Vibration Therapy for Cancer-Related Bone Diseases
Abstract
1. Cancers Affect Bone Health
2. Mechanical Stimulation and Specifically Vibration
3. Vibration Effects on Cancer Models and Bone-Cancer Cell Interactions
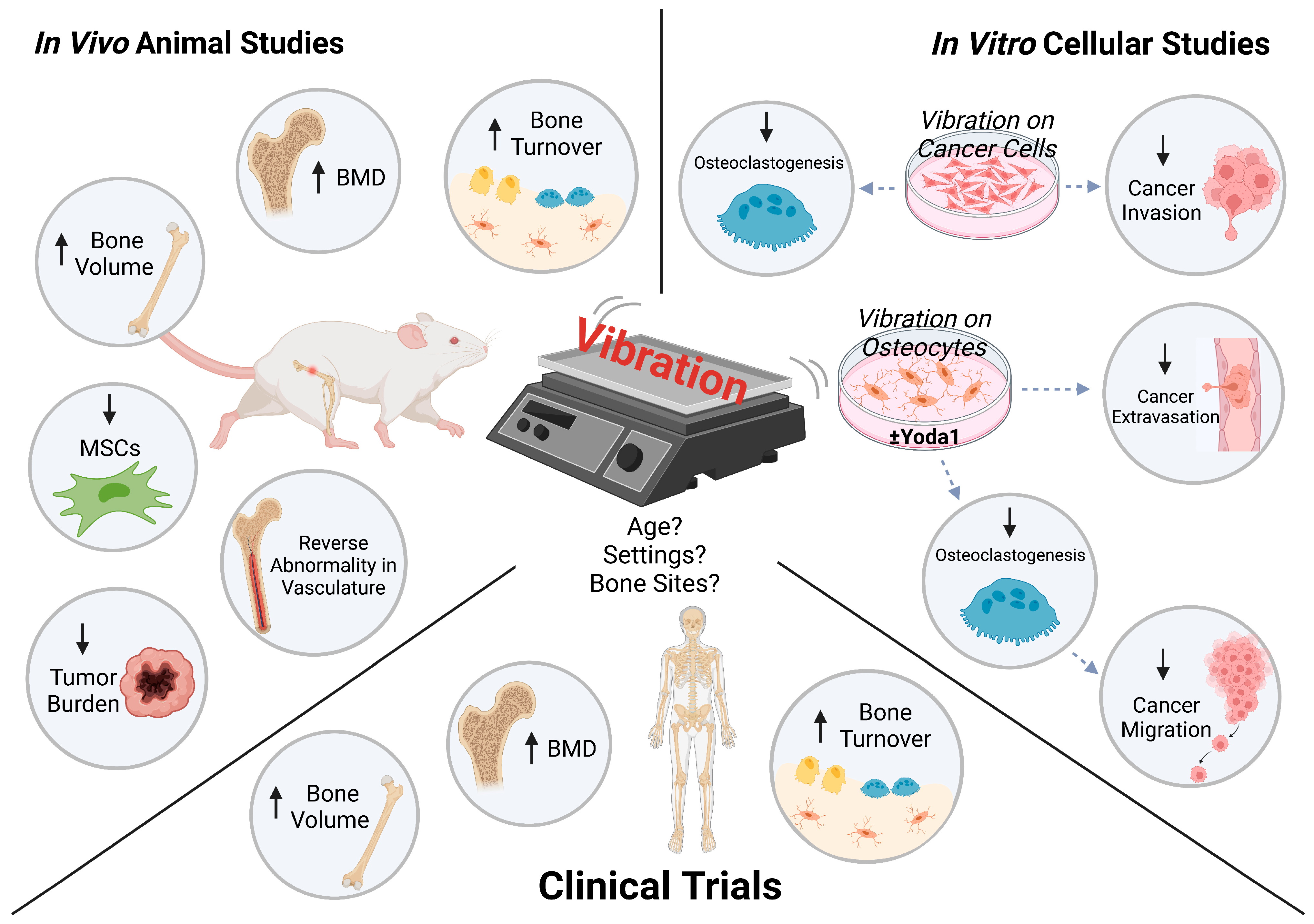
3.1. Safe to Perform
3.2. Bone Mineral Density (BMD) and Bone Volume
3.3. Bone Remodeling and Turnover Markers
3.4. Tumor Burden and Progression
3.5. Vascularization
3.6. Mesenchymal Stem Cell (MSC) Population
4. Parameters Contributing towards Vibration Efficacy
4.1. Age
4.2. Vibration Settings
4.2.1. Magnitude and Frequency
4.2.2. Rest Periods
4.3. Bone Site-Specific
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ALP | Alkaline phosphatase |
| BMC | Bone mineral content |
| BMD | Bone mineral density |
| BMPs | Bone morphogenetic proteins |
| BSAP | Bone-specific alkaline phosphatase |
| COX2 | Cyclooxygenase 2 |
| CTX | C-terminal telopeptide |
| DKK1 | Dickkopf-related protein 1 |
| ET1 | Endothelin 1 |
| FASL | First apoptosis signal ligand |
| FGFs | Fibroblast growth factors |
| G-CSF | Granulocyte colony-stimulating factor |
| G6PD | Glucose-6-phosphate dehydrogenase |
| HMLF | High-magnitude low-frequency |
| HUVECs | Human umbilical vein endothelial cells |
| IGFs | Insulin-like growth factors |
| IL11 | Interleukin 11 |
| IL6 | Interleukin 6 |
| LCN | Lacunocanalicular network |
| LINC | Linker of nucleoskeleton and cytoskeleton |
| LMHF | Low-magnitude high-frequency |
| LMLF | Low-magnitude low-frequency |
| MSCs | Mesenchymal stem cells |
| NADP-ME1 | Nicotinamide adenine dinucleotide phosphate-dependent malic enzyme 1 |
| NTX | N-terminal telopeptide |
| OPG | Osteoprotegerin |
| P1NP | Procollagen type 1 N-terminal propeptide |
| PTHrP/PTHLH | Parathyroid hormone-related protein/hormone-like hormone |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| RUNX2 | Runt-related transcription factor 2 |
| SA-βgal | Senescence-associated beta-galactosidase |
| SREs | Skeletal-related events |
| TGF-β | Transforming growth factor-beta |
| TRACP5b | Tartrate-resistant acid phosphatase 5b |
| VEGFs | Vascular endothelial growth factors |
| WBV | Whole body vibration |
| YAP | Yes-associated protein |
References
- Pulido, C.; Vendrell, I.; Ferreira, A.R.; Casimiro, S.; Mansinho, A.; Alho, I.; Costa, L. Bone metastasis risk factors in breast cancer. Ecancermedicalscience 2017, 11, 715. [Google Scholar] [CrossRef]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Haider, A.; Rashid, S.; Dakhilalla, A.; Al-Nabet, M.H.; Paget, S. Paget’s “seed and soil” theory of cancer metastasis: An idea whose time has come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Liu, X.; Cheng, K.; Sheng, J.; Kong, J.; Liu, T. Pre-metastatic niche formation in different organs induced by tumor extracellular vesicles. Front. Cell Dev. Biol. 2021, 9, 733627. [Google Scholar] [CrossRef]
- Furesi, G.; Rauner, M.; Hofbauer, L.C. Emerging players in prostate cancer–bone niche communication. Trends Cancer 2021, 7, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, E.G.; Delgado-Calle, J. The Emerging role of osteocytes in cancer in bone. JBMR Plus 2019, 3, e10186. [Google Scholar] [CrossRef]
- Hiraga, T. Bone metastasis: Interaction between cancer cells and bone microenvironment. J. Oral Biosci. 2019, 61, 95–98. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Anderson, J.; Cregor, M.D.; Hiasa, M.; Chirgwin, J.M.; Carlesso, N.; Yoneda, T.; Mohammad, K.S.; Plotkin, L.I.; Roodman, G.D.; et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 2016, 76, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.V.; Lam, C.; Dalmia, S.; Gao, P.; Young, J.; Middleton, K.; Liu, C.; Xu, H.; You, L. Mechanical regulation of breast cancer migration and apoptosis via direct and indirect osteocyte signaling. J. Cell. Biochem. 2018, 119, 5665–5675. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.V.; Xu, L.; Mei, X.; Middleton, K.; You, L. Mechanically stimulated osteocytes reduce the bone-metastatic potential of breast cancer cells in vitro by signaling through endothelial cells. J. Cell. Biochem. 2019, 120, 7590–7601. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.; Kiely, P.A.; Hoey, D.A. Mechanically stimulated osteocytes promote the proliferation and migration of breast cancer cells via a potential CXCL1/2 mechanism. Biochem. Biophys. Res. Commun. 2021, 534, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Song, X.; Seaman, K.; You, L. Microfluidic co-culture platforms for studying osteocyte regulation of other cell types under dynamic mechanical stimulation. Curr. Osteoporos. Rep. 2022, 20, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Song, X.; Ke, Y.; Raha, A.; Wu, Y.; Wasi, M.; Wang, L.; Geng, F.; You, L. Yoda1 enhanced low-magnitude high-frequency vibration on osteocytes in regulation of MDA-MB-231 breast cancer cell migration. Cancers 2022, 14, 3395. [Google Scholar] [CrossRef]
- Song, X.; Lin, C.-Y.; Mei, X.; Wang, L.; You, L. Reduction of breast cancer extravasation via vibration activated osteocyte regulation. iScience 2022, 25, 105500. [Google Scholar] [CrossRef] [PubMed]
- Yip, R.K.H.; Rimes, J.S.; Capaldo, B.D.; Vaillant, F.; Mouchemore, K.A.; Pal, B.; Chen, Y.; Surgenor, E.; Murphy, A.J.; Anderson, R.L.; et al. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nat. Commun. 2021, 12, 6920. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, K.; Stegen, S.; van Gastel, N.; Carmeliet, G. The vasculature: A vessel for bone metastasis. Bonekey Rep. 2015, 4, 742. [Google Scholar] [CrossRef][Green Version]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Tadi, P. Aromatase Inhibitors; StatPearls Publishing: Tampa, FL, USA, 2022; ISBN 9781444113730. [Google Scholar]
- Anagnostis, P.; Bosdou, J.K.; Vaitsi, K.; Goulis, D.G.; Lambrinoudaki, I. Estrogen and bones after menopause: A reappraisal of data and future perspectives. Hormones 2000, 20, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lester, J.; Coleman, R. Bone loss and the aromatase inhibitors. Br. J. Cancer 2005, 93, S16–S22. [Google Scholar] [CrossRef]
- Ho, S.-M. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol. 2003, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Vandenput, L.; Boonen, S.; Lindberg, M.K.; Bouillon, R.; Ohlsson, C. Androgens and bone. Endocr. Rev. 2004, 25, 389–425. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology 2002, 60, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L.; Khosla, S. Androgens and bone. Steroids 2009, 74, 296–305. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Stucci, S.; Tucci, M.; Silvestris, F. Cancer treatment-induced bone loss (CTIBL): Pathogenesis and clinical implications. Cancer Treat. Rev. 2015, 41, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Wissing, M.D. Chemotherapy- and irradiation-induced bone loss in adults with solid tumors. Curr. Osteoporos. Rep. 2015, 13, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Murali, B.; Ren, Q.; Luo, X.; Faget, D.V.; Cole, T.; Ricci, B.; Thotala, D.; Monahan, J.; Van Deursen, J.M.; et al. Therapy-induced senescence drives bone loss. Cancer Res. 2020, 80, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Wang, J.; Bai, J.; Zhai, J.; Zhu, G. Radiation induces primary osteocyte senescence phenotype and affects osteoclastogenesis invitro. Int. J. Mol. Med. 2021, 47, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kuai, F.; Shi, Q.; Yang, H. Doxorubicin restrains osteogenesis and promotes osteoclastogenesis in vitro. Am. J. Transl. Res. 2020, 12, 5640–5654. [Google Scholar] [PubMed]
- Park, H.J.; Yoon, S.Y.; Park, J.N.; Suh, J.H.; Choi, H.S. Doxorubicin Induces Bone Loss by Increasing Autophagy through a Mitochondrial ROS/TRPML1/TFEB Axis in Osteoclasts. Antioxidants 2022, 11, 1476. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Lin, T.; Tribble, M.B.; Zhu, J.; Altman, A.R.; Tseng, W.J.; Zhang, Y.; Akintoye, S.O.; Cengel, K.; Liu, X.S.; et al. PTH1-34 alleviates radiotherapy-induced local bone loss by improving osteoblast and osteocyte survival. Bone 2014, 67, 33–40. [Google Scholar] [CrossRef]
- Chandra, A.; Lin, T.; Young, T.; Tong, W.; Ma, X.; Tseng, W.J.; Kramer, I.; Kneissel, M.; Levine, M.A.; Zhang, Y.; et al. Suppression of sclerostin alleviates radiation-induced bone loss by protecting bone-forming cells and their progenitors through distinct mechanisms. J. Bone Miner. Res. 2017, 32, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Gelmon, K.A.; Pritchard, K.I.; Paterson, A.H.G. Bone-targeted agents and skeletal-related events in breast cancer patients with bone metastases: The state of the art. Curr. Oncol. 2012, 19, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Woo, S. bin Biophosphonate-related osteonecrosis of the jaws. Dent. Clin. N. Am. 2008, 52, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Qaisi, M.; Hargett, J.; Loeb, M.; Brown, J.; Caloss, R. Denosumab related osteonecrosis of the jaw with spontaneous necrosis of the soft palate: Report of a life threatening case. Case Rep. Dent. 2016, 2016, 5070187. [Google Scholar] [CrossRef]
- Kanker, W.; Fonds, O. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007; ISBN 9780972252225. [Google Scholar]
- Hackshaw-McGeagh, L.E.; Perry, R.E.; Leach, V.A.; Qandil, S.; Jeffreys, M.; Martin, R.M.; Lane, J.A. A systematic review of dietary, nutritional, and physical activity interventions for the prevention of prostate cancer progression and mortality. Cancer Causes Control. 2015, 26, 1521–1550. [Google Scholar] [CrossRef]
- Bonn, S.E.; Sjölander, A.; Lagerros, Y.T.; Wiklund, F.; Stattin, P.; Holmberg, E.; Grönberg, H.; Bälter, K. Physical activity and survival among men diagnosed with prostate cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, 57–64. [Google Scholar] [CrossRef]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A systematic review of exercise systematic reviews in the cancer literature. PM&R 2017, 9, S347–S384. [Google Scholar] [CrossRef]
- Sheill, G.; Guinan, E.M.; Peat, N.; Hussey, J. Considerations for exercise prescription in patients with bone metastases: A comprehensive narrative review. PM&R 2018, 10, 843–864. [Google Scholar] [CrossRef]
- Lynch, M.E.; Brooks, D.; Mohanan, S.; Lee, M.J.; Polamraju, P.; Dent, K.; Bonassar, L.J.; van der Meulen, M.C.H.; Fischbach, C. In vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J. Bone Miner. Res. 2013, 28, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pei, S.; Wasi, M.; Parajuli, A.; Yee, A.; You, L.; Wang, L. Moderate tibial loading and treadmill running, but not overloading, protect adult murine bone from destruction by metastasized breast cancer. Bone 2021, 153, 116110. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Tseng, S.Y.; Chen, C.N.; Liao, W.C.; Wang, C.H.; Lee, M.C.; Hsu, P.S. Effect of 6 months of whole body vibration on lumbar spine bone density in postmenopausal women: A randomized controlled trial. Clin. Interv. Aging 2013, 8, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, L.C.; De Oliveira, R.G.; De Almeida Pires-Oliveira, D.A. Effects of whole-body vibration versus pilates exercise on bone mineral density in postmenopausal women: A randomized and controlled clinical trial. J. Geriatr. Phys. Ther. 2019, 42, E23–E31. [Google Scholar] [CrossRef] [PubMed]
- Gusi, N.; Raimundo, A.; Leal, A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: A randomized controlled trial. BMC Musculoskelet. Disord. 2006, 7, 92. [Google Scholar] [CrossRef]
- Bianchi, M.L.; Vai, S.; Baranello, G.; Broggi, F.; Judex, S.; Hangartner, T.; Rubin, C. Low-intensity vibration protects the weight-bearing skeleton and suppresses fracture incidence in boys with Duchenne muscular dystrophy: A prospective, randomized, double-blind, placebo-controlled clinical trial. JBMR Plus 2022, 6, e10685. [Google Scholar] [CrossRef] [PubMed]
- DiVasta, A.D.; Feldman, H.A.; Rubin, C.T.; Gallagher, J.S.; Stokes, N.; Kiel, D.P.; Snyder, B.D.; Gordon, C.M. The ability of low-magnitude mechanical signals to normalize bone turnover in adolescents hospitalized for anorexia nervosa. Osteoporos. Int. 2017, 28, 1255–1263. [Google Scholar] [CrossRef]
- Mogil, R.J.; Kaste, S.C.; Ferry, R.J.; Hudson, M.M.; Mulrooney, D.A.; Howell, C.R.; Partin, R.E.; Srivastava, D.K.; Robison, L.L.; Ness, K.K. Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors a randomized clinical trial. JAMA Oncol. 2016, 2, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Almstedt, H.C.; Grote, S.; Korte, J.R.; Perez Beaudion, S.; Shoepe, T.C.; Strand, S.; Tarleton, H.P. Combined aerobic and resistance training improves bone health of female cancer survivors. Bone Rep. 2016, 5, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.K.; Peddle-McIntyre, C.J.; Galvão, D.A.; Hunt, C.; Spry, N.; Newton, R.U. Whole body vibration exposure on markers of bone turnover, body composition, and physical functioning in breast cancer patients receiving aromatase inhibitor therapy: A randomized controlled trial. Integr. Cancer Ther. 2018, 17, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Seefried, L.; Genest, F.; Strömsdörfer, J.; Engelmann, B.; Lapa, C.; Jakob, F.; Baumann, F.T.; Sperlich, B.; Jundt, F. Impact of whole-body vibration exercise on physical performance and bone turnover in patients with monoclonal gammopathy of undetermined significance. J. Bone Oncol. 2020, 25, 100323. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Lippi, L.; Ammendolia, A.; Cisari, C.; Venetis, K.; Sajjadi, E.; Fusco, N.; Invernizzi, M. Physical exercise with or without whole-body vibration in breast cancer patients suffering from aromatase inhibitor—Induced musculoskeletal symptoms: A pilot randomized clinical study. J. Pers. Med. 2021, 11, 1369. [Google Scholar] [CrossRef] [PubMed]
- Oschwald, V.; Prokop, A.; Maas, V.; Streckmann, F.; Bloch, W.; Baumann, F.T.; Daeggelmann, J. Whole-body vibration training for inpatient children and adolescents receiving chemotherapy for first cancer diagnosis: An exploratory feasibility study. Ger. J. Exerc. Sport Res. 2022, 53, 30–36. [Google Scholar] [CrossRef]
- Pagnotti, G.M.; Adler, B.J.; Green, D.E.; Chan, M.E.; Frechette, D.M.; Shroyer, K.R.; Beamer, W.G.; Rubin, J.; Rubin, C.T. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone 2012, 51, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Pagnotti, G.M.; Chan, M.E.; Adler, B.J.; Shroyer, K.R.; Rubin, J.; Bain, S.D.; Rubin, C.T. Low intensity vibration mitigates tumor progression and protects bone quantity and quality in a murine model of myeloma. Bone 2016, 90, 69–79. [Google Scholar] [CrossRef]
- Matsumoto, T.; Mukohara, A. Effects of whole-body vibration on breast cancer bone metastasis and vascularization in mice. Calcif. Tissue Int. 2022, 111, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wright, L.E.; Pagnotti, G.M.; Uzer, G.; Powell, K.M.; Wallace, J.M.; Sankar, U.; Rubin, C.T.; Mohammad, K.; Guise, T.A.; et al. Mechanical suppression of breast cancer cell invasion and paracrine signaling to osteoclasts requires nucleo-cytoskeletal connectivity. Bone Res. 2020, 8, 40. [Google Scholar] [CrossRef]
- Ota, T.; Chiba, M.; Hayashi, H. Vibrational stimulation induces osteoblast differentiation and the upregulation of osteogenic gene expression in vitro. Cytotechnology 2016, 68, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- García-López, S.; Villanueva, R.E.; Massó-Rojas, F.; Páez-Arenas, A.; Meikle, M.C. Micro-vibrations at 30 Hz on bone cells cultivated in vitro produce soluble factors for osteoclast inhibition and osteoblast activity. Arch. Oral Biol. 2020, 110, 104594. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Lackner, I.; Liedert, A.; Fischer, V.; Ignatius, A. Effects of low-magnitude high-frequency vibration on osteoblasts are dependent on estrogen receptor α signaling and cytoskeletal remodeling. Biochem. Biophys. Res. Commun. 2018, 503, 2678–2684. [Google Scholar] [CrossRef]
- Gao, H.; Zhai, M.; Wang, P.; Zhang, X.; Cai, J.; Chen, X.; Shen, G.; Luo, E.; Jing, D. Low-level mechanical vibration enhances osteoblastogenesis via a canonical Wnt signaling-associated mechanism. Mol. Med. Rep. 2017, 16, 317–324. [Google Scholar] [CrossRef]
- Pravitharangul, A.; Suttapreyasri, S.; Leethanakul, C. Iliac and mandible osteoblasts exhibit varied responses to LMHF vibration. Cell Biol. Int. 2018, 42, 1349–1357. [Google Scholar] [CrossRef]
- Lau, E.; Al-Dujaili, S.; Guenther, A.; Liu, D.; Wang, L.; You, L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone 2010, 46, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Fukunaga, T.; Sasaki, K.; Seiryu, M.; Yoshizawa, M.; Takeshita, N.; Takano-Yamamoto, T. Vibration enhances osteoclastogenesis by inducing RANKL expression via NF-κB signaling in osteocytes. Bone 2019, 123, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Uzer, G.; Brobst, K.E.; Xie, Z.; Sen, B.; Yen, S.S.; Styner, M.; Rubin, J. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci. Rep. 2015, 5, 11049. [Google Scholar] [CrossRef]
- Olcum, M.; Ozcivici, E. Daily application of low magnitude mechanical stimulus inhibits the growth of MDA-MB-231 breast cancer cells in vitro. Cancer Cell Int. 2014, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, M.O.; Grogan, J.A.; Markelc, B.; Beardo, A.; Enjalbert, R.; Kaeppler, J.; Daly, N.; Hetherington, J.; KrügerKr, T.; Maini, P.K.; et al. Abnormal morphology biases hematocrit distribution in tumor vasculature and contributes to heterogeneity in tissue oxygenation. Proc. Natl. Acad. Sci. USA 2020, 114, 27811–27819. [Google Scholar] [CrossRef]
- Bennewith, K.L.; Durand, R.E. Quantifying transient hypoxia in human tumor xenografts by flow cytometry. Cancer Res. 2004, 64, 6183–6189. [Google Scholar] [CrossRef]
- Harris, A.L. Hypoxia-a key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Reymond, N.; D’Água, B.B.; Ridley, A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 2013, 13, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Prasadam, I.; Zhou, Y.; Du, Z.; Chen, J.; Crawford, R.; Xiao, Y. Osteocyte-induced angiogenesis via VEGF-MAPK-dependent pathways in endothelial cells. Mol. Cell. Biochem. 2014, 386, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xu, Z.L.; Zhao, T.J.; Ye, L.H.; Zhang, X.D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008, 269, 67–77. [Google Scholar] [CrossRef]
- Rubin, C.T.; Capilla, E.; Luu, Y.K.; Busa, B.; Crawford, H.; Nolan, D.J.; Mittal, V.; Rosen, C.J.; Pessin, J.E.; Judex, S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl. Acad. Sci. USA 2007, 104, 17879–17884. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Q.; Liu, Y.; Zhang, L.; Li, D.; Zhu, Z.; Gan, X.; Yu, H. Vibration loading promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells via p38 MAPK signaling pathway. J. Biomech. 2018, 71, 67–75. [Google Scholar] [CrossRef]
- Pongkitwitoon, S.; Uzer, G.; Rubin, J.; Judex, S. Cytoskeletal configuration modulates mechanically induced changes in mesenchymal stem cell osteogenesis, morphology, and stiffness. Sci. Rep. 2016, 6, 34791. [Google Scholar] [CrossRef]
- Li, H.; Wu, W.; He, X.; Cao, C.; Yu, X.; Zeng, Y.; Li, L. Applying vibration in early postmenopausal osteoporosis promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells and suppresses postmenopausal osteoporosis progression. Biosci. Rep. 2019, 39, BSR20191011. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, T.; Yang, X.; Li, Y.; Xie, D.; Zheng, W.; Cui, H.; Deng, W.; Tan, X. Low-magnitude, high-frequency vibration promotes the adhesion and the osteogenic differentiation of bone marrow-derived mesenchymal stem cells cultured on a hydroxyapatite-coated surface: The direct role of Wnt/β-catenin signaling pathway activation. Int. J. Mol. Med. 2016, 38, 1531–1540. [Google Scholar] [CrossRef]
- Turner, S.; Torode, M.; Climstein, M.; Naughton, G.; Greene, D.; Baker, M.K.; Fiatarone Singh, M.A. A randomized controlled trial of whole body vibration exposure on markers of bone turnover in postmenopausal women. J. Osteoporos. 2011, 2011, 710387. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, C.S.; Johncola, A.J.; Batzdorf, A.S.; Jones, B.C.; al Mukaddam, M.; Sexton, K.; Shults, J.; Leonard, M.B.; Snyder, P.J.; Wehrli, F.W. Effect of low-intensity vibration on bone strength, microstructure, and adiposity in pre-osteoporotic postmenopausal women: A randomized placebo-controlled trial. J. Bone Miner. Res. 2021, 36, 673–684. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Kovtun, A.; Lackner, I.; Mödinger, Y.; Hacker, S.; Liedert, A.; Tuckermann, J.; Ignatius, A. Estrogen receptor α- (ERα), but not ERβ-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration. Bone 2018, 110, 11–20. [Google Scholar] [CrossRef]
- Ren, J.; Wang, X.H.; Wang, G.C.; Wu, J.H. 17β estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells. Bone 2013, 53, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Torvinen, S.; Kannus, P.; Sievänen, H.; Järvinen, T.A.H.; Pasanen, M.; Kontulainen, S.; Nenonen, A.; Järvinen, T.L.N.; Paakkala, T.; Järvinen, M.; et al. Effect of 8-month vertical whole body vibration on bone, muscle performance, and body balance: A randomized controlled study. J. Bone Miner. Res. 2003, 18, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zeng, Y.; Bao, M.; Wen, J.; Zhu, G.; Cao, C.; He, X.; Li, L. Low-magnitude vibration induces osteogenic differentiation of bone marrow mesenchymal stem cells via miR-378a-3p/Grb2 pathway to promote bone formation in a rat model of age-related bone loss. FASEB J. 2020, 34, 11754–11771. [Google Scholar] [CrossRef]
- Bas, G.; Loisate, S.; Hudon, S.F.; Woods, K.; Hayden, E.J.; Pu, X.; Beard, R.; Oxford, J.T.; Uzer, G. Low intensity vibrations augment mesenchymal stem cell proliferation and differentiation capacity during in vitro expansion. Sci. Rep. 2020, 10, 9369. [Google Scholar] [CrossRef]
- Wen, J.; Bao, M.; Tang, M.; He, X.; Yao, X.; Li, L. Low magnitude vibration alleviates age-related bone loss by inhibiting cell senescence of osteogenic cells in naturally senescent rats. Aging 2021, 13, 12031–12045. [Google Scholar] [CrossRef]
- Patton, D.M.; Bigelow, E.M.R.; Schlecht, S.H.; Kohn, D.H.; Bredbenner, T.L.; Jepsen, K.J. The relationship between whole bone stiffness and strength is age and sex dependent. J. Biomech. 2019, 83, 125–133. [Google Scholar] [CrossRef]
- Rubin, C.; Turner, A.S.; Mu¨ller, R.; Mu¨ller, M.; Mittra, E.; Mcleod, K.; Lin, W.; Qin, Y.-X. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J. Bone Miner. Res. 2002, 17, 349–357. [Google Scholar] [CrossRef]
- Rubin, C.; Turner, A.S.; Bain, S.; Mallinckrodt, C.; McLeod, K. Low mechanical signals strengthen long bones. Nature 2001, 412, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Turner, A.S.; Mallinckrodt, C.; Jerome, C.; Mcleod, K.; Bain, S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 2002, 30, 445–452. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Qi, M.C.; Xu, J.; Xu, J.; Liu, H.W.; Dong, W.; Li, J.Y.; Hu, M. Low-magnitude high-frequency loading, by whole-body vibration, accelerates early implant osseointegration in ovariectomized rats. Mol. Med. Rep. 2014, 10, 2835–2842. [Google Scholar] [CrossRef]
- Shi, H.F.; Cheung, W.H.; Qin, L.; Leung, A.H.C.; Leung, K.S. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 2010, 46, 1299–1305. [Google Scholar] [CrossRef]
- Vanleene, M.; Shefelbine, S.J. Therapeutic impact of low amplitude high frequency whole body vibrations on the osteogenesis imperfecta mouse bone. Bone 2013, 53, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.A.; Brodt, M.D.; Silva, M.J. Skeletal effects of whole-body vibration in adult and aged mice. J. Orthop. Res. 2010, 28, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.W.; Jacobs, C.R.; Donahue, H.J. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am. J. Physiol. Cell Physiol. 2001, 281, C1635–C1641. [Google Scholar] [CrossRef]
- Morrell, A.E.; Robinson, S.T.; Silva, M.J.; Guo, X.E. Mechanosensitive Ca2+ signaling and coordination is diminished in osteocytes of aged mice during ex vivo tibial loading. Connect. Tissue Res. 2020, 61, 389–398. [Google Scholar] [CrossRef]
- Schurman, C.A.; Verbruggen, S.W.; Alliston, T. Disrupted osteocyte connectivity and pericellular fluid flow in bone with aging and defective TGF- β signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2023999118. [Google Scholar] [CrossRef]
- Hemmatian, H.; Bakker, A.D.; Klein-Nulend, J.; van Lenthe, G.H. Aging, osteocytes, and mechanotransduction. Curr. Osteoporos. Rep. 2017, 15, 401–411. [Google Scholar] [CrossRef]
- Uda, Y.; Azab, E.; Sun, N.; Shi, C.; Pajevic, P.D. Osteocyte mechanobiology. Curr. Osteoporos. Rep. 2017, 15, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H.; et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015, 4, e07369. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of piezo1 by mechanical signals promotes bone anabolism. eLife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef]
- Wolff, J. Morphogenesis of bone. JAMA 1970, 213, 2260. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodelling; Springer: Berlin/Heidelberg, Germany, 1986. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Oliveira, R.G.; Pires-Oliveira, D.A.A. Effects of whole body vibration on bone mineral density in postmenopausal women: A systematic review and meta-analysis. Osteoporos. Int. 2016, 27, 2913–2933. [Google Scholar] [CrossRef]
- Slatkovska, L.; Alibhai, S.M.H.; Beyene, J.; Hu, H.; Demaras, A.; Cheung, A.M. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women a randomized trial. Ann. Intern. Med. 2011, 155, 668–679. [Google Scholar] [CrossRef]
- Knobf, M.T.; Jeon, S.; Smith, B.; Harris, L.; Kerstetter, J.; Thompson, A.S.; Insogna, K. Effect of a randomized controlled exercise trial on bone outcomes: Influence of adjuvant endocrine therapy. Breast Cancer Res. Treat. 2016, 155, 491–500. [Google Scholar] [CrossRef]
- Rubin, C.T.; Sommerfeldt, D.W.; Judex, S.; Qin, Y.-X. Inhibition of osteopenia by low magnitude, high-frequency mechanical stimuli. Drug Discov. Today 2001, 6, 848–858. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Silva, M.J. The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann. Biomed. Eng. 2006, 34, 1149–1156. [Google Scholar] [CrossRef]
- Judex, S.; Lei, X.; Han, D.; Rubin, C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 2007, 40, 1333–1339. [Google Scholar] [CrossRef]
- Zhang, R.; Gong, H.; Zhu, D.; Gao, J.; Fang, J.; Fan, Y. Seven day insertion rest in whole body vibration improves multi-level bone quality in tail suspension rats. PLoS ONE 2014, 9, e92312. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gong, H.; Huang, X.; Zhang, R.; Ma, R.; Zhu, D. Multi-level assessment of fracture calluses in rats subjected to low-magnitude high-frequency vibration with different rest periods. Ann. Biomed. Eng. 2016, 44, 2489–2504. [Google Scholar] [CrossRef] [PubMed]
- Sehmisch, S.; Galal, R.; Kolios, L.; Tezval, M.; Dullin, C.; Zimmer, S.; Stuermer, K.M.; Stuermer, E.K. Effects of low-magnitude, high-frequency mechanical stimulation in the rat osteopenia model. Osteoporos. Int. 2009, 20, 1999–2008. [Google Scholar] [CrossRef]
- Kivell, T.L. A review of trabecular bone functional adaptation: What have we learned from trabecular analyses in extant hominoids and what can we apply to fossils? J. Anat. 2016, 228, 569–594. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Styner, M.; Pagnotti, G.M.; Galior, K.; Wu, X.; Thompson, W.R.; Uzer, G.; Sen, B.; Xie, Z.; Horowitz, M.C.; Styner, M.A.; et al. Exercise regulation of marrow fat in the setting of PPARγ agonist treatment in female C57BL/6 mice. Endocrinology 2015, 156, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Pagnotti, G.M.; Rubin-Sigler, J.; Naeher, M.; Copes, L.E.; Judex, S.; Rubin, C.T.; Demes, B. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak external forces. J. Exp. Biol. 2015, 218, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Mougios, V.; Skraparlis, A.; Kabasakalis, A.; Mantzoros, C.S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism 2014, 63, 918–921. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Benedetti, L.; Galli, D.; Prè, D.; Silvani, G.; Crosetto, N.; Magenes, G.; Cusella De Angelis, M.G. Low-amplitude high frequency vibration down-regulates myostatin and atrogin-1 expression, two components of the atrophy pathway in muscle cells. J. Tissue Eng. Regen. Med. 2014, 8, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W. Hoi RCT of Vibration Effect on Vertebral BMD in Disabled Patients. Available online: https://clinicaltrials.gov/ct2/show/NCT04180267?term=vibration&cond=Bone+Diseases&draw=2&rank=7 (accessed on 1 March 2023).
- Clark, B. Assessment of Cortical Bone Mechanics Technology (CBMT) Fracture Discrimination Capability. Available online: https://clinicaltrials.gov/ct2/show/NCT05721898?term=vibration&cond=Bone+Diseases&draw=2&rank=31 (accessed on 1 March 2023).
- Feyzioğlu, Ö. The Effect of Wearable Vibration Therapy on Shoulder Functionality in Individuals Receiving Adjuvant Radiotherapy after Breast Cancer Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT05680116?term=vibration&cond=cancer&draw=2&rank=3 (accessed on 1 March 2023).
| Treatment | Vibration Magnitude and Frequency | Vibration Duration | Age | Cancer | Major Findings | |
|---|---|---|---|---|---|---|
| Mogil et al. 2016 [51] | WBV | 0.3 g; 32–37 Hz | 10 min/session; 2 sessions/day; 7 days/week for 1 year | Mean 14 | Pediatric cancer | - Total-body BMD ↑ - Tibial trabecular bone ↑ - Osteocalcin, P1NP, BSAP ↑ (trend) - RANKL ↑ - Circulating osteocalcin correlated with change in total-body BMD |
| Almstedt et al. 2016 [52] | Resistance cardio training + WBV | 20–25 Hz | 30–45 s/day; 3 days/week for 26 weeks | Mean 63 | Breast cancer | - BMD at spine, hips, and whole body ↑ - P1NP ↓ - CTX ↓ (trend) |
| Baker et al. 2018 [53] | WBV | 0.3 g; 27–32 Hz | 20 min/session; 3 sessions/week for 12 weeks | Mean 62 | Breast cancer | - No differences for markers of bone formation/resorption, physical functioning, body composition - No changes in BMD |
| Seefried et al. 2020 [54] | WBV | 1.5–3 mm; 7–30 Hz | 30 min/session; 2 sessions/week for 3 or 6 months | Median 62 | Precancer | - Physical functioning ↑ - No differences in tibial BMD - Sclerostin, NTX of collagen type 1, TRACP5b ↑ (trend) - DKK1, P1NP ↓ (trend) - Total ALP ↓ |
| de Sire et al. 2021 [55] | Exercise + WBV | 20.44 m/s2 (2.1 g with g = 9.81 m/s2); 30 Hz | 50–60 min/session; 3 sessions/week for 4 weeks | Mean 52 | Breast cancer | - Physical performance ↑ - Muscle strength ↑ - Pain ↓ |
| Vibration Magnitude and Frequency | Vibration Duration | Age | Cancer | Major Findings | |
|---|---|---|---|---|---|
| Pagnotti et al. 2012 [57] | 0.3 g; 90 Hz | 15 min/day; 5 days/week for a year | 3 months | Ovarian cancer | - Trabecular bone volume of proximal tibia and L5 vertebrae ↑ - L5 vertebrae was more plate-like - Marrow-derived MSCs ↓ - Overall tumor incidence and metastatic lesions ↓ (trend) |
| Pagnotti et al. 2016 [58] | 15 min/day; 5 days/week for 8 weeks | 7 weeks | Multiple myeloma | - Trabecular bone volume in the femur ↑ but not in the tibia - Cortical bone volume in the femur ↑ - Transcortical perforations in the femur ↓ - Trabecular bone volume and trabecular connectivity density in the L5 vertebrae ↑ - Serum TRACP5b ↓ - Tumor expansion and myeloma cells ↓ - Necrotic tumor of tibial marrow ↓ | |
| Matsumoto et al. 2022 [59] | 20 min/day; 5 days/week for 3 weeks | 8 weeks | Breast cancer | - Osteolytic bone loss ↓ - BMD of cortical and trabecular bones ↑ - Serum osteocalcin ↑ (trend) - Vessel diameter ↓, vessel number density ↑ (trend), and vessel diameter heterogeneity ↓ |
| Vibration Magnitude and Frequency | Vibration Duration | Cells Exposed to Vibration | Cancer | Experimental Set-Up | Major Findings | |
|---|---|---|---|---|---|---|
| Yi et al. 2020 [60] | 0.3 g; 90 Hz | 20 min/bout; 1 or 2 bouts/day for 3 days | MDA-MB-231, MCF-7 breast cancer | Conventional cell cultures | - PTHLH, IL11, RANKL ↓ - Osteoclastogenesis ↓ - FASL-mediated cancer apoptosis ↑ - Cancer invasion ↓ more with twice-daily vibration - Cancer cell stiffness ↑ | |
| Lin et al. 2022 [16] | 0.3 g; 60 Hz | 1 h | MLO-Y4 osteocytes; MDA-MB-231 breast cancer | MDA-MB-231 breast cancer | - Nuclear translocation of YAP ↑ - Vibration + Yoda1: nuclear translocation of YAP ↑↑ - Vibration ± Yoda1: osteoclastogenesis ↓ - Vibration + Yoda1: cancer migration ↓ | |
| Song et al. 2022 [17] | 1 h/day for 3 days | MLO-Y4 osteocytes; HUVECs; MDA-MB-231 breast cancer | Microfluidic platform | - COX-2, Piezo1 ↑ - RANKL and RANKL/OPG ↓ - Piezo1 knockdown in osteocytes: vibration-stimulation of COX-2, OPG ↓ - Cancer extravasation ↓ - Vibration + Yoda1: cancer extravasation ↓↓ on Day 2 but no on Day 4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Sassi, A.; Seaman, K.; Lin, C.-Y.; You, L. Vibration Therapy for Cancer-Related Bone Diseases. Vibration 2023, 6, 449-465. https://doi.org/10.3390/vibration6020028
Song X, Sassi A, Seaman K, Lin C-Y, You L. Vibration Therapy for Cancer-Related Bone Diseases. Vibration. 2023; 6(2):449-465. https://doi.org/10.3390/vibration6020028
Chicago/Turabian StyleSong, Xin, Amel Sassi, Kimberly Seaman, Chun-Yu Lin, and Lidan You. 2023. "Vibration Therapy for Cancer-Related Bone Diseases" Vibration 6, no. 2: 449-465. https://doi.org/10.3390/vibration6020028
APA StyleSong, X., Sassi, A., Seaman, K., Lin, C.-Y., & You, L. (2023). Vibration Therapy for Cancer-Related Bone Diseases. Vibration, 6(2), 449-465. https://doi.org/10.3390/vibration6020028






