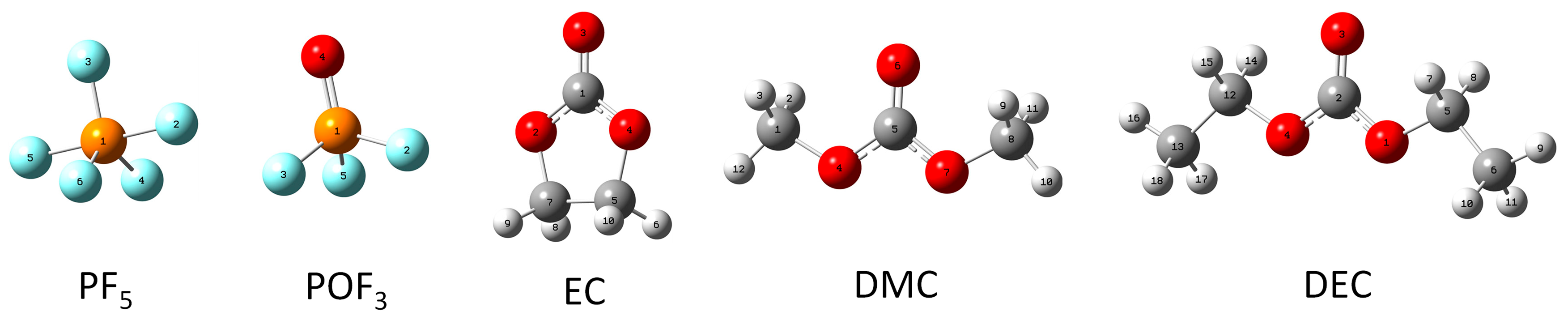
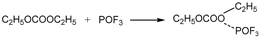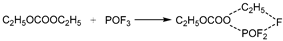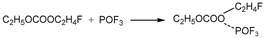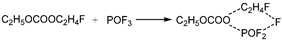1. Introduction
Under the overarching context of the globally accelerated transition toward a low-carbon energy system, lithium-ion batteries (LIBs) have emerged as the core technological enabler in the energy storage and conversion sector, primarily attributed to their inherent advantages of high energy density, long cycle life, and low self-discharge rate. These attributes have propelled their widespread adoption across critical domains, including electric vehicles, large-scale energy storage systems (ESSs), and portable electronic devices, thereby solidifying their pivotal role in modern energy infrastructure. Notably, the global LIB market demonstrated robust growth in 2024, with total shipments reaching 1545.1 GWh, representing a year-on-year increase of 28.5% [
1].
Meanwhile, behind the vigorous development of the LIB industry, safety issues concerning LIBs, particularly fire hazards, have become increasingly prominent. In recent years, LIB fires have shown a frequent occurrence globally, with multiple severe fire accidents attracting widespread attention. In May 2024, a fire broke out at the Gateway Energy Storage Station in California, USA, during which a large amount of toxic gases was released. The fire reignited twice and burned for six days, forcing the evacuation of nearby residents. In June 2024, a fire occurred at the Aricell lithium battery factory in Hwaseong City, Gyeonggi-do, South Korea, resulting in 23 deaths. In June 2025, the roll-on/roll-off cargo ship “Morning Midas” caught fire in the waters off Alaska, USA, destroying 800 electric vehicles, hybrid vehicles, and over 2200 other fuel-powered vehicles on board, eventually leading to the sinking of the ship. In the first half of 2025 alone, there have been at least 15 fire safety incidents in global civil aviation caused by LIB power banks carried by passengers, a significant increase compared with the same period in 2024. The fire risks in application scenarios of LIBs such as LIB energy storage stations, electric vehicles, electric bicycles, and electronic products cannot be ignored either, posing severe challenges to public life and property safety as well as the sustainable development of the industry.
LIBs fires are caused by thermal runaway. Thermal runaway of LIBs are a serious chain exothermic reaction process triggered by the coupling of multi-scale and multi-physical fields inside them, and their mechanisms involve the synergistic effect of electrode materials, electrolytes, and interface reactions [
2,
3]. When LIBs are subjected to mechanical abuse (such as crushing and piercing), electrical abuse (overcharging and short circuit), or thermal abuse (high ambient temperature), they first trigger the thermal self-exothermic decomposition reactions of the solid electrolyte interphase (SEI) film on the surface of their negative electrode, releasing gases such as CO
2, C
2H
4, CO, and H
2 along with some heat. As the temperature rises to 120–200 °C, the lithium intercalated in the negative electrode reacts with organic solvents in the electrolyte (such as ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC)), generating Li
2CO
3 and flammable gases like, CO
2, CO, and H
2 and releasing a large amount of heat. Meanwhile, lithium salts in the electrolyte (such as LiPF
6) undergo thermal decomposition reactions, and the products further combine with the electrolyte and promote oxidative decomposition reactions, releasing toxic gases such as HF, PF
5, and POF
3 and heat [
4,
5,
6,
7]. As the temperature continues to rise, the separator inside the battery melts, leading to internal short circuits. When the temperature further increases to 200–300 °C, positive electrode materials (such as Li
2CoO
2, LiFePO
4, and LiNi
xCo
yMn
zO
3) decompose, releasing O
2 which reacts with organic solvents in the electrolyte and releasing a large amount of heat. At this stage, the electrolyte continues to decompose to produce toxic gases such as HF and CO, which form a flammable gas mixture with the products of electrode reactions and deflagrate at high temperatures [
8,
9].
The above reactions form a positive feedback cycle of “exothermic reaction, temperature rise, reaction intensification”, eventually causing rupturing of the battery casing, electrolyte volatilization, and flame jetting while releasing a large amount of fire gases. During the thermal runaway and combustion process of LIBs, most of the fire gases are derived from the thermal decomposition and oxidation reactions of the electrolyte. Meanwhile, the reaction temperature has a significant impact on the modes of thermal decomposition and oxidation reactions, as well as the types and quantities of products generated. In addition, during the thermal runaway and combustion process of LIBs, the rapid evaporation, oxidation, and thermal decomposition of the electrolyte lead to a sharp increase in the internal pressure of the battery, resulting in the destruction of the internal pole piece structure and causing changes in the internal flow field of the battery. The rise in the internal pressure of the battery, along with the thermal decomposition and oxidation reactions of the electrolyte and the migration of fire gases, further affect the combustion reaction mode and intensify the thermal runaway process [
10,
11,
12].
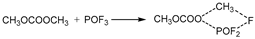
LiPF
6 is the most widely used lithium salt in LIB electrolytes. Its core function is to dissociate into Li
+ and PF
6− in carbonate-based solvents, providing a conductive carrier for the migration of lithium ions between the positive and negative electrodes, forming a high ionic conductivity system, and ensuring the battery’s charge–discharge efficiency. Meanwhile, LiPF
6 participates in the formation of the SEI film on the negative electrode; at high concentrations, it can promote the generation of inorganic components such as LiF and can optimize interface stability, and it exhibits good compatibility with aluminum current collectors, being suitable for high-voltage cathode materials. The thermal instability of LiPF
6 is one of the key factors inducing thermal runaway in LIBs [
13,
14,
15,
16]. LiPF
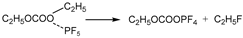
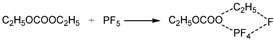
6 begins to decompose into LiF and PF
5 at approximately 107 °C. As a strong Lewis acid, PF
5 catalyzes the decomposition of solvents, releasing a large amount of heat. Furthermore, PF
5 reacts with trace amounts of water to generate HF and POF
3. HF damages the SEI film and corrodes electrodes, exacerbating side reactions [
17,
18,
19,
20]. PF
5 and POF
3 continuously promote solvent decomposition through autocatalytic chain reactions and synergize with thermal decomposition of EC, DMC, and DEC, leading to a sudden rise in the temperature of the battery and ultimately triggering combustion or explosion. The massive generation of fire gases such as HF, CO, CO
2, and C
2H
4 during this process further intensifies the increase in internal pressure of the battery and the spread of thermal runaway [
21,
22,
23,
24].
Relevant studies have shown that PF
5 and POF
3, the thermal decomposition products of LiPF
6, can catalyze the chain oxidation reactions of organic solvents, reducing the critical temperature for thermal runaway by 10–30 °C. Many scholars have conducted experimental studies on the reaction mechanisms between LiPF
6 and electrolyte components such as EC, DMC, DEC, and EMC and proposed preliminary thermal decomposition reaction mechanisms [
25,
26,
27]. In the field of LIB fire research, the kinetic studies on the thermal decomposition and oxidation reactions of electrolytes are still in their infancy. The existing experimental data and reaction models are relatively simplistic, lacking detailed chemical reaction mechanism models, intermediate products, and reaction kinetic parameters. The analysis of the synergistic decomposition effects in mixed solvent systems and the dynamic evolution of free radical chain reactions is insufficient; most models are simplified to main reaction pathways, making it difficult to quantify the energy changes during thermal runaway and the generation rates of fire products. Consequently, combustion kinetic analysis and fire numerical simulations for LIBs still exhibit significant inaccuracies, which constrain the design and development of high-safety LIBs.
In recent years, there have been many research studies on the fire numerical simulation of LIBs using CFD software. Fire numerical models have been developed to build up synthetic data from fire experiments at different scales of LIBs such as cell, pack, vehicle, and energy storage systems [
28,
29,
30]. They can provide fast predictions of the consequences of fire development. A key challenge for numerical modeling is the representation of the fire performance of LIBs properties. At present, the numerical simulation process for LIB fires usually involves directly inputting the heat release law and fire gas release law of a specific type of battery measured in the laboratory into the CFD program for calculation. This simulation process ignores the influence of different sizes and different combustion conditions on fire behavior, and the simulation results usually have a certain deviation from the actual situation. This is because research on the combustion and kinetic mechanism of LIBs is still in its infancy, and an accurate and complete detailed chemical reaction mechanism model for thermal decomposition and oxidation has not yet been established.
The kinetic simulation of electrolyte combustion reactions forms the foundation for research on LIBs fires, relying heavily on the accurate description of chemical reaction mechanisms and precise prediction of thermodynamic and kinetic data of reactions. In recent years, with the advancement of quantum chemical methods and computational capabilities, theoretical calculation approaches have been increasingly widely applied to predict and analyze chemical reaction mechanisms under combustion conditions [
31]. In our previous research studies, we employed quantum chemical calculation methods to elaborate in detail on the thermal decomposition reaction mechanisms, oxidation reaction mechanisms, and fire gas generation patterns of main electrolyte components (EC, DMC, and DEC), as well as the catalytic effect of Li
+ on the thermal decomposition reactions of electrolytes during the thermal runaway process of LIBs [
21,
32].
In this paper, we employ quantum chemical calculation methods to further analyze the reaction mechanisms between PF5 and POF3 (thermal decomposition products of LiPF6) and the main electrolyte components (EC, DMC, and DEC) in LIB electrolyte systems. Additionally, we explore the theoretical basis for improving the oxidation resistance and flame retardancy of LIBs by using fluorinated electrolytes. By clarifying these mechanisms, which represent the core contributions and innovations of this study, we aim to establish a solid theoretical foundation for the combustion reaction mechanisms of electrolytes and provide theoretical support for subsequent in-depth research on LIB fire mechanisms, as well as the construction of numerical models to enhance battery fire resistance.
3. Results and Discussion
The medium environment in which LIB electrolyte molecules reside exerts a considerable impact on their oxidative property, reductive property, and thermal stability [
21,
35]. In the air environment, the electrolyte evaporates into a gaseous state, leading to a significant increase in intermolecular distances, and the interactions between solvent molecules become almost negligible. Compared with the air environment, the electrostatic intermolecular interactions within the electrolyte are stronger, and the solvent effect is prominent. The solvent effect refers to the phenomenon in liquid phase reactions where the physical and chemical properties of the solvent affect the reaction equilibrium and reaction rate, whose essence lies in the fact that solvent molecules alter the electrostatic interactions between groups. In the solvent environment, some thermal decomposition reaction pathways and their reaction energies may undergo changes. Therefore, in this paper, the reaction mechanisms of EC, DMC, and DEC with PF
5 and POF
3 in both the electrolyte environment and the air environment are analyzed, respectively.
In the electrolyte, LiPF
6 undergoes decomposition reactions during the charge–discharge or thermal runaway processes of LIBs, generating substances such as PF
5 and POF
3. The thermal decomposition reactions of LiPF
6 upon combination with H
2O are the key factors inducing LIBs’ aging and thermal runaway. In our previous work, we have analyzed the reaction pathways and mechanism of LiPF
6 with H
2O in detail, and they are shown in the
Supplementary Information of this paper,
Figure S1a [
21]. Similarly, EC and DMC can also undergo analogous reactions, and these reaction mechanisms are shown in
Figure S1b,c.
Both PF
5 and POF
3 exhibit strong oxidizing properties. The PF
5 molecule adopts a trigonal bipyramidal structure, where the central P atom is surrounded by F atoms. Given the extremely high electron-withdrawing ability of F atoms, they strongly attract the electron cloud of the central P atom through an inductive effect, leading to a significant reduction in the electron cloud density of the P atom. This confers PF
5 a stronger ability to gain electrons, i.e., a stronger oxidizing property. The POF
3 molecule, on the other hand, has a tetrahedral structure with stable P=O double bonds. Compared with PF
5, the electron cloud density of POF
3’s central P atom is significantly higher, resulting in a relatively weaker oxidizing property. (The HOMO and LUMO of PF
5 and POF
3 are shown in
Figure S2.)
3.1. Reactions of EC with PF5 and POF3
As one of the most core components in an LIB electrolyte, EC plays crucial roles such as dissolving lithium salts, facilitating ion conduction, and stabilizing electrode interfaces in the electrolyte. The dielectric constant of EC is much higher than that of other carbonate solvents such as DMC and DEC. And its high dielectric constant enables EC to efficiently dissolve ionic lithium salts and promote the dissociation of LiPF6 into freely mobile ions of Li+ and PF6−, which provides a high ion concentration in the electrolyte. The oxygen atoms (carbonyl oxygen and cyclic ether oxygen) in EC molecules can form coordinate bonds with Li+, PF5, and POF3, and its strong coordination ability allows it to occupy a core position in the solvation sheath.
3.1.1. Reactions of EC with PF5
Figure 2a shows the thermal decomposition reaction pathway of EC combining with PF
5 in the electrolyte environment. The symbol of TS in the figure represents the transition state species. Firstly, absorbing 185.37 kJ/mol of heat, the cyclic ether oxygen atom in the EC molecule and its adjacent methylene carbon atom combine with the phosphorus atom and fluorine atom in the PF
5 molecule, respectively. Meanwhile, the PF
5 molecule promotes a stretching of the C-O bond in the cyclic structure of the EC molecule to transform into TS1, which is a transition state. Subsequently, the cyclic structure of the EC molecule is broken at the C-O bond, where it combines with PF
5, and the P-F single bond where the F atom linking EC in the PF
5 molecule cleaves, generating a chain group FCH
2CH
2OCOOPF
4 and releasing 90.52 kJ/mol of energy. Then, the group of FCH
2CH
2OCOOPF
4 could undergo further thermal decomposition reactions divided into two concurrent pathways:
(1) In the first pathway, absorbing 344.71 kJ/mol of heat, for the group of FCH2CH2OCOOPF4, the C-O bond linking POF4 cleaves and generates groups of POF4 and FCH2CH2OCO-. Herein, absorbing 126.56 kJ/mol of heat, the group of FCH2CH2OCO- transforms into TS2, with the C-O bond stretching at the C atom of FCH2CH2-. And then, TS2 breaks into CO2 and FCH2CH2-, releasing 210.86 kJ/mol of energy. The FCH2CH2- combines with a free H atom in the electrolyte to form a FCH2CH3 molecule.
(2) In the second pathway, absorbing 352.18 kJ/mol of heat, for the group of FCH2CH2OCOOPF4, the C-O bond linking the group of FCH2CH2- cleaves and generates groups of FCH2CH2- and PF4OCOO-. Then the group of PF4OCOO- transforms into TS3, with the C-O bond stretching at the C atom being connected to POF4, absorbing 55.73 kJ/mol of heat. And then TS3 breaks into POF4 and CO2, releasing 147.45 kJ/mol of energy.
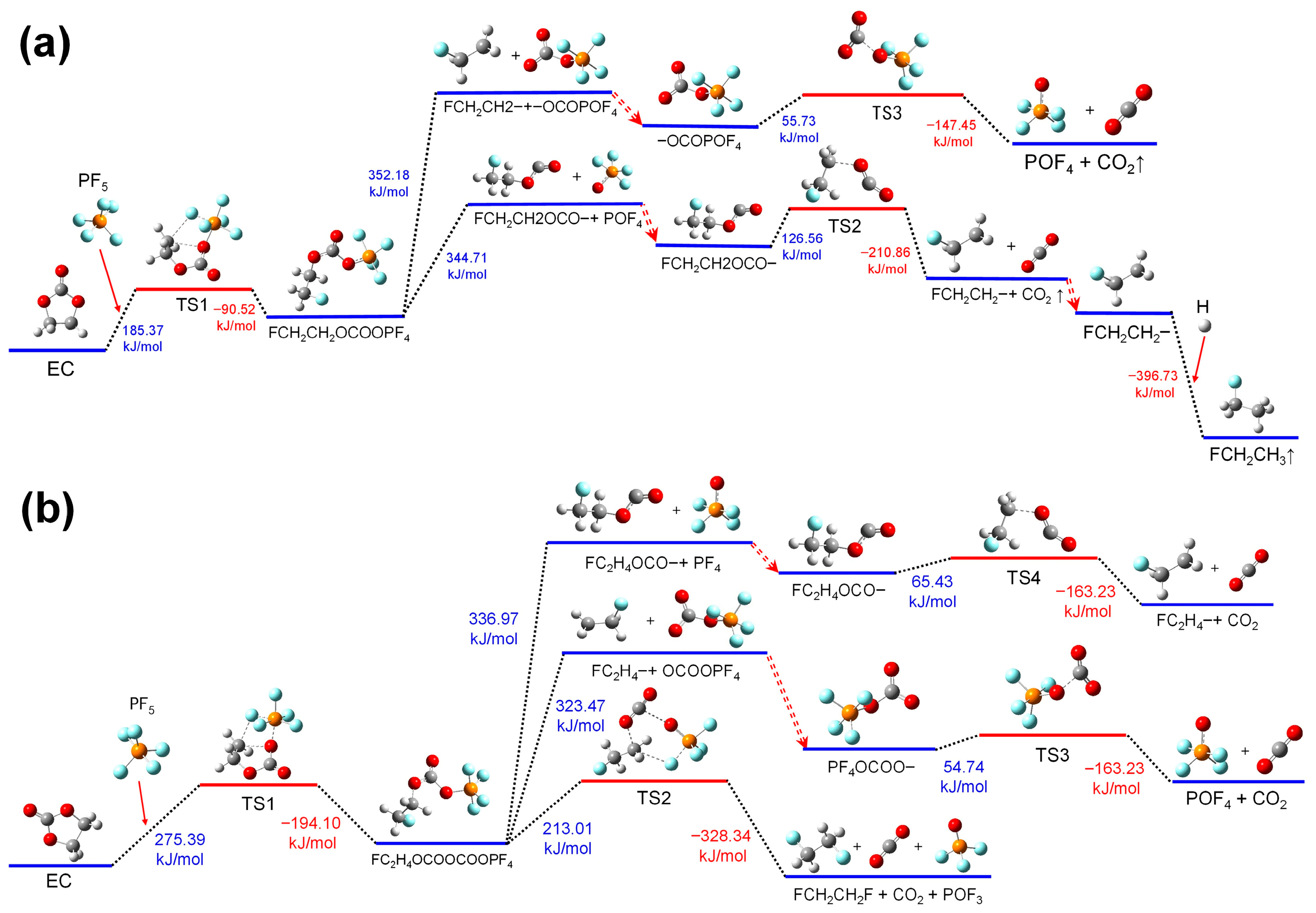
Figure 2b shows the thermal decomposition reaction pathway of EC combining with PF
5 in the air environment. And these reactions are more complex than those in the electrolyte environment. Consistent with the reactions in the electrolyte environment, one F atom and the P atom in the PF
5 molecule combine with the cyclic ether O atom and its adjacent methylene’s C atom in the EC molecule, respectively. Absorbing heat, the group transform into a state species of TS1. And then, TS1 undergoes ring-opening to generate a chain group of FCH
2CH
2OCOOPF
4 with an energy release.
In the air environment, there are three reaction pathways for FCH2CH2OCOOPF4. Among them, the first two reaction pathways are the same as those in the electrolyte environment: the FCH2CH2OCOOPF4 group undergoes bond cleavage at the C-O bond linking groups of FCH2CH2- and the PF4O-, respectively. However, due to the disappearance of the solvation effect in the electrolyte environment, the reaction energy has changed.
In the third pathway, the group of FCH2CH2OCOOPF4 absorbs heat and undergoes a reorganization reaction. The C-O bond linking the group of FCH2CH2- and the C-O bond linking the group of POF4- stretch simultaneously. Meanwhile, a F atom in the group of POF4- will combine with the -CH2- of FCH2CH2- and the P-F bond linking this F atom stretch together. After this reorganization process, the group of FCH2CH2OCOOPF4 transforms into TS2. Then, the TS2 decomposes into FCH2CH2F, CO2, and POF3, releasing energy.
In summary, the PF
5 molecule combines with the EC molecule, promoting its ring-opening and thermal decomposition reactions. This promoting effect is particularly significant in the electrolyte environment. The minimum energy required for the ring-opening reactions of EC is 290.89 kJ/mol [
21,
32], while after combining with PF
5, the ring-opening energy of EC decreases to 185.37 kJ/mol, representing a reduction of 36.27%. Therefore, PF
5 can accelerate the decomposition of the electrolyte during the thermal runaway process.
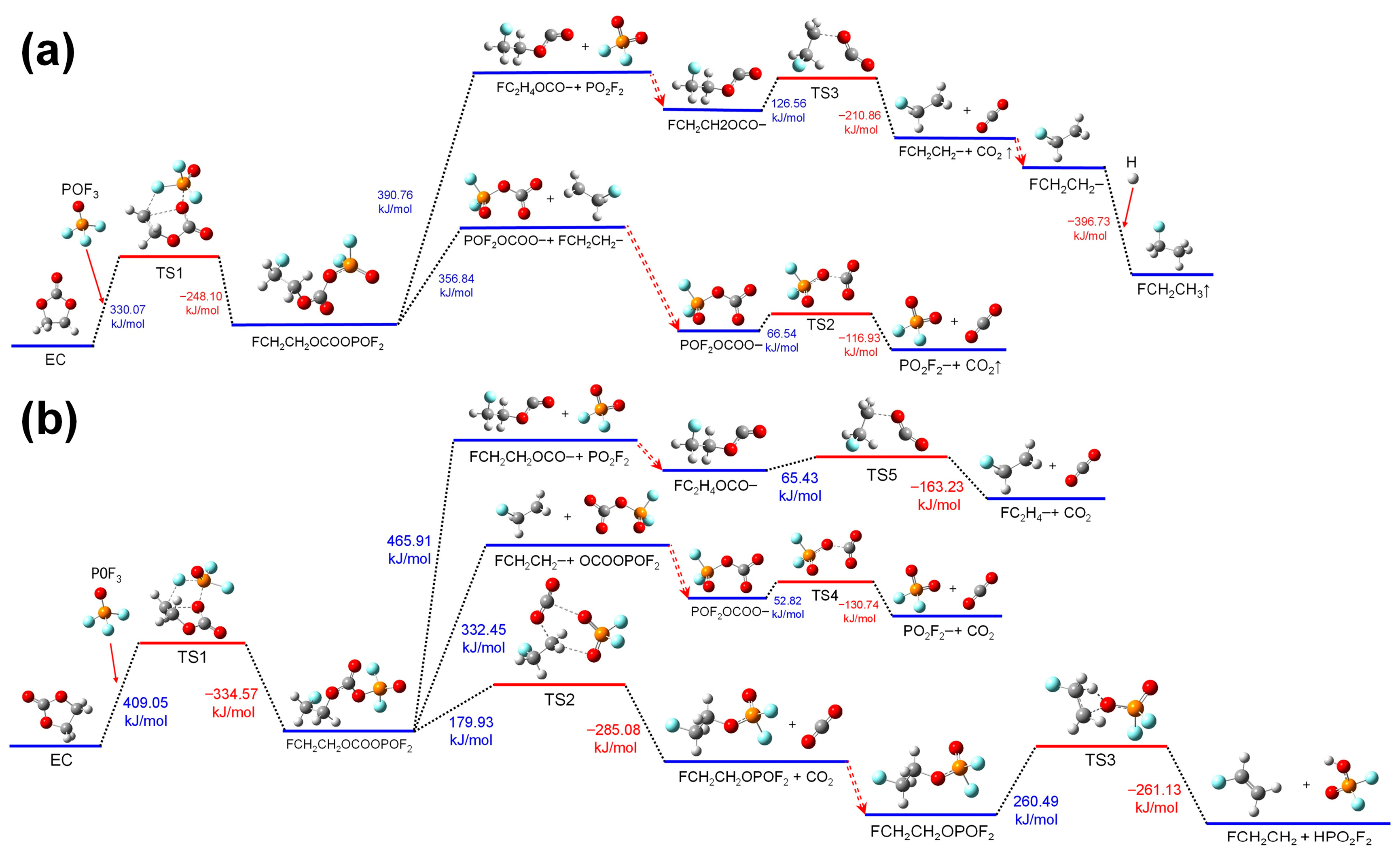
3.1.2. Reactions of EC with POF3
Figure 3 shows the thermal decomposition reaction pathway of EC combining with POF
3. The reaction mechanisms of EC with POF
3 in electrolyte and air environments are similar to those of EC with PF
5. Due to the fact that the oxidizability of POF
3 is weaker than that of PF
5, the heat absorbed during the ring-opening reactions after POF
3 combines with EC is higher than that after PF
5 combines with EC, whether in the electrolyte environment or the air environment. The energy consumed during the further thermal decomposition reactions of the chain group of FCH
2CH
2OCOOPOF
2 generated after ring-opening is significantly higher than that of the FCH
2CH
2OCOOPF
4 group formed after PF
5 promotes the ring-opening of EC. Thus, POF
3 exhibits a weaker catalytic effect on promoting the thermal decomposition reactions of EC than that of PF
5.
In the air environment, in the third reaction pathway of the thermal decomposition process that occurs after EC combines with POF3, there are certain differences between the reorganization process of the FCH2CH2OCOOPOF2 group compared with that of the FCH2CH2OCOOPF4 group. After absorbing heat, for the group of FCH2CH2OCOOPOF2, the C-O bond linking FCH2CH2- and the C-O bond connecting with POF2- stretch simultaneously, and the P=O bond in the POF2- group stretches as well. Meanwhile, the O atom of POF2- combines with the -CH2- group at the end of the FCH2CH2- group to form the transition state substance of TS2. Then, the TS2 further decomposes into FCH2CH2OPOF2 and CO2. In this process, the FCH2CH2- group and the PO2F2- group undergo reorganization to form the group of FCH2CH2OPOF2. Absorbing heat, for the group of FCH2CH2OPOF2, the H atom on the FCH2- group migrates to the O atom on the PO2F2 group. Meanwhile, with the C-O bond linking the FCH2CH2- group stretching, the FCH2CH2OPOF2 transforms into TS3. Then, the TS3 further decomposes into FCHCH2 and HPO2F2, releasing energy.
3.2. Reactions of DMC with PF5 and POF3
DMC ranks among the most critical solvents in LIB electrolytes. As a representative of linear carbonate solvents, DMC exhibits prominent features including low viscosity, low melting point, and excellent electrochemical stability. These intrinsic properties enable it to broaden the thermal stability window of the electrolyte, optimize the low-temperature performance of batteries, reduce electrolyte viscosity, mitigate the migration resistance of Li+ ions, and thereby enhance the charge–discharge efficiency as well as rate capability.
3.2.1. Reactions of DMC with PF5
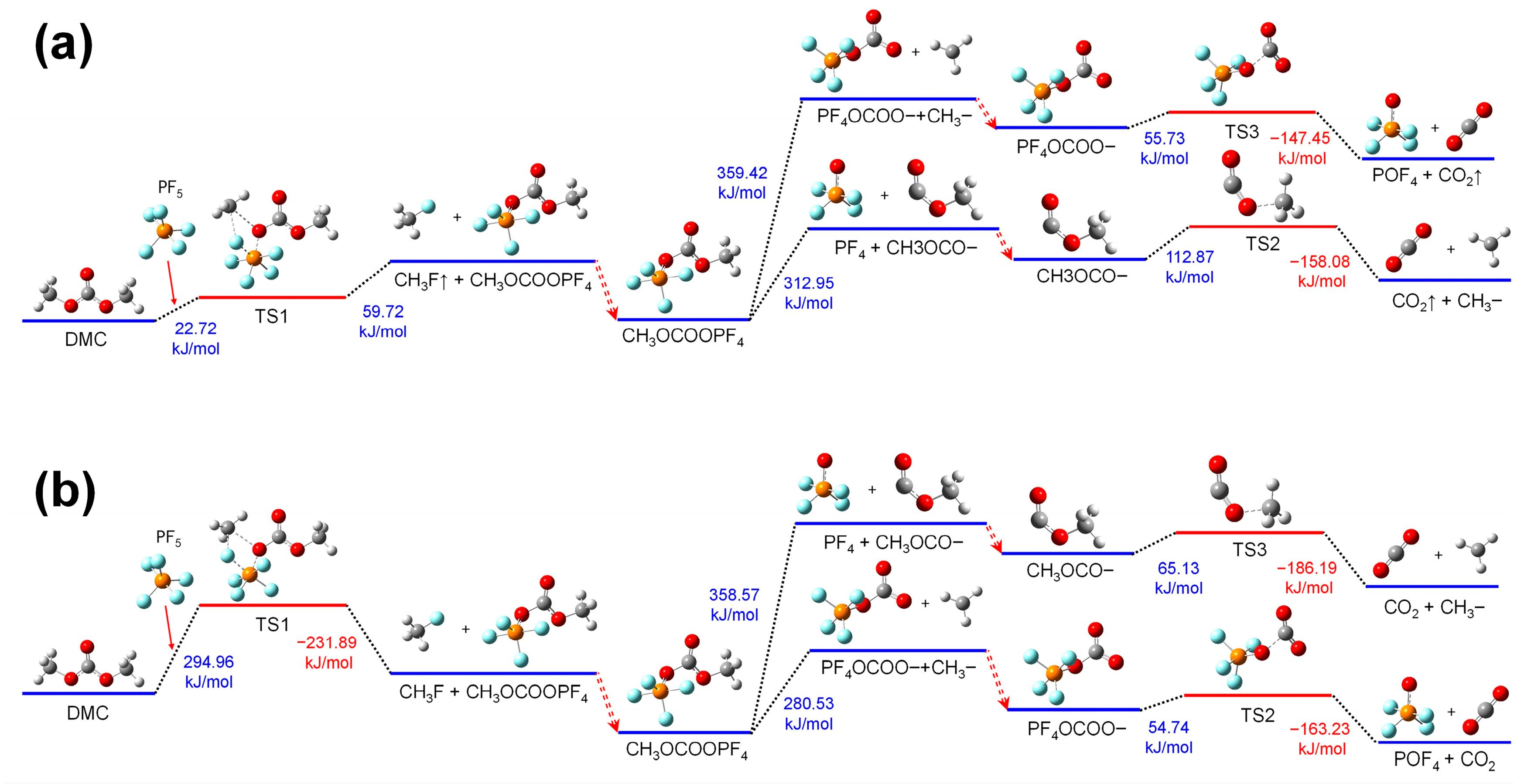
Figure 4a shows the thermal decomposition reaction pathway of DMC combining with PF
5 in the electrolyte environment. Firstly, the O atom in the CH
3O- group of the DMC molecule binds to the P atom in PF
5, while the C atom of the same CH
3O- binds to the F atom in the former PF
5. Absorbing energy, the C-O bond in the CH
3O- group and the P-F bond in PF
5 stretch, thereby forming the transition state substance TS1. Subsequently, the TS1 decomposes into CH
3F and CH3OCOOPF
4. Then, the group of CH3OCOOPF
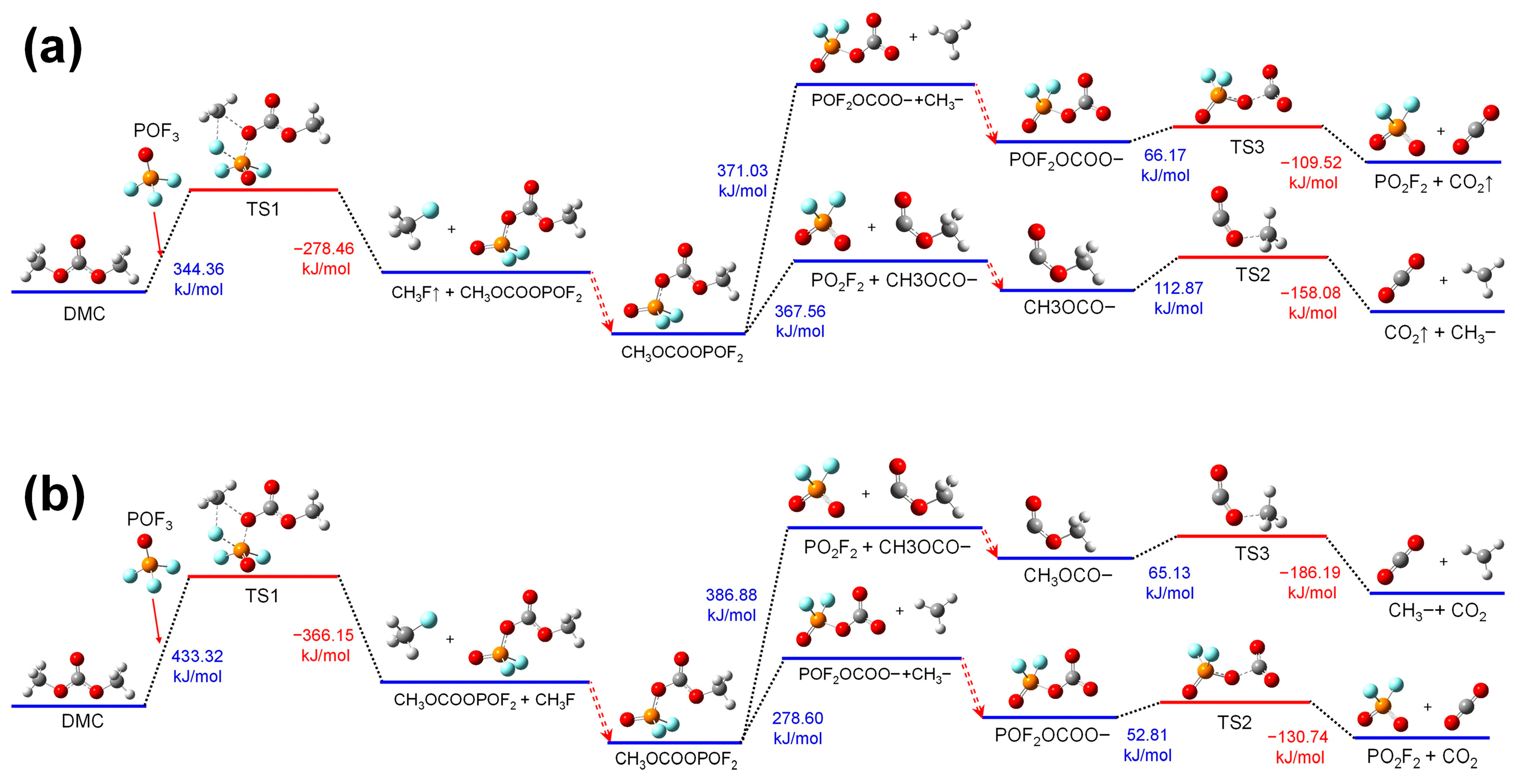
4 could undergo further thermal decomposition reactions divided into two concurrent pathways:
(1) In the first pathway, absorbing heat, the group of CH3OCOOPF4 breaks at the C-O bond linking POF4 and CH3OCO-. And then upon a further heat absorption, the C-O bond linking CH3- and -OCO- in CH3OCO- stretches. And the CH3OCOOPF4 transforms into TS2, and then it decomposes into CO2 and CH3-, releasing energy.
(2) In the second pathway, absorbing heat, the group of CH3OCOOPF4 breaks at the C-O bind linking POF4OCO- and CH3-. And then upon a further heat absorption, the group of POF4OCO- transforms into TS3 by the P-O bond, which links POF4 and -OCO- stretching. Then, TS3 decomposes into POF4 and CO2, releasing energy.
Figure 4b shows the thermal decomposition reaction pathway of DMC combining with PF
5 in the air environment. And these reactions are consistent with those in the electrolyte environment, except there are some differences in the energy changes during the reaction process. The energy barriers for thermal decomposition for DMC combining with PF
5 reduce significantly compared with those for DMC thermal decomposed independently. And this effect is more pronounced in the electrolyte environment than in the air environment.
3.2.2. Reactions of DMC with POF3
Figure 5 shows the thermal decomposition reaction pathway of DMC combining with POF
3 in the electrolyte environment and the air environment.
The thermal decomposition reaction mechanisms of DMC combining with POF3 in both the electrolyte and air environments are analogous to those of DMC combining with PF5. Given that the oxidizing property of POF3 is weaker than that of PF5, the energy absorbed in the ring-opening reactions of DMC combining with POF3 is higher than that when DMC combines with PF5.
3.3. Reactions of DEC with PF5 and POF3
DEC also plays multiple crucial roles in LIB electrolytes. Similar to DMC, as another linear carbonate solvent, DEC has a low viscosity characteristic, and it significantly enhances the fluidity of the electrolyte. Although DEC and DMC are structurally similar, the differences in their molecular structures result in significant variations in their thermal stability. The essential cause of the difference in their thermal stability lies in the structural effects induced by the groups of CH
3- and C
2H
5-. A longer carbon chain of the C
2H
5- in the DEC molecule endows its more complex reaction characteristics [
26].
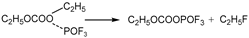
3.3.1. Reactions of DEC with PF5
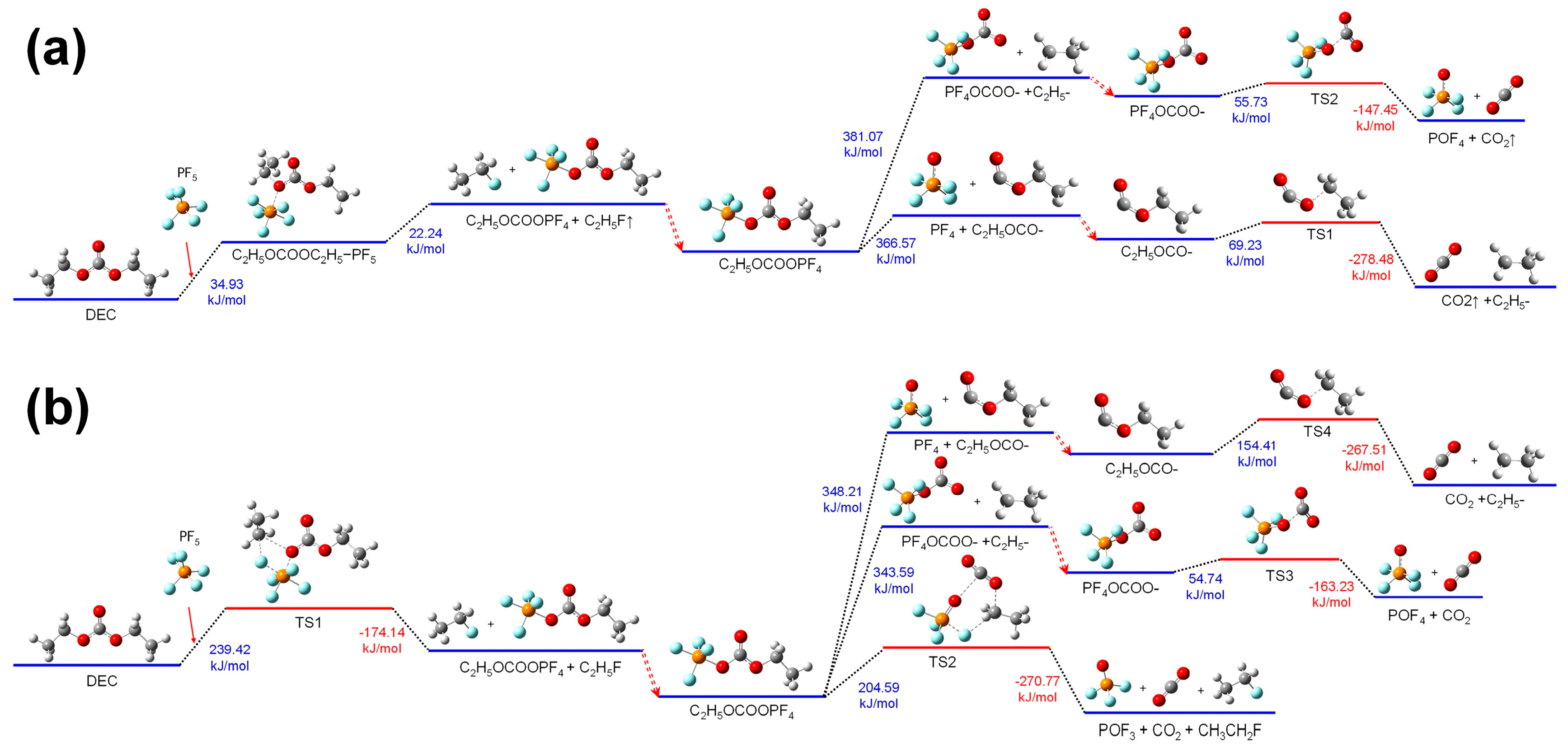
Figure 6a shows the thermal decomposition reaction pathway of DEC combining with PF
5 in the electrolyte environment. The O atom in the ethoxy group of DEC combines with the P atom in PF
5, forming a group of C
2H
5OCOOC
2H
5-PF
5. Absorbing heat, the group of C
2H
5OCOOC
2H
5-PF
5 undergoes a reaction where one F atom from PF
5 combines with the C
2H
5- from DEC, followed by a further decomposition into C
2H
5F and C
2H
5OCOOPF
4. Then the C
2H
5OCOOPF
4 undergoes further thermal decomposition reactions divided into two concurrent pathways.
(1) In the first pathway, absorbing heat, the group of C2H5OCOOPF4 cleaves at the C-O bond linking PF4O- and produces groups of POF4 and C2H5OCO-. Then, for the group of C2H5OCO-, absorbing heat, the C-O bond linking C2H5- and -OCO- stretches to transform into TS1. And the TS1 breaks into C2H5- and CO2, releasing energy.
(2) In the second pathway, absorbing heat, the group of C2H5OCOOPF4 cleaves at the C-O bond, on another side, linking C2H5-, and produces other groups of C2H5- and PF4OOCO-. Then, for the group of PF4OOCO-, absorbing heat, the C-O bond linking PF4O- and -OCO- stretches to transforms into TS2. And the TS2 breaks into POF4- and CO2, releasing energy.
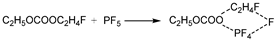
Figure 6b shows the thermal decomposition reaction pathway of DEC combining with PF
5 in the air environment. It differs slightly from that in the electrolyte. Absorbing heat, the O atom in the C
2H
5O- group of DEC binds to the P atom in PF
5, while the C atom combines with a F atom in PF
5. Meanwhile, the C-O bond linking C
2H
5- and the P-F bond at the F atom connecting with DEC stretch to transform into a transition state TS1. Then, the TS1 breaks into C
2H
5F and C
2H
5OCOOPF
4, releasing energy. C
2H
5OCOOPF
4 undergoes further thermal decomposition through three pathways. The first two of these pathways are identical to those in the electrolyte environment, where the C
2H
5OCOOPF
4 group undergoes C-O bond cleavage at the C
2H
5- site and the PF
4O site, respectively. However, due to the absence of the solvation effect of the electrolyte environment, their reaction energy has changed.
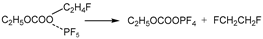
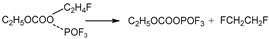
In the third pathway, absorbing heat, the group of C2H5OCOOPF4 undergoes reorganization reactions. The C-O bonds at the C2H5- site and PF4O- site stretch. Simultaneously, a F atom in PF4O- combines with the C atom of the -CH2- in C2H5-, while the P-F bond at this corresponding F atom stretches. Then, the group of C2H5OCOOPF4 transforms into TS2.
The C2H5OCOOPF4 group absorbs energy and undergoes reorganization. The C-O bond at the C2H5− site and the C-O bond at the POF4− site stretch simultaneously, while the P-F bond in the POF4− group stretches. The corresponding fluorine atom combines with the methylene group of the C2H5− group, forming the transition state TS2. Then, TS2 breaks into POF3, CO2, and C2H5F. This reaction process is similar to the third reaction pathway of the thermal decomposition of EC combining with PF5 in the air environment.
The reaction mechanisms of thermal decomposition of DEC combined with PF
5 in electrolyte and air environments are similar to those of DMC. DEC is more likely to combine with PF
5 and undergo the first step of thermal decomposition to generate fluorinated hydrocarbons and chain phosphate groups. This is because the bond energy of the C-C bond in the C
2H
5− group of DEC (approximately 347 kJ/mol) is lower than that of the C-H bond in the CH
3− group of DMC (approximately 413 kJ/mol). At high temperatures, the C-C bond in DEC is more prone to cleavage, leading to molecular decomposition [
36]. During the further thermal decomposition process of the chain phosphate groups of C
2H
5OCOOPF
4, the hyperconjugation effect of C
2H
5− in C
2H
5OCOOPF
4 enhances its local thermal stability, resulting in slightly higher energy consumption for some of its subsequent thermal decomposition reactions compared with CH
3OCOOPF
4 produced by DMC’s reactions with PF
5.
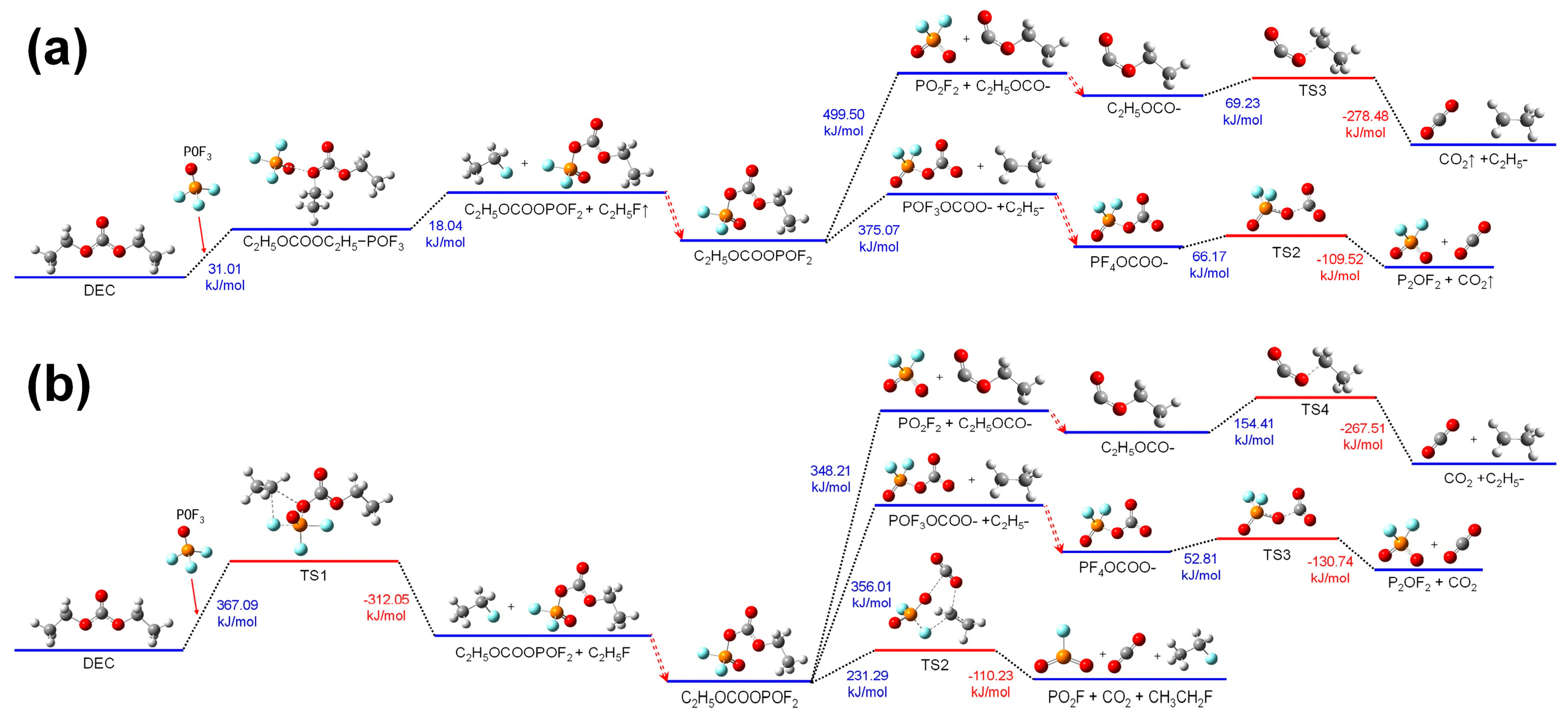
3.3.2. Reactions of DEC with POF3
Figure 7 shows the thermal decomposition reaction pathway of DEC combining with POF
3 in the electrolyte environment and the air environment.
The thermal decomposition reaction mechanisms of DEC combining with POF3 in both electrolyte and air environments are analogous to those of DEC combining with POF3. Compared with DMC, DEC is more prone to undergoing thermal decomposition after combining with POF3. This is attributed to the stronger electron-donating property of the C2H5- in DEC, which enables DEC to more easily combine with POF3 in a solution environment and undergo further decomposition reactions.
In summary, both PF
5 and POF
3 can promote the thermal decomposition reactions of EC, DMC, and DEC, whether in the electrolyte environment or the air environment. The chain structure of DMC and DEC makes them more prone to combining with PF
5 and POF
3. DEC is more prone to combining with PF
5 and POF
3 and undergoing thermal decomposition reactions than DMC. Compared with DMC, after combining with PF
5 or POF
3, EC and DEC could undergo recombination reactions with lower energy consumption. Therefore, PF
5 and POF
3 exhibit a more significant promoting effect on their thermal decomposition of EC and DEC. And this regularity is consistent with the results of experimental studies on the thermal analysis of electrolytes [
20,
24].
The reaction mechanism model for the thermal decomposition of the electrolyte catalyzed by PF5 and POF3 proposed by this research group has promoted a leap in the field of lithium-ion battery thermal runaway research from phenomenological observation to mechanism-based regulation. PF5 and POF3 activate the ester groups of carbonate molecules in the electrolyte through a Lewis acid catalytic mechanism, which reduces the activation energy for C-O bond cleavage, accelerates the formation of low-carbon chain aldehyde and ketone products, and further triggers fluorination reactions, ultimately leading to the formation of an acid catalysis chain propagation vicious cycle. This insight fills the theoretical gap regarding how lithium salt decomposition products quantitatively affect the thermal stability of electrolytes and corrects the previous one-sided research perspective of only focusing on the thermal stability of the lithium salts themselves. Based on the understanding of the active sites and catalytic pathways of PF5 and POF3 in the reaction model, we can develop electrolyte additives by targeting the Lewis acid activity of PF5, which can block the catalytic reaction sites through coordination. This mechanism-guided research and developed model replaces the traditional trial-and-error method and significantly improves the efficiency of electrolyte modification. Meanwhile, the intermediate products from the reactions of PF5 and POF3 with the electrolyte can serve as molecular-level indicators for thermal runaway early warning and protection. This provides a theoretical basis for the development of high sensitivity gas sensors and the construction of multi-level early warning systems, thereby enabling the early identification and intervention of thermal runaway risks.
3.4. The Inhibitory Effects of Fluorinated Electrolytes on the Promotions of PF5 and POF3
Compared with EC, DMC, and DEC, fluorinated carbonate electrolytes exhibit better thermal stability. The strong electron-withdrawing effect of F atoms in fluorinated carbonate electrolytes enhances the antioxidant capacity of the electrolytes and significantly strengthens the promoting effect on the thermal decomposition reactions of PF
5 and POF
3. In this part, we analyze the energy consumption of the fluorinated electrolytes, FEC, FDMC, and FDEC, during their combination with PF
5 and POF
3. The molecular models of FEC, FDMC, and FDEC are shown in
Figure S3.
3.4.1. Inhibitory Effects of FEC for Thermal Decomposition
The energy consumption during the reactions of EC and FEC with PF
5 and POF
3 are shown in
Table 2 and
Figure S4a.
Compared with the EC molecule, in the FEC molecule, one H atom on the -CH2- group in its cyclic structure is replaced by a F atom. The FEC molecule presents an asymmetric structure different from a centrally symmetric structure of an EC molecule. Therefore, there are two different combining modes between FEC and PF5 as well as POF3. The first reaction mode is the same as that of EC. PF5 or POF3 molecules combine with the -CH2- and -O- on the side of FEC that does not contain F atoms, followed by a ring-opening reaction. And the other reaction mode is that PF5 or POF3 molecules combine with the -CHF- and -O- on the F-containing side of FEC, followed by a ring-opening reaction. Compared with EC, whether in the electrolyte or the air environment, the energy consumption during FEC combining with PF5 or POF3 increases. Therefore, the antioxidant capacity of FEC during the thermal runaway process is significantly better than that of EC.
3.4.2. Inhibitory Effects of FDMC for Thermal Decomposition
The energy consumption during the reactions of DMC and FDMC with PF
5 and POF
3 are shown in
Table 3 and
Figure S4b.
Compared with the DMC molecule, in the FDMC molecule, one H atom on the terminal CH3- group of its chain structure is replaced by a F atom. FDMC also has two combining modes with PF5 or POF3. In the first mode, the reaction process and energy consumption of PF5 or POF3 combining with the C atom and O atom on the -CH3 side in FDMC are almost the same as those in DMC. And in the second mode, PF5 or POF3 combines with the C atom and O atom on the FCH2- side in FDMC. Compared with DMC, in the electrolyte environment, the energy consumed by the combination of FDMC with PF5 and POF3 increases by 733.67% and 36.33%, respectively; in the air environment, the energy consumed by the combination of FDMC with PF5 and POF3 rises by 105.73% and 74.46%, respectively. So, the antioxidant capacity of FDMC during the thermal runaway process is significantly superior to that of DMC.
3.4.3. Inhibitory Effects of FDEC for Thermal Decomposition
The energy consumption during the reactions of DEC and FDEC with PF
5 and POF
3 are shown in
Table 4 and
Figure S4c.
Compared with DMC, DEC has more fluorinated substitution sites. In this part, the reactivity of the FDEC molecule (C2H5OCOOCH2CH2F), where the H atom at the terminal CH3- position of DEC is replaced by a F atom, is analyzed.
In the electrolyte environment, both of the DEC and FDEC molecules first combine with PF5 or POF3 and then continue to undergo thermal decomposition reactions. Compared with DEC, the energy consumed during the binding process of FDEC with PF5 or POF3 decreases slightly, but the energy consumed in the subsequent thermal decomposition reactions increases by 212.40 kJ/mol and 208.71 kJ/mol, respectively (rising by 571.74% and 631.22%, respectively). Therefore, the antioxidant capacity of FDEC during the thermal runaway process is significantly improved compared with that of DEC. In the air environment, both DEC and FDEC combine with PF5 or POF3 and absorb heat, transforming into transition state substances. The energies absorbed during these processes and their further thermal decomposition reactions increase by 6.33% and 6.10%, respectively.
In summary, fluorinated electrolytes can significantly increase the energy required for their binding process with PF5 and POF3, inhibiting the promoting effect on their thermal decomposition during thermal runaway of LIBs. Therefore, in the design of LIB systems and the preparation of electrolytes, the usage of fluorinated electrolytes can significantly improve LIBs’ thermal stability and enhance their safety performance.
In LIBs, the conventional lithium salt LiPF6 is recognized as one of the critical factors contributing to the risk of electrolyte thermal runaway. When the battery temperature rises, LiPF6 tends to decompose readily, generating reactive species such as PF5 and POF3. These two substances further catalyze the thermal decomposition of the electrolyte, forming a vicious cycle of catalysis-accelerated decomposition and exacerbating the potential hazard of battery thermal runaway.
If LiPF6 is replaced with more stable lithium salts such as LiFSI, LiBF, or LiTFSI, the formation of PF5 and POF3 can be eliminated at the source. Such alternative lithium salts do not contain labile P-F bonds in their molecular structures, so they are less prone to decomposing and producing reactive catalytic substances under high-temperature conditions, fundamentally breaking the chain of reactive species generation-catalyzed electrolyte decomposition.
Meanwhile, alternative lithium salts can also optimize the interfacial stability of the electrolyte, reduce the side reactions between the electrolyte and electrodes at high temperatures, and further decrease the heat generation rate. This dual effect not only inhibits the thermal decomposition process of the electrolyte but also significantly enhances the overall thermal stability of LIBs. It provides crucial support for the safe operation of LIBs in high-temperature environments and holds great significance for the safety performance upgrading in fields such as new energy vehicles and energy storage systems.