Foaming Capability, Structural Stability, and Fire Extinguishing Performance Optimization of Short-Chain Fluorocarbon Foam by Modulating Gas–Liquid Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PFH-BZ-Based Foam Extinguishing Agent
2.3. Experimental System and Procedures
3. Results and Discussion
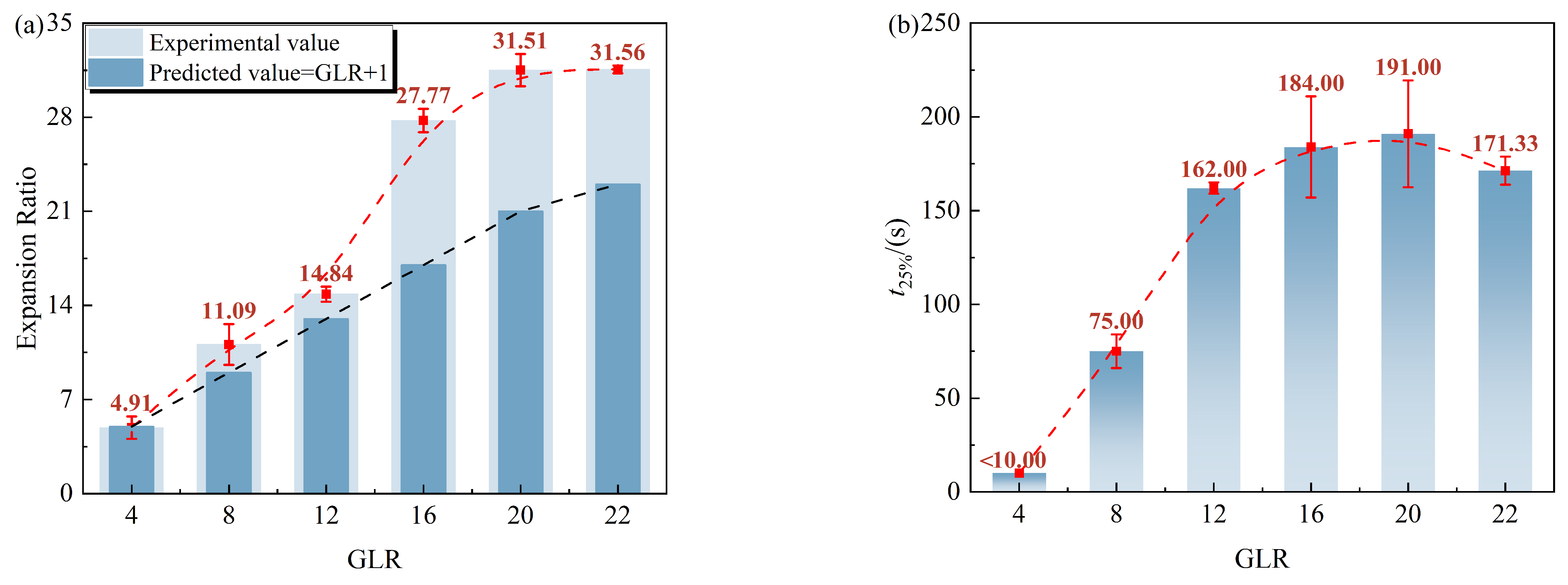
3.1. Effect of GLR on Foaming Capability and Drainage Characteristics
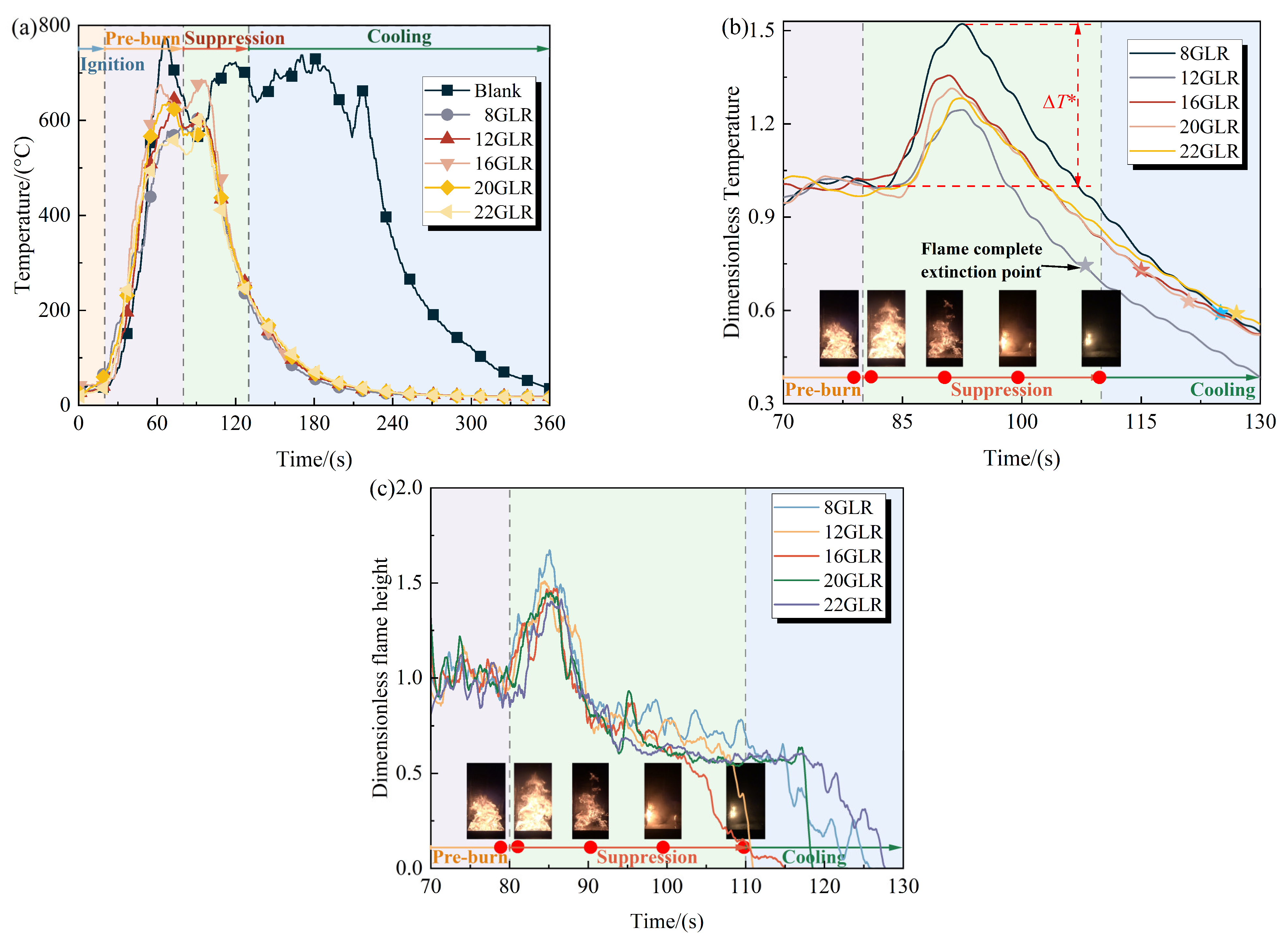
3.2. Effect of GLR on Foam Fire Extinguishing Performance
3.3. Mechanism of GLR on Foam Performance
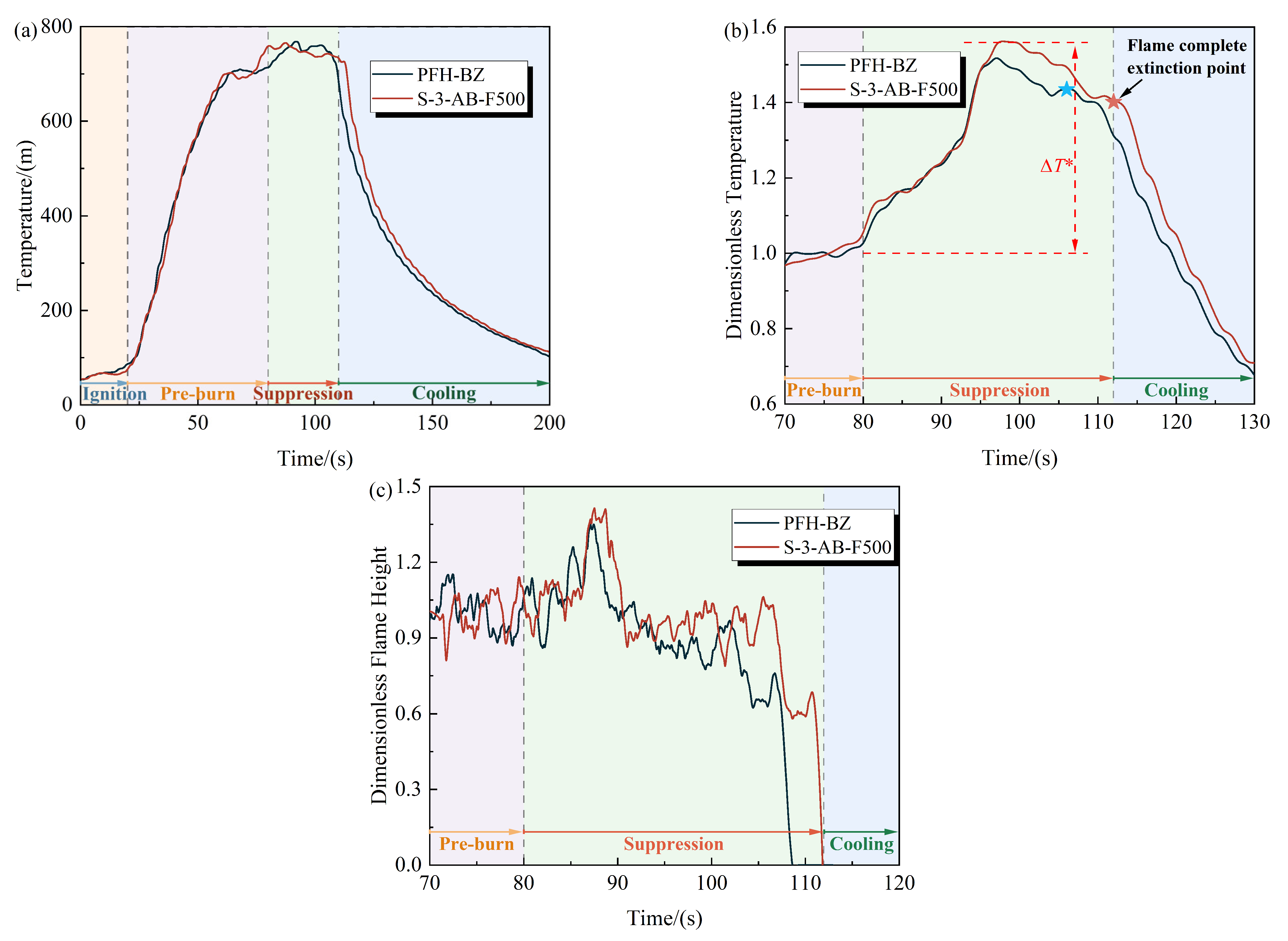
3.4. Comparison Evaluation of Burn-Back Resistance Between PFH-BZ and Commercial Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shebeko, Y.N.; Bolodian, I.A.; Molchanov, V.P.; Deshevih, Y.I.; Gordienko, D.M.; Smolin, I.M.; Kirillov, D.S. Fire and Explosion Risk Assessment for Large-Scale Oil Export Terminal. J. Loss Prev. Process Ind. 2007, 20, 651–658. [Google Scholar] [CrossRef]
- Ginestet, S.; Le Bot, C. Evaporation Flow Assessment from Petroleum Product Storage Tanks Exposed to Fire Conditions. Oil Gas Sci. Technol. 2018, 73, 27. [Google Scholar] [CrossRef]
- Zavareh, R.D.; Dana, T.; Roayaei, E.; Monavari, S.M.; Jozi, S.A. The Environmental Risk Assessment of Fire and Explosion in Storage Tanks of Petroleum Products. Sustainability 2022, 14, 10747. [Google Scholar] [CrossRef]
- Fadeyibi, I.O.; Jewo, P.I.; Opoola, P.; Babalola, O.S.; Ugburo, A.; Ademiluyi, S.A. Burns and Fire Disasters from Leaking Petroleum Pipes in Lagos, Nigeria: An 8-Year Experience. Burns 2011, 37, 145–152. [Google Scholar] [CrossRef]
- Chaudhary, A.; Gupta, A.; Kumar, S.; Kumar, R. Pool Fires of Jatropha Biodiesel and Their Blends with Petroleum Diesel. Exp. Therm. Fluid Sci. 2019, 101, 175–185. [Google Scholar] [CrossRef]
- Okamoto, K.; Yamasaki, H.; Matsuoka, I.; Ichikawa, T.; Matsuoka, H.; Saeki, Y.; Honma, M. Burning Behavior and Fire Hazards of Petroleum Liquid Combustible Spills. J. Loss Prev. Process Ind. 2024, 90, 105346. [Google Scholar]
- Laundess, A.J.; Rayson, M.S.; Dlugogorski, B.Z.; Kennedy, E.M. Small-Scale Test Protocol for Firefighting Foams DEF(AUST)5706: Effect of Bubble Size Distribution and Expansion Ratio. Fire Technol. 2010, 47, 149–162. [Google Scholar]
- Mawhinney, J.R. Fixed Fire Protection Systems in Tunnels: Issues and Directions. Fire Technol. 2013, 49, 477–508. [Google Scholar]
- Li, J.; Li, Y.; Bi, Q.; Li, Y.; Chow, W.; Cheng, C.; To, C.; Chow, C. Performance Evaluation on Fixed Water-Based Firefighting System in Suppressing Large Fire in Urban Tunnels. Tunn. Undergr. Space Technol. 2019, 84, 56–69. [Google Scholar] [CrossRef]
- Zabelin, I.V.; Shkola, M.V.; Shlegel, N.E.; Strizhak, P.A. Using Hydrate Foam to Extinguish Petroleum Product Tank Fires. J. Loss Prev. Process Ind. 2025, 98, 105730. [Google Scholar]
- Zhao, W.; Xu, Z.; Yan, L. Optimizing Xanthan Gum for Enhanced Fire Extinguishing Performance of Eco-Friendly Short-Chain Fluorocarbon Surfactant Foam. Fire 2025, 8, 463. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Zhang, T.; Wang, Q.; Li, Y.; Kang, Q. Performance of Foam Agents on Pool Fires at High Altitudes. Fire Technol. 2021, 58, 1285–1304. [Google Scholar] [CrossRef]
- Dahlbom, S.; Mallin, T.; Bobert, M. Fire Test Performance of Eleven PFAS-Free Class B Firefighting Foams Varying Fuels, Admixture, Water Types and Foam Generation Techniques. Fire Technol. 2022, 58, 1639–1665. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; Zhao, J.; Zhang, Y.; Hu, L. Experimental Research on the Effectiveness of Different Types of Foam of Extinguishing Methanol/Diesel Pool Fires. Combust. Sci. Technol. 2024, 196, 1791–1809. [Google Scholar] [CrossRef]
- Kim, A.K.; Crampton, G.P. Evaluation of the Fire Suppression Effectiveness of Manually Applied Compressed-Air-Foam (CAF) System. Fire Technol. 2009, 48, 549–564. [Google Scholar] [CrossRef]
- Arvidson, M.; Mindykowski, P. Fire Testing of Alternative Fixed Fire-Extinguishing Systems for Ro-Ro Spaces Onboard Ships. Ships Offshore Struct. 2023, 18, 423–428. [Google Scholar] [CrossRef]
- Singh, R.; Ray, S.K. Study on Control of Disastrous Open Fires in Underground Coalmines. J. Sci. Ind. Res. 2004, 63, 1010–1018. [Google Scholar]
- Tian, F.; Wang, K.; Fang, J.; Shah, H.R.; Lang, X.; Mu, S.; Wang, J.; Wang, J. Suppression Behavior Difference Between Compressed Air/Nitrogen Foam over Liquid Fuel Surface Under Constant Radiation Heat Flux. Fire Technol. 2022, 60, 1225–1243. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wu, M.; Shao, Z.; Li, Y.; Zhao, Y.; Zhang, K. Carbon Dioxide Sealing-Based Inhibition of Coal Spontaneous Combustion: A Temperature-Sensitive Micro-Encapsulated Fire-Retardant Foamed Gel. Fuel 2020, 266, 117036. [Google Scholar] [CrossRef]
- Wang, X.; Liao, Y.; Lin, L. Experimental Study on Fire Extinguishing with a Newly Prepared Multi-Component Compressed Air Foam. Chin. Sci. Bull. 2009, 54, 492–496. [Google Scholar] [CrossRef]
- Shang, F.; Zhang, J.; Zhu, T.; Su, W.; Li, K.; Zou, Y.; Guo, Y. Analysis of Fire Extinguishing Performance and Mechanisms in Transformer Oil Pool Fires by Large-Scale Compressed Nitrogen Foam: Impact of Different Nozzle Pressures. J. Fire Sci. 2024, 42, 281–296. [Google Scholar] [CrossRef]
- Kang, W.; Yan, L.; Ding, F.; Guo, X.; Xu, Z. Experimental Study on Fire-Extinguishing Efficiency of Protein Foam in Diesel Pool Fire. Case Stud. Therm. Eng. 2019, 16, 100557. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, F.; Zhang, S.; Wang, L.; Ke, Y.; Huang, J.; Su, W.; Liu, R.; Sheng, Y. Research on Key Parameters and Fire Extinguishing Effectiveness of Compressed Air Foam System Used for UHV Substation. Fire Technol. 2024, 61, 1969–1990. [Google Scholar] [CrossRef]
- Jeon, K.S.; Kim, H.S.; Sim, J.U.; Yoo, Y.H.; Park, J.O. CFD Analysis Study on Aqueous Film Foaming Foam Injection Optimization to Respond to Oil Fires in Naval Ship Compartment. Appl. Chem. Eng. 2024, 35, 239–247. [Google Scholar]
- Isabelle, C.; Sylvie, C.A.; Florence, E.; François, G.; Reinhard, H.; Olivier, P.; Florence, R.; Arnaud, S.J. Foams: Structure and Dynamics, 1st ed.; Oxford University Press: Oxford, UK, 2013; pp. 113–134. [Google Scholar]
- Lorenceau, E.; Louvet, N.; Rouyer, F.; Pitois, O. Permeability of Aqueous Foams. Eur. Phys. J. E 2009, 28, 293–304. [Google Scholar] [CrossRef]
- Isert, N.; Maret, G.; Aegerter, C.M. Coarsening Dynamics of Three-Dimensional Levitated Foams: From Wet to Dry. Eur. Phys. J. E 2013, 36, 116. [Google Scholar] [CrossRef]
- Badve, M.; Barigou, M. Local Description of Foam Flow, Deformation and Pressure Drop in Narrow Constricted Channels. Int. J. Multiph. Flow 2020, 128, 103279. [Google Scholar] [CrossRef]
- Ding, F.; Kang, W.; Yan, L.; Xu, Z.; Guo, X. Influence of Gas–Liquid Ratio on the Fire-Extinguishing Efficiency of Compressed Gas Protein Foam in Diesel Pool Fire. J. Therm. Anal. Calorim. 2020, 146, 1465–1472. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, Z.; Yan, L. Zwitterionic Short-Chain Fluorocarbon Surfactant: Synthesis, Synergy with Hydrocarbon Surfactants, and Effects of Inorganic Salts on Surface Activity and Foam Performance. Surf. Interfaces 2025, 72, 107125. [Google Scholar] [CrossRef]
- GB 15308-2006; Foam Extinguishing Agent. Standardization Administration of the People’s Republic of China: Beijing, China, 2006.
- Qi, D.; Wang, L.; Ji, J.; Li, M. Dimensionless analytical solutions for steady-state fire smoke spread through high-rise shaft. Fire Saf. J. 2017, 93, 12–20. [Google Scholar] [CrossRef]
- Liu, Z.; Carpenter, D.; Kim, A.K. Cooling Characteristics of Hot Oil Pool by Water Mist during Fire Suppression. Fire Saf. J. 2008, 43, 269–281. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Jin, C.; Xu, P.; Wang, J.; Xie, Q. Experimental Investigation of Flow Patterns and Rheological Characteristics of Compressed Air Foam in Horizontal Tube. Phys. Fluids 2024, 36, 033118. [Google Scholar] [CrossRef]
- Koehler, S.A.; Hilgenfeldt, S.; Stone, H.A. A Generalized View of Foam Drainage: Experiment and Theory. Langmuir 2000, 16, 6327–6341. [Google Scholar] [CrossRef]
- Stevenson, P. Dimensional Analysis of Foam Drainage. Chem. Eng. Sci. 2006, 61, 4503–4510. [Google Scholar] [CrossRef]
- Garo, J.P.; Koseki, H.; Vantelon, J.P.; Fernandez-Pello, C. Combustion of Liquid Fuels Floating on Water. Therm. Sci. 2007, 11, 119–140. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Wu, C.; Li, C.; Chen, X.; Lu, S. Collision Dynamics of a Single Water Droplet Impinging on a High-Temperature Pool of Oil. Acta Mech. 2017, 229, 1567–1577. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Z.; Zheng, G.; Chen, P. Study on the Effect of Mist Flux on Water Mist Fire Extinguishing. Fire Saf. J. 2022, 130, 103601. [Google Scholar] [CrossRef]
- Ebrahim, M.; Elkenani, B.; Ortega, A. Transient Surface Temperatures upon the Impact of a Single Droplet onto a Heated Surface in the Film Evaporation Regime. Int. J. Heat Mass Transf. 2022, 186, 122463. [Google Scholar] [CrossRef]




| GLR | System Pressure/(MPa) | Gas Flow Rate/(L/h) | Liquid Flow Rate/(L/h) |
|---|---|---|---|
| 4 | 0.70 | 180 | 45 |
| 8 | 0.70 | 360 | 45 |
| 12 | 0.70 | 540 | 45 |
| 16 | 0.70 | 720 | 45 |
| 20 | 0.70 | 900 | 45 |
| 22 | 0.70 | 1000 | 45 |
| GLR | Extinction Time /(s) | Maximum Increase in T*/(%) | Maximum Increase in H*/(%) | Rcool /(%/s) |
|---|---|---|---|---|
| 8 | 47.73 ± 1.91 | 43.09 ± 9.26 | 57.08 ± 14.45 | 2.54 ± 0.40 |
| 12 | 27.49 ± 2.31 | 34.51 ± 13.86 | 51.82 ± 1.34 | 3.45 ± 0.28 |
| 16 | 35.37 ± 0.71 | 32.36 ± 5.77 | 51.75 ± 6.60 | 2.38 ± 0.11 |
| 20 | 41.21 ± 2.77 | 27.91 ± 6.22 | 44.49 ± 0.44 | 2.21 ± 0.01 |
| 22 | 46.93 ± 1.24 | 26.07 ± 3.19 | 43.30 ± 2.59 | 2.07 ± 0.04 |
| Foam Extinguishing Agent | PFH-BZ Foam | S-3-AB-F500 Foam |
|---|---|---|
| Extinction time/(s) | 26.16 | 31.80 |
| Burn-back time/(s) | 662.37 | 695.54 |
| Maximum increase in T*/(%) | 52.66 | 56.27 |
| Maximum increase in H*/(%) | 35.18 | 41.65 |
| Rcool/(%/s) | 1.02 | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhao, W.; Xu, Z.; Yan, L. Foaming Capability, Structural Stability, and Fire Extinguishing Performance Optimization of Short-Chain Fluorocarbon Foam by Modulating Gas–Liquid Ratio. Fire 2026, 9, 59. https://doi.org/10.3390/fire9020059
Zhao W, Xu Z, Yan L. Foaming Capability, Structural Stability, and Fire Extinguishing Performance Optimization of Short-Chain Fluorocarbon Foam by Modulating Gas–Liquid Ratio. Fire. 2026; 9(2):59. https://doi.org/10.3390/fire9020059
Chicago/Turabian StyleZhao, Wenjun, Zhisheng Xu, and Long Yan. 2026. "Foaming Capability, Structural Stability, and Fire Extinguishing Performance Optimization of Short-Chain Fluorocarbon Foam by Modulating Gas–Liquid Ratio" Fire 9, no. 2: 59. https://doi.org/10.3390/fire9020059
APA StyleZhao, W., Xu, Z., & Yan, L. (2026). Foaming Capability, Structural Stability, and Fire Extinguishing Performance Optimization of Short-Chain Fluorocarbon Foam by Modulating Gas–Liquid Ratio. Fire, 9(2), 59. https://doi.org/10.3390/fire9020059







