Acute Vascular Response of Hand to Force and Vibration
Abstract
1. Introduction
2. Methods
2.1. Participants
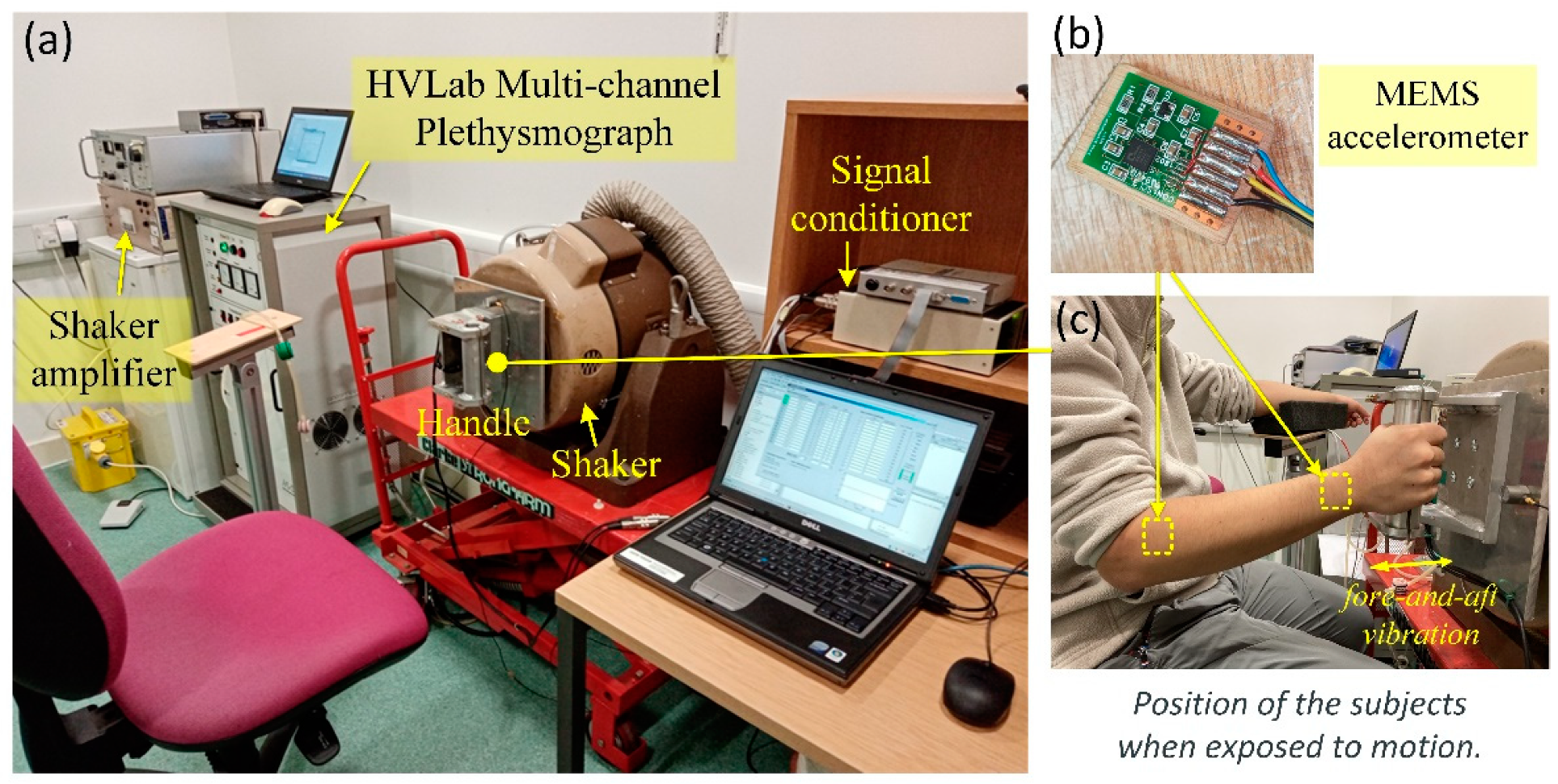
2.2. Measurements of Finger Circulation
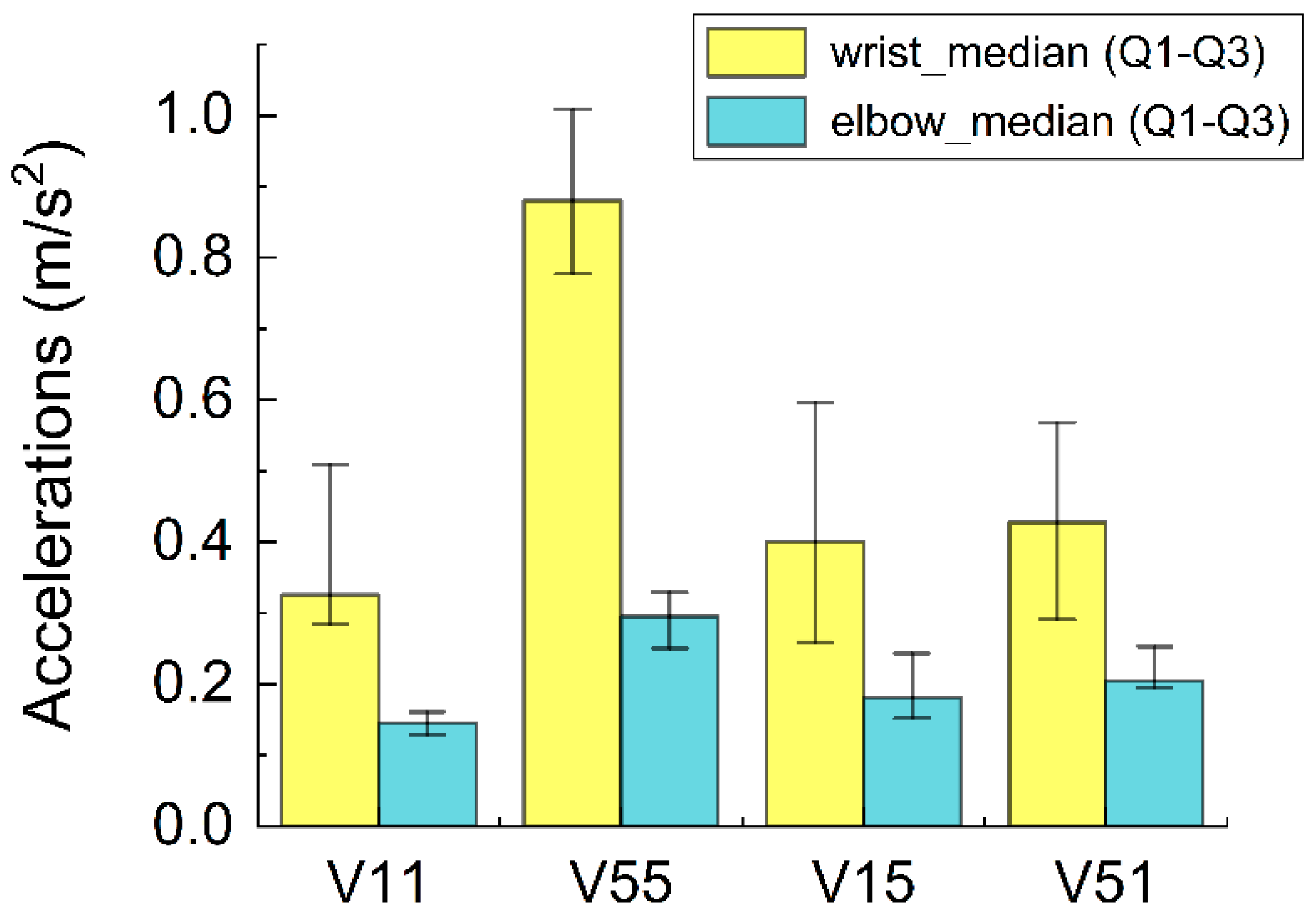
2.3. Motion Stimuli and Force Range
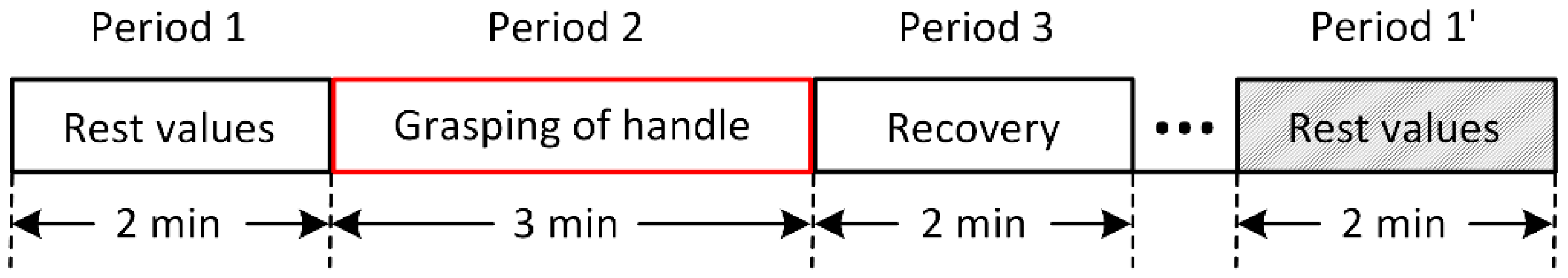
2.4. Experimental Procedure
- Period 1: no force and no vibration (2 min): measurement of FBF and FST;
- Period 2: force (and vibration) (3 min): measurement of FBF, FST, and acceleration;
- Period 3: no force and no vibration (2 min): measurement of FBF and FST.
2.5. Statistical Analysis
3. Results
3.1. Finger Circulation during Pre-Exposure Period
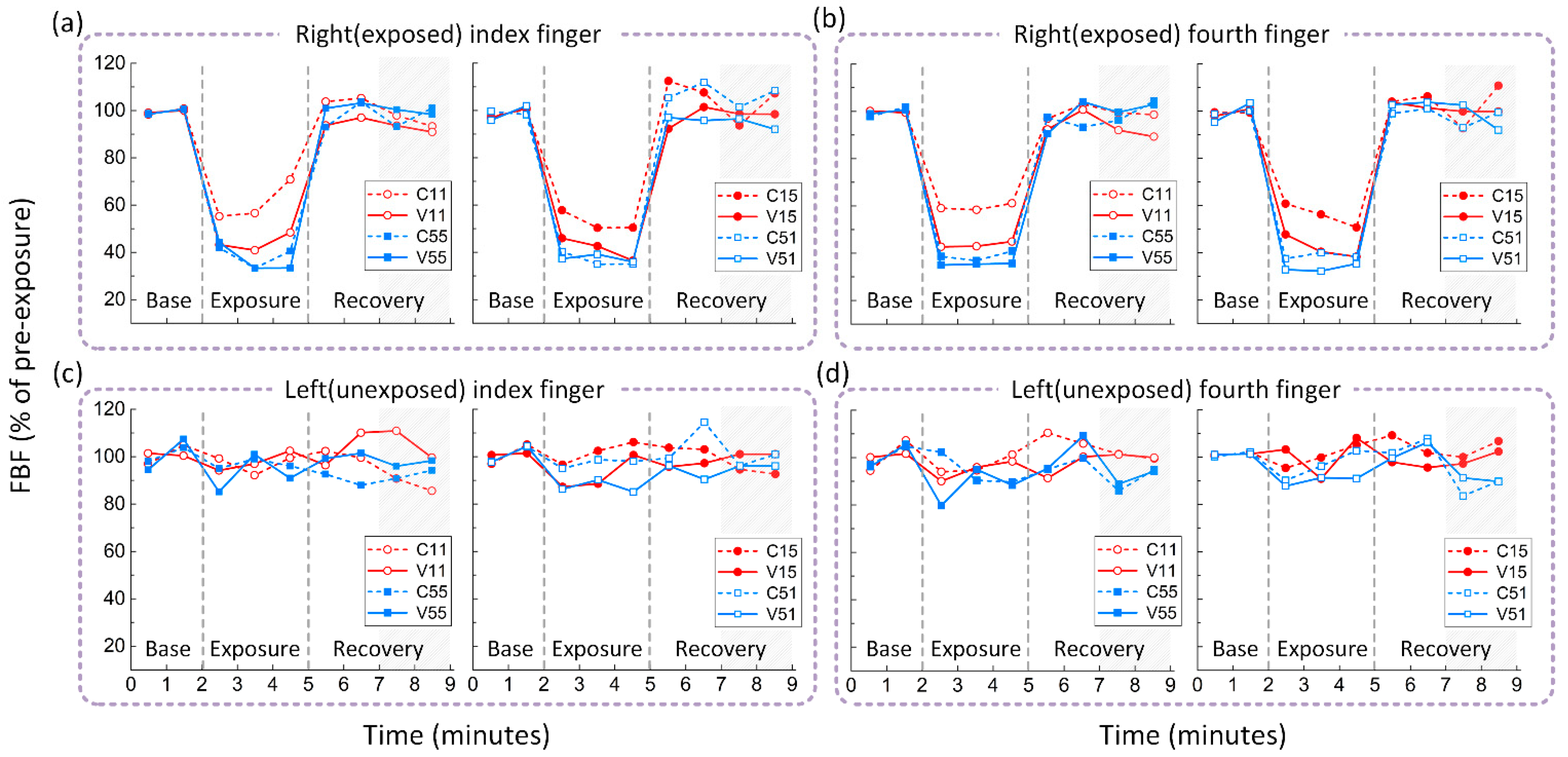
3.2. Effects of Hand Force on Finger Circulation
3.3. Combined Effects of Hand Force and Vibration on Finger Circulation
4. Discussion
4.1. Vascular Response to Hand Force
4.2. Vascular Response to Vibration
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gemne, G. Diagnostics of hand-arm system disorders in workers who use vibrating tools. Occup. Environ. Med. 1997, 54, 90. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.J.; Erdreich, J. Handbook of Human Vibration; Acoustical Society of America: Melville, NY, USA, 1991. [Google Scholar]
- Griffin, M.J.; Bovenzi, M. The diagnosis of disorders caused by hand-transmitted vibration: Southampton Workshop 2000. Int. Arch. Occup. Environ. Health 2002, 75, 1–5. [Google Scholar] [CrossRef] [PubMed]
- IIDB02-New Cases of Non-Lung Diseases in England, Wales and Scotland by Disease. Available online: https://www.hse.gov.uk/statistics/tables/iidb02.xlsx (accessed on 25 February 2022).
- Health and Safety Executive Hand-Arm Vibration—Key Messages. Available online: https://www.hse.gov.uk/vibration/hav/keymessages.htm (accessed on 25 February 2022).
- Bovenzi, M. Exposure-response relationship in the hand-arm vibration syndrome: An overview of current epidemiology research. Int. Arch. Occup. Environ. Health 1998, 71, 509–519. [Google Scholar] [CrossRef]
- Bovenzi, M.; Zadini, A.; Franzinelli, A.; Borgogni, F. Occupational musculoskeletal disorders in the neck and upper limbs of forestry workers exposed to hand-arm vibration. Ergonomics 1991, 34, 547–562. [Google Scholar] [CrossRef]
- Bovenzi, M.; Lindsell, C.; Griffin, M. Response of finger circulation to energy equivalent combinations of magnitude and duration of vibration. Occup. Environ. Med. 2001, 58, 185–193. [Google Scholar] [CrossRef]
- Krajnak, K.; Miller, G.; Waugh, S. Contact area affects frequency-dependent responses to vibration in the peripheral vascular and sensorineural systems. J. Toxicol. Environ. Health 2018, 81, 6–19. [Google Scholar] [CrossRef]
- Bovenzi, M.; Welsh, A.J.L.; Della Vedova, A.; Griffin, M.J. Acute effects of force and vibration on finger blood flow. Occup. Environ. Med. 2006, 63, 84–91. [Google Scholar] [CrossRef]
- Griffin, M.J.; Welsh, A.J.; Bovenzi, M. Acute response of finger circulation to force and vibration applied to the palm of the hand. Scand. J. Work Environ. Health 2006, 32, 383–391. [Google Scholar] [CrossRef][Green Version]
- Thompson, A.J.; Griffin, M.J. Effect of the magnitude and frequency of hand-transmitted vibration on finger blood flow during and after exposure to vibration. Int. Arch. Occup. Environ. Health 2009, 82, 1151–1162. [Google Scholar] [CrossRef]
- Ye, Y.; Griffin, M.J. Changes in finger blood flow induced by low magnitude localised vibration of the thenar eminence of the hand. In Proceedings of the 44th United Kingdom Conference on Human Responses to Vibration, Loughborough, UK, 7–9 September 2009. [Google Scholar]
- Radwin, G.R.; Armstrong, T.J.; Chaffin, D.B. Power hand tool vibration effects on grip exertions. Ergonomics 1987, 30, 833–855. [Google Scholar] [CrossRef]
- BS EN ISO 21420:2020; Protective Gloves. General Requirements and Test Methods. International Organization for Standardization: London, UK, 2020.
- Greenfield, A.; Whitney, R.; Mowbray, J. Methods for the investigation of peripheral blood flow. Br. Med. Bull. 1963, 19, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Welsh, A.; Griffin, M. Multi-Channel and Single-Channel Venous Occlusion Plethysmography for Finger Blood Flow Measurement. 2004. Available online: http://resource.isvr.soton.ac.uk/staff/pubs/PubPDFs/Pub10501.pdf (accessed on 25 February 2022).
- ISO 5349-1; Mechanical Vibration—Measurement and Evaluation of Human Exposure to Hand-Transmitted Vibration—Part 1: General Requirements. International Organization for Standardization: Geneva, Switzerland, 2001.
- Sandover, J.; Louw, J. Acute effects of grip force and vibration on finger temperature. In Proceedings of the 6th International Conference on Hand-Arm Vibration, Bonn, Germany, 19–22 May 1992. [Google Scholar]
- Scheffer, M.; Dupuis, H. Effects of combined hand-arm vibration and cold on skin temperature. Int. Arch. Occup. Environ. Health 1989, 61, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Hartung, E.; Dupuis, H.; Scheffer, M. Effects of grip and push forces on the acute response of the hand-arm system under vibrating conditions. Int. Arch. Occup. Environ. Health 1993, 64, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Miyakita, T.; Miura, H.; Futatsuka, M. Hand-Arm Vibration, Noise, Temperature and Static Load. Kurume Med. J. 1990, 37, S73–S83. [Google Scholar] [CrossRef]
- Riedel, S. Consideration of grip and push forces for the assessment of vibration exposure. Cent. Eur. J. Public Health 1995, 3, 139–141. [Google Scholar] [PubMed]
- Welcome, D.; Rakheja, S.; Dong, R.; Wu, J.; Schopper, A. An investigation on the relationship between grip, push and contact forces applied to a tool handle. Int. J. Ind. Ergon. 2004, 34, 507–518. [Google Scholar] [CrossRef]
- Marcotte, P.; Aldien, Y.; Boileau, P.; Rakheja, S.; Boutin, J. Effect of handle size and hand–handle contact force on the biodynamic response of the hand–arm system under zh-axis vibration. J. Sound Vib. 2005, 283, 1071–1091. [Google Scholar] [CrossRef]
- Adewusi, S.A.; Rakheja, S.; Marcotte, P.; Boutin, J. Vibration transmissibility characteristics of the human hand–arm system under different postures, hand forces and excitation levels. J. Sound Vib. 2010, 329, 2953–2971. [Google Scholar] [CrossRef]
- Saha, S.; Kalra, P. A review on hand-arm vibration exposure and vibration transmissibility from power hand tools to hand-arm system. Int. J. Hum. Factors Ergon. 2016, 4, 10–46. [Google Scholar] [CrossRef]
- Bovenzi, M.; Lindsell, C.J.; Griffin, J.M. Acute vascular responses to the frequency of vibration transmitted to the hand. Occup. Environ. Med. 2000, 57, 422–430. [Google Scholar] [CrossRef]
- Ye, Y.; Griffin, M.J. Effects of temperature on reductions in finger blood flow induced by vibration. Int. Arch. Occup. Environ. Health 2011, 84, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bader, D.; Barnhill, R.; Ryan, T. Effect of Externally Applied Skin Surface Forces on Tissue Vasculature. Arch. Phys. Med. Rehabil. 1986, 67, 807–811. [Google Scholar] [PubMed]
- Bovenzi, M.; Lindsell, C.J.; Griffin, M.J. Magnitude of acute exposures to vibration and finger circulation. Scand. J. Work Environ. Health 1999, 278–284. [Google Scholar] [CrossRef] [PubMed]

| Vibration group | G10 + F10 + HAV (V11) | G50 + F50 + HAV (V55) | G10 + F50 + HAV (V15) | G50 + F10 + HAV (V51) |
| Control group | G10 + F10 (C11) | G50 + F50 (C55) | G10 + F50 (C15) | G50 + F10 (C51) |
| (a) Alterations in finger blood flow (% of pre-exposure). | ||||
| C11 | V11 | C55 | V55 | |
| Left (unexposed) finger | 99.94% (88.47–104.91%) | 95.93% (89.61–102.76%) | 90.62% (86.65–99.30%) | 88.61% (74.32–103.90%) |
| Right (exposed) finger | 59.72% ** (47.42–72.95%) | 47.16% ** (32.06–61.41%) | 43.72% ** (26.46–51.83%) | 41.11% ** (30.90–46.19%) |
| C15 | V15 | C51 | V51 | |
| Left (unexposed) finger | 100.93% (93.09–112.05%) | 92.62% (83.92–109.33%) | 97.86% (77.49–106.45%) | 83.26% (77.70–99.16%) |
| Right (exposed) finger | 51.96% ** (43.48–75.24%) | 45.16% ** (37.54–55.55%) | 39.61% ** (34.87–48.80%) | 40.38% ** (25.87–61.40%) |
| (b) Alterations in finger skin temperature (% of pre-exposure). | ||||
| C11 | V11 | C55 | V55 | |
| Left (unexposed) finger | 100.64% (100.22–101.17%) | 100.22% (99.61–101.84%) | 99.24% (97.43–100.74%) | 98.36% (97.92–99.05%) |
| Right (exposed) finger | 98.05% (96.07–99.37%) | 96.57% (93.95–98.03%) | 95.95% * (94.45–96.07%) | 96.20% * (95.15–97.19%) |
| C15 | V15 | C51 | V51 | |
| Left (unexposed) finger | 100.77% (100.40–101.69%) | 99.74% (98.01–99.94%) | 100.48% (99.40–101.36%) | 99.66% (98.34–99.95%) |
| Right (exposed) finger | 97.56% ** (97.02–98.46%) | 96.54% ** (95.89–97.98%) | 96.24% ** (95.48–97.49%) | 96.00% ** (95.33–96.42%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Ye, Y. Acute Vascular Response of Hand to Force and Vibration. Vibration 2022, 5, 153-164. https://doi.org/10.3390/vibration5010010
Gao S, Ye Y. Acute Vascular Response of Hand to Force and Vibration. Vibration. 2022; 5(1):153-164. https://doi.org/10.3390/vibration5010010
Chicago/Turabian StyleGao, Shuxiang, and Ying Ye. 2022. "Acute Vascular Response of Hand to Force and Vibration" Vibration 5, no. 1: 153-164. https://doi.org/10.3390/vibration5010010
APA StyleGao, S., & Ye, Y. (2022). Acute Vascular Response of Hand to Force and Vibration. Vibration, 5(1), 153-164. https://doi.org/10.3390/vibration5010010







