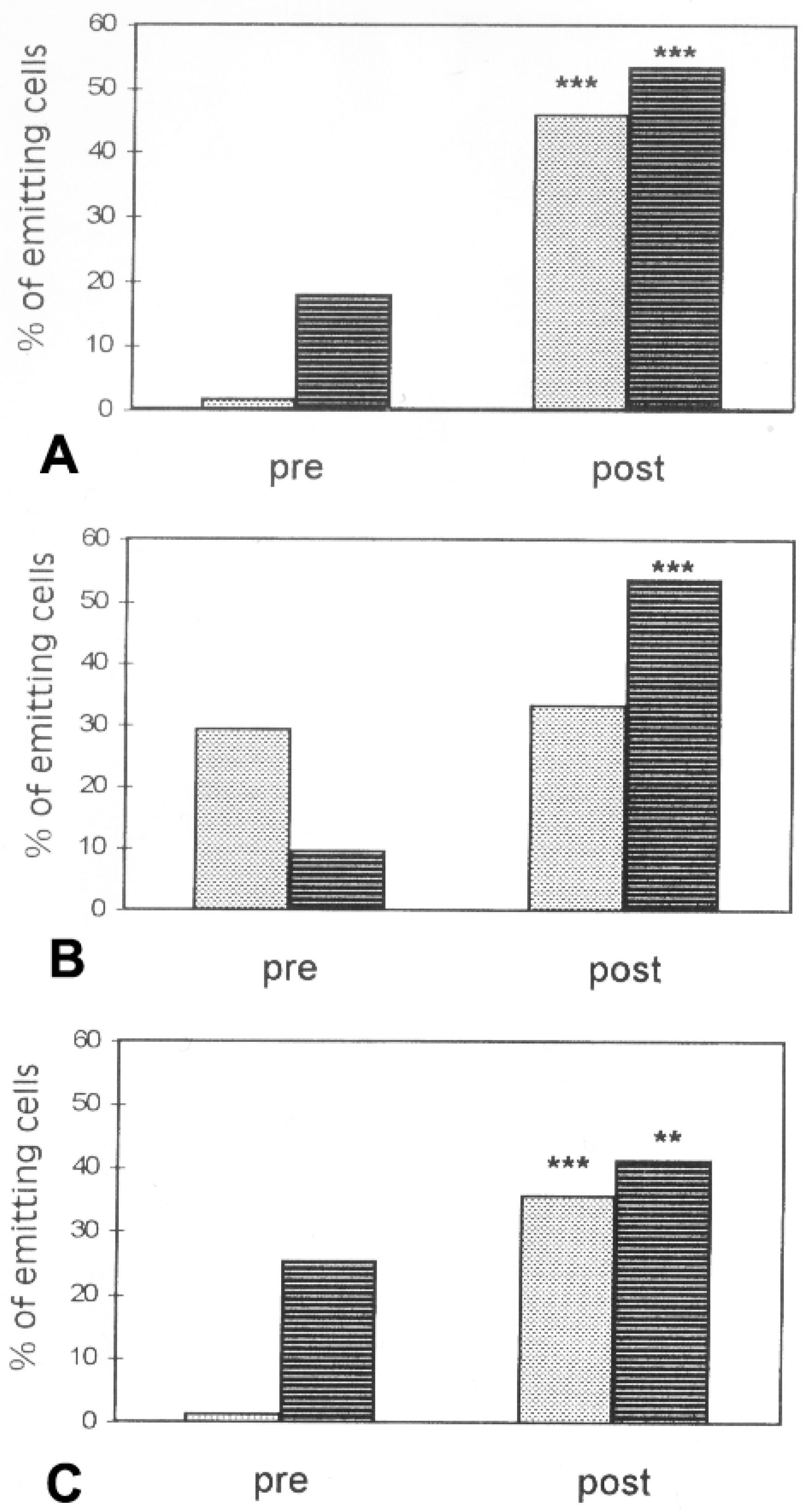
6. Environmental Effects on Glial Response to Injury and Dye Coupling
7. Summary and Conclusions
Acknowledgments
Conflicts of Interest
References
- Kettenmann, H.; Ransom, B.R. Neuroglia; Oxford University Press: New York, NY, USA, 2005; ISBN 0-19-515222-0. [Google Scholar]
- Verkhratsky, A.; Butt, A. Glial Physiology and Pathophysiology; Wiley-Blackwell: Oxford, UK, 2013; ISBN 978-0-470-97852-8. [Google Scholar]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A. Interlaminar astroglial processes in the cerebral cortex of adult monkeys but not of adult rats. Acta Anat. 1996, 155, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A. A columnar-supporting mode of astroglial architecture in the cerebral cortex of adult primates? Neurobiology 2001, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A. The interlaminar glia: From serendipity to hypothesis. Brain Struct. Funct. 2017, 222, 1109–1129. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Fuchs, E.; Härtig, W.; Marotte, L.R.; Puissant, V. “Rodent-like” and “primate-like” types of astroglial architecture in the adult cerebral cortex of mammals: A comparative study. Anat. Embryol. 2000, 201, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Sherwood, C.; Hof, P. Interlaminar astroglial processes in the cerebral cortex of great apes. Anat. Embryol. 2004, 429, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Lipina, S.; Yáñez, A.; Puissant, V. Postnatal development of interlaminar astroglial processes in the cerebral cortex of primates. Int. J. Dev. Neurosci. 1997, 15, 823–833. [Google Scholar] [CrossRef]
- Aiello, L.C.; Wheeler, P.T. The Expensive-Tissue Hypotesis: The brain and the digestive system in human and primate evolutiom. Curr. Anthropol. 1995, 36, 199–221. [Google Scholar] [CrossRef]
- Dunbar, R.I.M. The social brain hypothesis and its implications for social evolution. Ann. Hum. Biol. 2009, 36, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.M. Astrocytes and the evolution of the human brain. Med. Hypotheses 2014, 82, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Zilles, K.; Schlaug, G.; Matelli, M.; Luppino, G.; Schleicher, A.; Qü, M.; Dabringhaus, A.; Seitz, R.; Roland, P.E. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J. Anat. 1995, 187, 515–537. [Google Scholar] [PubMed]
- Zilles, K.; Palomero-Gallagher, N. Multiple transmitter receptors in regions and layers of the human cerebral cortex. Front. Neuroanat. 2017, 11, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.M.; Yao, J.; Cao, X. Genomics: Disclose the influence of human specific genetic variation on the evolution and development of cerebral cortex. Hereditas 2016, 38, 957–970. [Google Scholar] [PubMed]
- Muntané, G.; Santpere, G.; Verendeev, A.; Sherwood, C. Interhemispheric gene expression differences in the cerebral cortex of humans and macaque monkeys. Brain Struct. Funct. 2017, 222, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Silver, D.L. Enhancing our brains: Genomic mechanisms underlying cortical evolution. Semin. Cell Dev. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Reisin, H.D.; Jones, M.; Bentham, C. Development of interlaminar astroglial processes in the cerebral cortex of control and Down’s syndrome human cases. Exp. Neurol. 2005, 193, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Gayol, S.; Yáñez, A.; Marco, P. Immunocytochemical and electron microscope observations on astroglial interlaminar processes in the primate neocortex. J. Neurosci. Res. 1997, 48, 352–357. [Google Scholar] [CrossRef]
- Grosche, J.; Matyash, V.; Moller, T.; Verkhratsky, A.; Reichenbach, A.; Kettenmann, H. Microdomains for neuron–glia interaction: Parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 1999, 2, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Potokar, M.; Kreft, M.; Andersson, J.D.; Pangrsic, T.; Chowdhury, H.H.; Pekny, M.; Zorec, R. Cytoskeleton and vesicle mobility in astrocytes. Traffic 2007, 8, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Yáñez, A.; Lipina, S. Disruption of immunoreactive glial fibrillary acidic protein patterns in the Cebus apella striate cortex following loss of visual input. J. Brain Res. 1999, 39, 447–451. [Google Scholar]
- Reisin, H.; Colombo, J.A. Glial changes in primate cerebral cortex following long-term sensory deprivation. Brain Res. 2004, 1000, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Feldman, M.L. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. IV Terminations upon spiny dendrites. J. Neurocytol. 1977, 6, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Biane, J.S.; Takashima, Y.; Scanziani, M.; Conner, J.M.; Tuszynski, M.H. Thalamocortical projections onto behaviorally relevant neurons exhibit plasticity during adult motor learning. Neuron 2016, 89, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Quinn, B.; Puissant, V. Disruption of astroglial interlaminar processes in Alzheimer’s disease. Brain Res. Bull. 2002, 58, 235–242. [Google Scholar] [CrossRef]
- Colombo, J.A.; Yañez, A.; Lipina, S. Interlaminar astroglial processes in the cerebral cortex of non-human primates: Response to injury. J. Brain Res. 1997, 38, 503–512. [Google Scholar]
- Colombo, J.A.; Reisin, H.D.; Miguel-Hidalgo, J.J.; Rajkowska, G. Cerebral cortex astroglia and the brain of a genius: A propos of A. Einstein’s. Brain Res. Rev. 2006, 52, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Reisin, H.D.; Colombo, J.A. Considerations on the astroglial architecture and the columnar organization of the cerebral cortex. Cell. Mol. Neurobiol. 2002, 22, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Napp, M.I.; Yañez, A.; Reisin, H. Tissue printing of astroglial interlaminar processes from human and non-human primate cerebral cortex. Brain Res. Rev. 2001, 55, 561–565. [Google Scholar] [CrossRef]
- Barres, B.A.; Koroshetz, W.J.; Chun, L.L.Y.; Corey, D.P. Ion channel expression by white matter glia: The type 1 astrocyte. Neuron 1990, 5, 527–544. [Google Scholar] [CrossRef]
- Gayol, S.; Pannicke, T.; Reichenbach, E.; Colombo, J.A. Cell–cell coupling in cultures of striatal and cortical astrocytes of the monkey Cebus apella. J. Brain Res. 1999, 4, 473–478. [Google Scholar]
- Lanosa, X.A.; Reisin, H.D.; Santacroce, I.; Colombo, J.A. Astroglial dye-coupling: An in vitro analysis of regional and interspecies differences in rodents and primates. Brain Res. 2008, 1240, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Bentham, C. Immunohistochemical analysis of subcortical white matter astroglia of infant and adult primates, with a note on resident neurons. Brain Res. 2006, 1100, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A. Cellular complexity in subcortical white matter: A distributed control circuit? Brain Struct. Funct. 2018, 223, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Napp, M.I. Ex vivo astroglial-induced radial glia express in vivo markers. J. Neurosci. Res. 1996, 46, 674–677. [Google Scholar] [CrossRef]
- Colombo, J.A.; Napp, M.I. Forebrain and midbrain astrocytes promotes neuritogenesis in cultured chromaffin cells. Restor. Neurol. Neurosci. 1994, 7, 111–117. [Google Scholar] [PubMed]
- Hunter, K.E.; Hatten, M.E. Radial glial cell transformation to astrocytes in bidirectional regulation by a diffusible factor in embryonic forebrain. Proc. Natl. Acad. Sci. USA 1995, 92, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Napp, M.I. Cerebrospinal fluid from l-dopa-treated Parkinson’s disease patients is dystrophic for various neural cell types ex vivo: Effects of astroglia. Exp. Neurol. 1998, 154, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Uceda, G.; Colombo, J.A.; Michelena, P.; López, M.G.; García, A.G. Rat striatal astroglia induce morphological and neurochemical changes in adult bovine, adrenergic-enriched adrenal chromaffin cells in vitro. Restor. Neurol. Neurosci. 1995, 8, 129–136. [Google Scholar] [PubMed]
- Lipina, S.J.; Colombo, J.A. Dissociated functional recovery in parkinsonian monkeys following transplantation of astroglial cells. Brain Res. 2001, 911, 176–180. [Google Scholar] [CrossRef]
- Lipina, S.J.; Colombo, J.A. Premorbid exercising in specific cognitive tasks prevents impairment of performance in parkinsonian monkeys. Brain Res. 2007, 1134, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.C.; Krech, D.; Rosenzweig, M.R. The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neurol. 1964, 123, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Globus, A.; Rosenzweig, M.R.; Bennett, E.L.; Diamond, M.C. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J. Comp. Physiol. Psychol. 1973, 82, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sirevaag, A.M.; Greenough, W.T. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987, 424, 320–332. [Google Scholar] [CrossRef]
- Lanosa, X.A.; Santacroce, I.; Colombo, J.A. Exposure to environmental enrichment prior to a cerebral cortex stab wound attenuates the postlesional astroglia response in rats. Neuron Glia Biol. 2011, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, I. Plasticity of Astroglial Networks in the Cerebral Cortex of the Rat: Response to Environmental Enrichment and Physicochemical Variables. Ph.D. Thesis, University of Buenos Aires, Buenos Aires, Argentina, 2017. [Google Scholar]


© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, J.A. Interlaminar Glia and Other Glial Themes Revisited: Pending Answers Following Three Decades of Glial Research. Neuroglia 2018, 1, 7-20. https://doi.org/10.3390/neuroglia1010003
Colombo JA. Interlaminar Glia and Other Glial Themes Revisited: Pending Answers Following Three Decades of Glial Research. Neuroglia. 2018; 1(1):7-20. https://doi.org/10.3390/neuroglia1010003
Chicago/Turabian StyleColombo, Jorge A. 2018. "Interlaminar Glia and Other Glial Themes Revisited: Pending Answers Following Three Decades of Glial Research" Neuroglia 1, no. 1: 7-20. https://doi.org/10.3390/neuroglia1010003
APA StyleColombo, J. A. (2018). Interlaminar Glia and Other Glial Themes Revisited: Pending Answers Following Three Decades of Glial Research. Neuroglia, 1(1), 7-20. https://doi.org/10.3390/neuroglia1010003



