The Effects of Burning Intensity on the Soil C-Related Properties and Mineralogy of Two Contrasting Forest Soils from Chilean National Parks
Abstract
1. Introduction
2. Materials and Methods
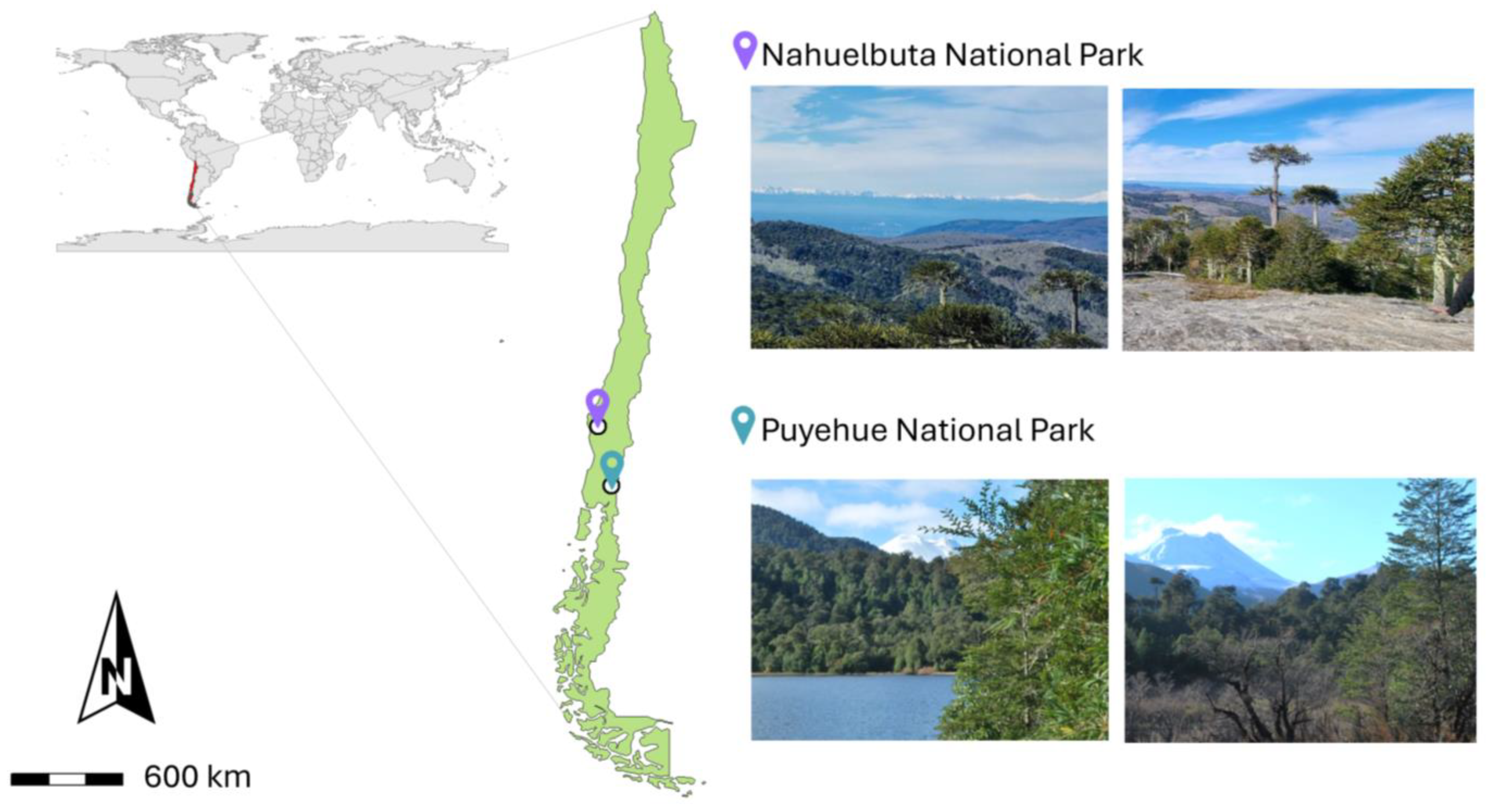
2.1. Sampling Sites
2.2. Heating Experiment
2.3. Chemical and Physical Soil Analyses
2.4. Mineralogical Analyses
2.5. Statistical Analysis
3. Results and Discussion
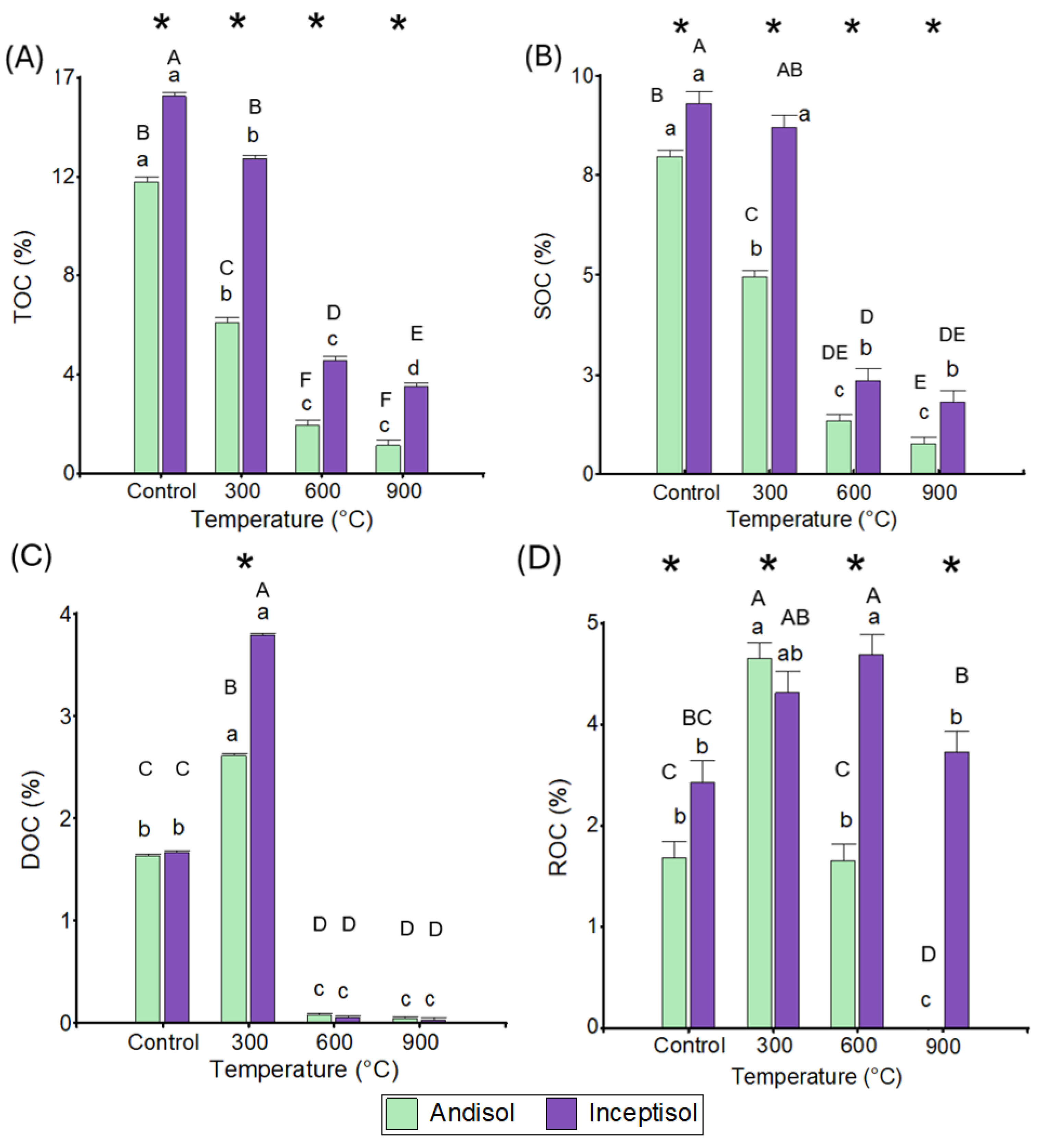
3.1. Organic Carbon Fractions
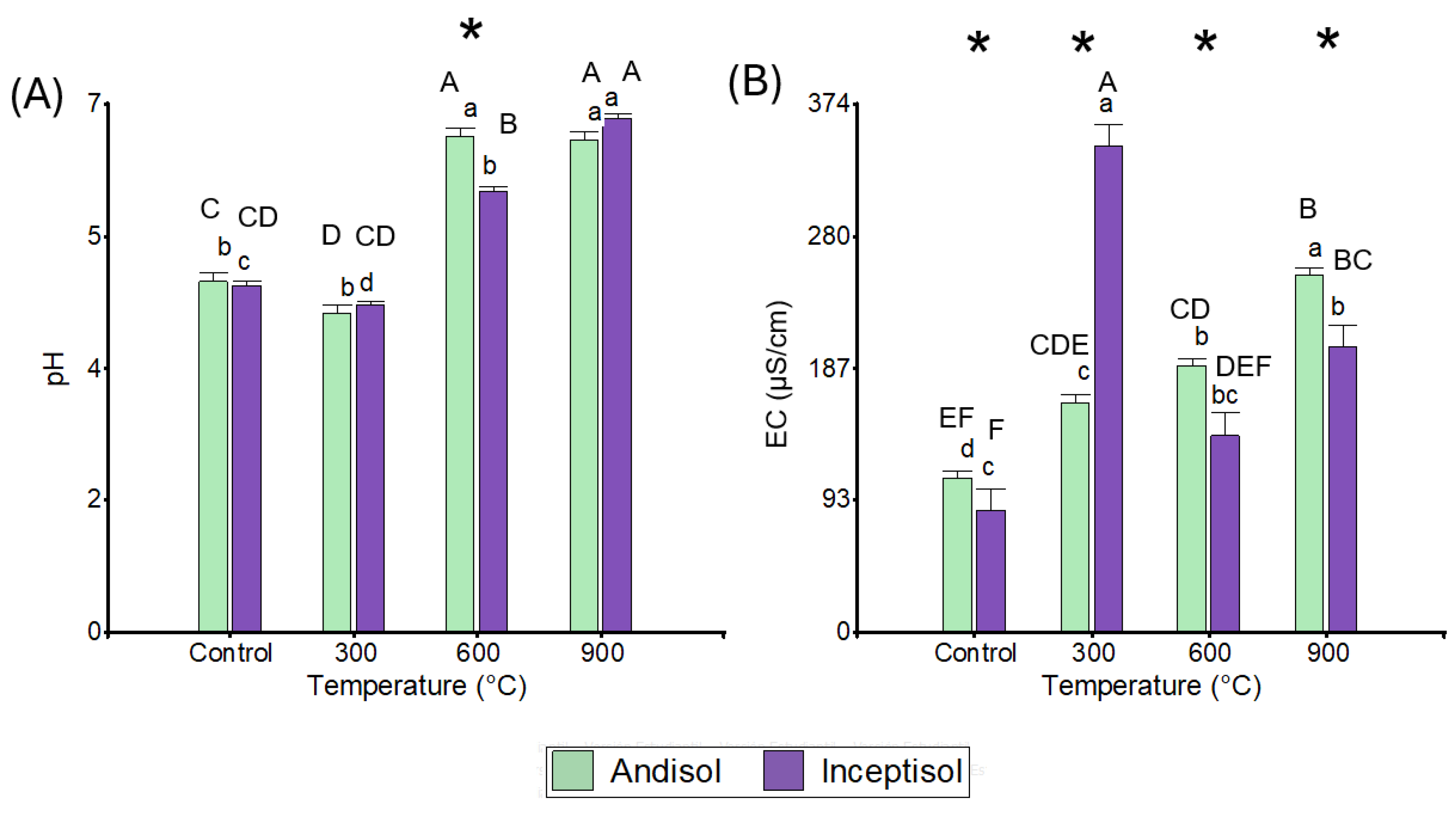
3.2. pH and Electrical Conductivity
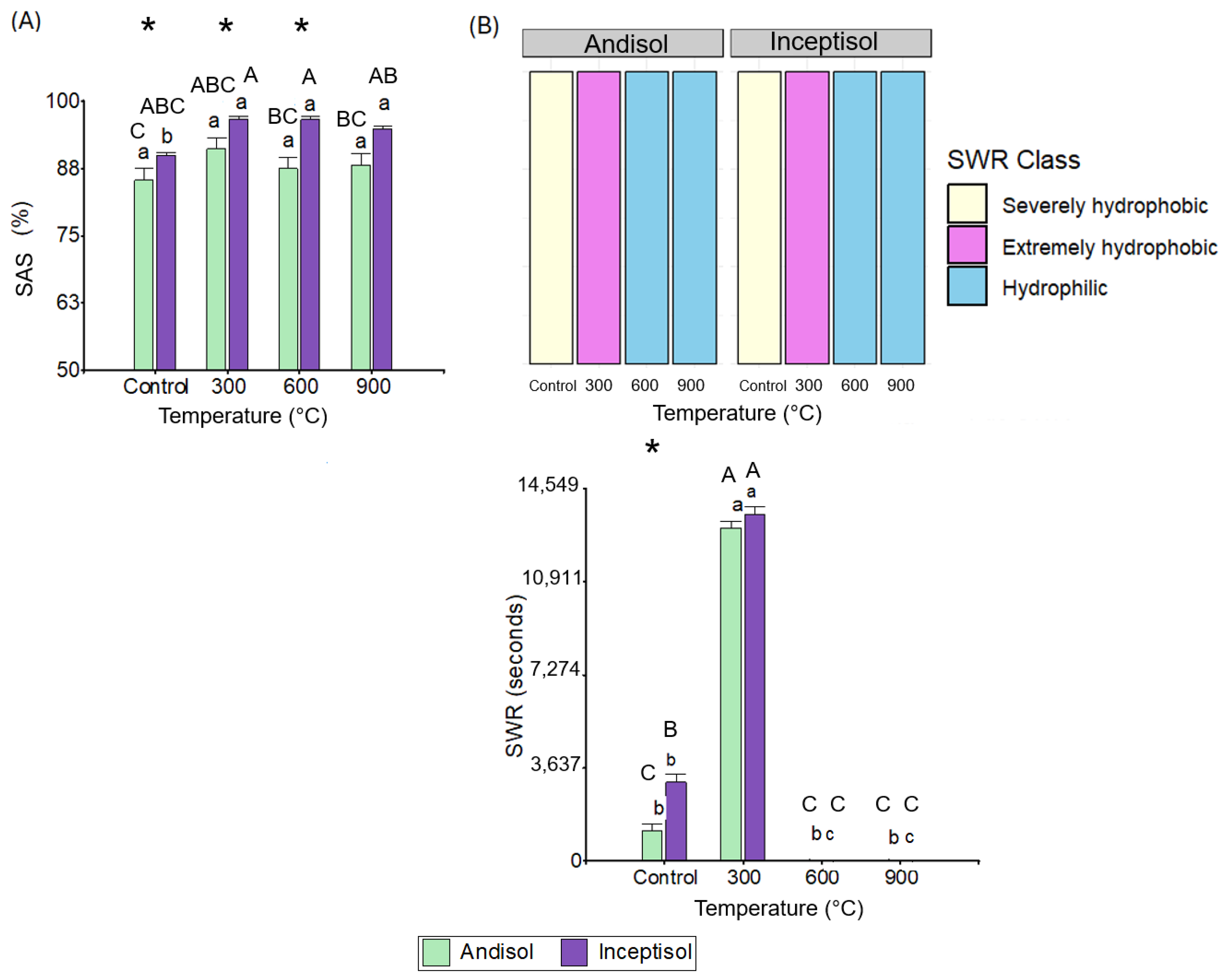
3.3. Soil Aggregate Stability and Soil Water Repellency
3.4. Mineralogical Modifications
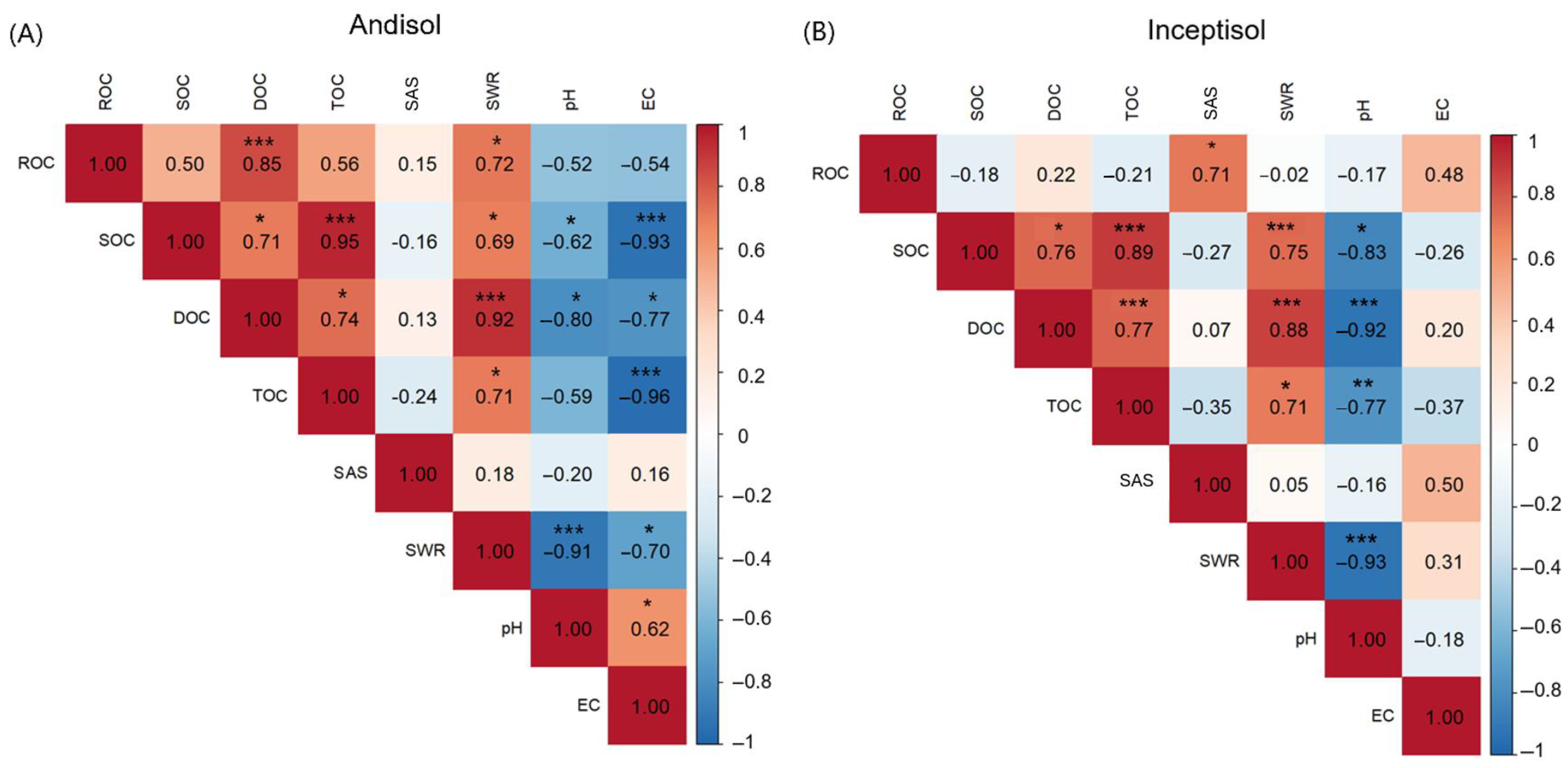
3.5. Spearman Correlations Among Soil Properties
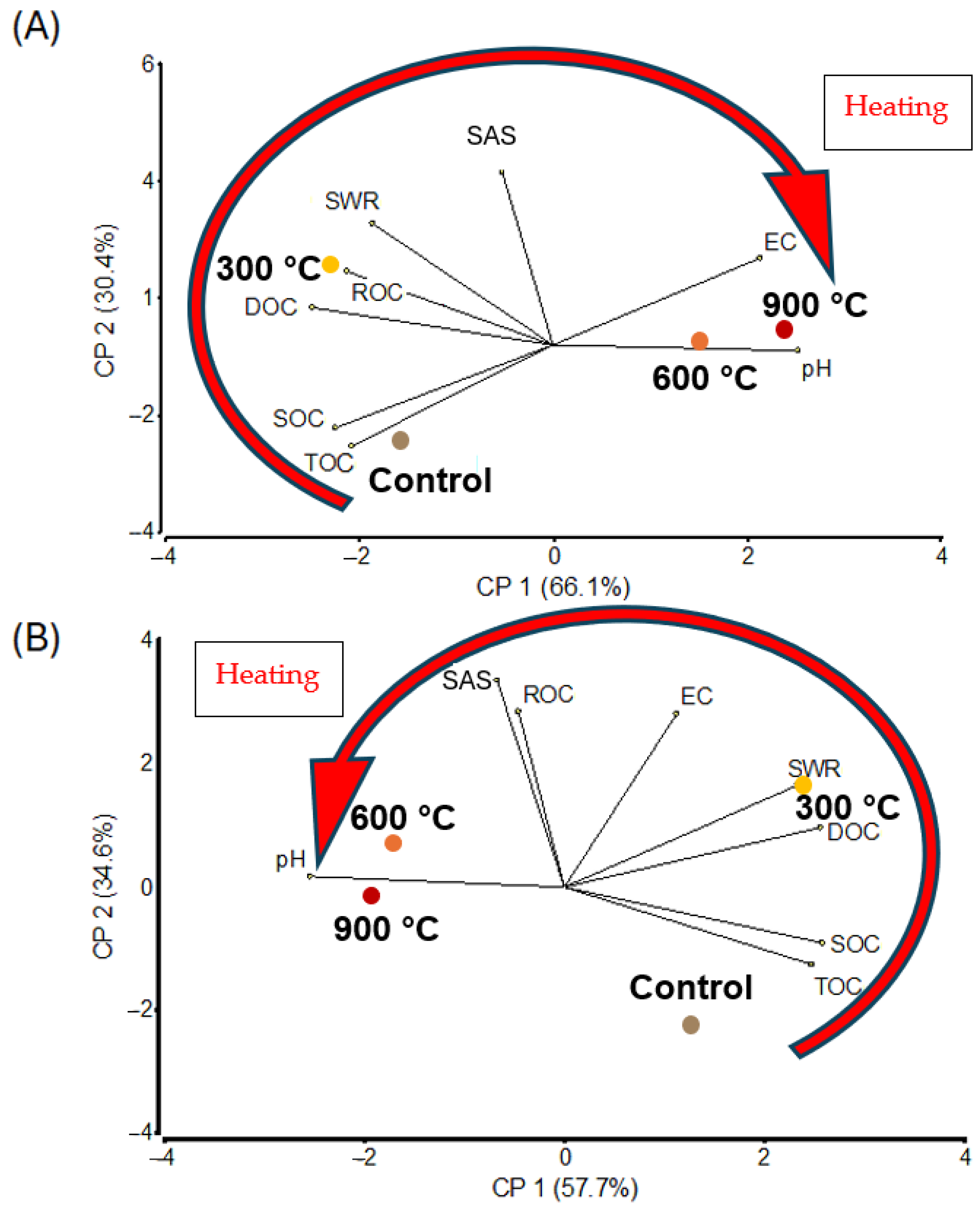
3.6. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO, ITPS, GSBI, CBD and EC. State of Knowledge of Soil Biodiversity: Status, Challenges and Potentialities; Report 2020; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Randerson, J.T.; Chen, Y.; Van Der Werf, G.R.; Rogers, B.M.; Morton, D.C. Global Burned Area and Biomass Burning Emissions from Small Fires. J. Geophys. Res. Biogeosci. 2012, 117, 4012. [Google Scholar] [CrossRef]
- González, M.E.; Muñoz, A.A.; González-Reyes, Á.; Christie, D.A.; Sibold, J. Fire History in Andean Araucaria–Nothofagus Forests: Coupled Influences of Past Human Land-Use and Climate on Fire Regimes in North-West Patagonia. Int. J. Wildland Fire 2020, 29, 649–660. [Google Scholar] [CrossRef]
- Beinroth, F.; Luzio, W.L.; Maldonado, F.P.; Esvaran, H. Taxonomy and Management of Andisols. In Proceedings of the Sixth International Soil Classification Workshop, Chile and Ecuador. Part II: Tour guide for Chile, Santiago, Chile, 9–20 January 1984; Sociedad Chilena de la Ciencia del Suelo: Santiago, Chile, 1985. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Certini, G. Effects of Fire on Properties of Forest Soils: A Review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Dathe, J.; Davinic, M.; Perry, G.; Wester, D.; Schwilk, D.W.; Rideout-Hanzak, S. Forest Wildfire and Grassland Prescribed Fire Effects on Soil Biogeochemical Processes and Microbial Communities: Two Case Studies in the Semi-Arid Southwest. Appl. Soil Ecol. 2016, 99, 118–128. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire Intensity, Fire Severity and Burn Severity: A Brief Review and Suggested Usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Pereira, P.; Martínez-Murillo, J.F.; Francos, M. Environments Affected by Fire. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2019; Volume 4, pp. 119–155. [Google Scholar]
- Badía-Villas, D.; González-Pérez, J.A.; Aznar, J.M.; Arjona-Gracia, B.; Martí-Dalmau, C. Changes in Water Repellency, Aggregation and Organic Matter of a Mollic Horizon Burned in Laboratory: Soil Depth Affected by Fire. Geoderma 2014, 213, 400–407. [Google Scholar] [CrossRef]
- Badía-Villas, D.; López-García, S.; Martí, C.; Ortíz-Perpiñá, O.; Girona-García, A.; Casanova-Gascón, J. Burn Effects on Soil Properties Associated to Heat Transfer under Contrasting Moisture Content. Sci. Total Environ. 2017, 601–602, 1119–1128. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire Effects on Belowground Sustainability: A Review and Synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire Effects on Soil Aggregation: A Review. Earth Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Araya, S.N.; Meding, M.; Berhe, A.A. Thermal Alteration of Soil Physico-Chemical Properties: A Systematic Study to Infer Response of Sierra Nevada Climosequence Soils to Forest Fires. SOIL 2016, 2, 351–366. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The Effect of Fire on Soil Organic Matter—A Review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.M. Fire-Induced Changes in Soil and Implications on Soil Sorption Capacity and Remediation Methods. Appl. Sci. 2019, 9, 3447. [Google Scholar] [CrossRef]
- Reynard-Callanan, J.; Pope, G.; Gorring, M.; Feng, H. Effects of High-Intensity Forest Fires on Soil Clay Mineralogy. Phys. Geogr. 2010, 31, 407–422. [Google Scholar] [CrossRef]
- Rivas, Y.; Matus, F.; Rumpel, C.; Knicker, H.; Garrido, E. Black Carbon Contribution in Volcanic Soils Affected by Wildfire or Stubble Burning. Org. Geochem. 2012, 47, 41–50. [Google Scholar] [CrossRef]
- Yusiharni, E.; Robert, J.G. Soil Minerals Recover after They Are Damaged by Bushfires. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; International Union of Soil Sciences: Vienna, Austria, 2010; pp. 104–107. [Google Scholar]
- Zihms, S.G.; Switzer, C.; Karstunen, M.; Tarantino, A. Understanding the Effects of High Temperature Processes on the Engineering Properties of Soils. In Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering, Paris, France, 2–6 September 2013; pp. 3427–3430. [Google Scholar]
- Inbar, A.; Lado, M.; Sternberg, M.; Tenau, H.; Ben-Hur, M. Forest Fire Effects on Soil Chemical and Physicochemical Properties, Infiltration, Runoff, and Erosion in a Semiarid Mediterranean Region. Geoderma 2014, 221–222, 131–138. [Google Scholar] [CrossRef]
- Ebel, B.A.; Moody, J.A.; Martin, D.A. Hydrologic Conditions Controlling Runoff Generation Immediately after Wildfire. Water Resour. Res. 2012, 48, 3529. [Google Scholar] [CrossRef]
- Shakesby, R.A.; Doerr, S.H.; Walsh, R.P.D. The Erosional Impact of Soil Hydrophobicity: Current Problems and Future Research Directions. J. Hydrol. 2000, 231–232, 178–191. [Google Scholar] [CrossRef]
- Neris, J.; Santamarta, J.C.; Doerr, S.H.; Prieto, F.; Agulló-Pérez, J.; García-Villegas, P. Post-Fire Soil Hydrology, Water Erosion and Restoration Strategies in Andosols: A Review of Evidence from the Canary Islands (Spain). IForest 2016, 9, 583–592. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; MacDonald, L.H. Soil Water Repellency: A Key Factor in Post-Fire Erosion. In Fire Effects on Soils and Restoration Strategies; Cerdà, A., Robichaud., P.R., Eds.; CRC Press: Boca Ratón, FL, USA, 2009. [Google Scholar]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A Review of the Effects of Forest Fire on Soil Properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Fowler, J.A.; Nelson, A.R.; Bechtold, E.K.; Paul, R.; Wettengel, A.M.; McNorvell, M.A.; Stevens-Rumann, C.S.; Fegel, T.S.; Anderson, E.; Rhoades, C.C.; et al. Pile Burns as a Proxy for High Severity Wildfire Impacts on Soil Microbiomes. Geoderma 2024, 448, 116982. [Google Scholar] [CrossRef]
- Bernhard, N.; Moskwa, L.M.; Schmidt, K.; Oeser, R.A.; Aburto, F.; Bader, M.Y.; Baumann, K.; von Blanckenburg, F.; Boy, J.; van den Brink, L.; et al. Pedogenic and Microbial Interrelations to Regional Climate and Local Topography: New Insights from a Climate Gradient (Arid to Humid) along the Coastal Cordillera of Chile. Catena 2018, 170, 335–355. [Google Scholar] [CrossRef]
- Reyes, J.; Thiers, O.; Gerding, V.; Donoso, P. Effect of Scarification on Soil Change and Establishment of and Artificial Forest Regeneration under Nothofagus Spp. in Southern Chile. J. Soil Sci. Plant Nutr. 2014, 14, 115–127. [Google Scholar] [CrossRef]
- Buol, S.W.; Southard, R.J.; Graham, R.C.; McDaniel, P.A. Soil Genesis and Classification, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Sharma, K.L.; Sharma, S.C.; Bawa, S.S.; Singh, S.; Chandrika, D.S.; Grace, J.K.; Rao, C.S.; Sankar, G.R.M.; Ravindrachary, G.; Munnalal; et al. Effects of Conjunctive Nutrient Management on Soil Fertility and Overall Soil Quality Index in Submountainous Inceptisol Soils under Rainfed Maize (Zea mays L.)–Wheat (Triticum Aestivum) System. Commun. Soil Sci. Plant Anal. 2015, 46, 47–61. [Google Scholar] [CrossRef]
- Matus, F.; Salazar, O.; Aburto, F.; Zamorano, D.; Nájera, F.; Jovanović, R.; Guerra, C.; Reyes-Rojas, L.; Seguel, O.; Pfeiffer, M.; et al. Perspective of Soil Carbon Sequestration in Chilean Volcanic Soils. npj Mater. Sustain. 2024, 2, 32. [Google Scholar] [CrossRef]
- Bertrand, S.; Fagel, N. Nature, Origin, Transport and Deposition of Andosol Parent Material in South-Central Chile (36–42° S). Catena 2008, 73, 10–22. [Google Scholar] [CrossRef]
- Valle, S.R.; Carrasco, J. Soil Quality Indicator Selection in Chilean Volcanic Soils Formed under Temperate and Humid Conditions. Catena 2018, 162, 386–395. [Google Scholar] [CrossRef]
- Balfour, V.N.; Woods, S.W. The Hydrological Properties and the Effects of Hydration on Vegetative Ash from the Northern Rockies, USA. Catena 2013, 111, 9–24. [Google Scholar] [CrossRef]
- Blank, R.R.; Allen, F.L.; Young, J.A. Influence of Simulated Burning of Soil-Litter From Low Sagebrush, Squirreltail, Cheatgrass, and Medusahead on Water-Soluble Anions and Cations. Int. J. Wildland Fire 1996, 6, 137–143. [Google Scholar] [CrossRef]
- Guerin, K.; Murphy, D.; Löhr, S.C.; Nothdurft, L. Experimental Constraints on the Role of Temperature and Pyrogenic Mineral Assemblage in Wildfire-Induced Major and Trace Element Mobilisation. Geochim. Cosmochim. Acta 2024, 386, 18–32. [Google Scholar] [CrossRef]
- Jiménez-Pinilla, P.; Mataix-Solera, J.; Arcenegui, V.; Delgado, R.; Martín-García, J.M.; Lozano, E.; Martínez-Zavala, L.; Jordán, A. Advances in the Knowledge of How Heating Can Affect Aggregate Stability in Mediterranean Soils: A XDR and SEM-EDX Approach. Catena 2016, 147, 315–324. [Google Scholar] [CrossRef]
- Thomaz, E.L.; Fachin, P.A. Effects of Heating on Soil Physical Properties by Using Realistic Peak Temperature Gradients. Geoderma 2014, 230–231, 243–249. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis, Part 3: Chemical Methods; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on Ignition as a Method for Estimating Organic and Carbonate Content in Sediments: Reproducibility and Comparability of Results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Ghani, A.; Dexter, M.; Perrott, K.W. Hot-Water Extractable Carbon in Soils: A Sensitive Measurement for Determining Impacts of Fertilisation, Grazing and Cultivation. Soil Biol. Biochem. 2003, 35, 1231–1243. [Google Scholar] [CrossRef]
- Rovira, P.; Ramón Vallejo, V. Labile, Recalcitrant, and Inert Organic Matter in Mediterranean Forest Soils. Soil Biol. Biochem. 2007, 39, 202–215. [Google Scholar] [CrossRef]
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.T. Water Repellency of Sieve Fractions from Sandy Soils and Relationships with Organic Material and Soil Structure. In Soil Structure/Soil Biota Interrelationships; Elsevier: Amsterdam, The Netherlands, 1993; pp. 105–118. [Google Scholar] [CrossRef]
- Kemper, W.D.; Koch, E.J. Aggregate Stability of Soils from Western United States and Canada: Measurement Procedure, Correlations with Soil Constituents; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 1966. [Google Scholar] [CrossRef]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer V: New York, NY, USA, 1996. [Google Scholar]
- Środoń, J.; Drits, V.A.; McCarty, D.K.; Hsieh, J.C.C.; Eberl, D.D. Quantitative X-Ray Diffraction Analysis of Clay-Bearing Rocks from Random Preparations. Clays Clay Miner. 2001, 49, 514–528. [Google Scholar] [CrossRef]
- Martín-Ramos, J.D. Using XPowder: A Software Package for Powder X-Ray Diffraction Analysis. D.L. GR-1001/04. Open J. Geol. 2020, 10. [Google Scholar]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 1980; ISBN 9780903056373. [Google Scholar]
- Joint Committee on Powder Diffraction Standards, A.S. for T. and M. Selected Powder Diffraction Data for Minerals, 1st ed.; Joint Committee on Powder Diffraction Standards: Swarthmore, PA, USA, 1974. [Google Scholar]
- Santín, C.; Doerr, S.H. Fire Effects on Soils: The Human Dimension. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150171. [Google Scholar] [CrossRef]
- Aznar, J.M.; González-Pérez, J.A.; Badía, D.; Martí, C. At What Depth Are The Properties of a Gypseous Forest Topsoil Affected By Burning? Land Degrad. Dev. 2016, 27, 1344–1353. [Google Scholar] [CrossRef]
- Sapkota, D.; Rawal, J.; Pudasaini, K.; Hu, L. A Laboratory Study of the Effects of Wildfire Severity on Grain Size Distribution and Erosion in Burned Soils. Fire 2025, 8, 46. [Google Scholar] [CrossRef]
- Pereira, J.S.; Badía-Villas, D.; Martí-Dalmau, C.; Mora, J.L.; Donzeli, V.P. Fire Effects on Biochemical Properties of a Semiarid Pine Forest Topsoil at Cm-Scale. Pedobiologia 2023, 96, 150860. [Google Scholar] [CrossRef]
- De La Cruz Domínguez, J.C.; Reyna, A.; Aguirre Gutiérrez, T.; Moreno, R.; De La Cruz Domínguez, J.C.; Alfaro Reyna, T.; Aguirre Gutiérrez, C.A.; Manuel, V.; Delgado Balbuena, J. Effects of Prescribed Burns on Soil Respiration in Semi-Arid Grasslands. Fire 2024, 7, 450. [Google Scholar] [CrossRef]
- Dou, X.; Köster, K.; Sun, A.; Li, G.; Yue, Y.; Sun, L.; Ding, Y. Temporal Dynamics of Soil Dissolved Organic Carbon in Temperate Forest Managed by Prescribed Burning in Northeast China. Environ. Res. 2023, 237, 117065. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.M.; Alexander, H.D.; Kielland, K.; Mann, P.J.; Natali, S.M.; Ruess, R.W. Fire Severity Effects on Soil Carbon and Nutrients and Microbial Processes in a Siberian Larch Forest. Glob. Change Biol. 2018, 24, 5841–5852. [Google Scholar] [CrossRef]
- Salgado, L.; Alvarez, M.G.; Díaz, A.M.; Gallego, J.R.; Forján, R. Impact of Wildfire Recurrence on Soil Properties and Organic Carbon Fractions. J. Environ. Manag. 2024, 354, 120293. [Google Scholar] [CrossRef]
- Bravo-Escobar, A.V.; O’Donnell, A.J.; Middleton, J.A.; Grierson, P.F. Differences in Dissolved Organic Matter (DOM) Composition of Soils from Native Eucalypt Forests and Exotic Pine Plantations Impacted by Wildfire in Southwest Australia. Geoderma Reg. 2024, 37, e00793. [Google Scholar] [CrossRef]
- Wang, J.J.; Dahlgren, R.A.; Chow, A.T. Controlled Burning of Forest Detritus Altering Spectroscopic Characteristics and Chlorine Reactivity of Dissolved Organic Matter: Effects of Temperature and Oxygen Availability. Environ. Sci. Technol. 2015, 49, 14019–14027. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Guan, P.; Zhang, P.; Mo, X.; Yin, G.; Qu, B.; Xu, S.; He, C.; Shi, Q.; et al. Temperature Thresholds of Pyrogenic Dissolved Organic Matter in Heating Experiments Simulating Forest Fires. Environ. Sci. Technol. 2023, 57, 17291–17301. [Google Scholar] [CrossRef]
- Gui, H.; Wang, J.; Hu, M.; Zhou, Z.; Wan, S. Impacts of Fire on Soil Respiration and Its Components: A Global Meta-Analysis. Agric. For. Meteorol. 2023, 336, 109496. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z. Recalcitrant Carbon Controls the Magnitude of Soil Organic Matter Mineralization in Temperate Forests of Northern China. For. Ecosyst. 2018, 5, 17. [Google Scholar] [CrossRef]
- Hrenović, J.; Kisić, I.; Delač, D.; Durn, G.; Bogunović, I.; Mikulec, M.; Pereira, P. Short-Term Effects of Experimental Fire on Physicochemical and Microbial Properties of a Mediterranean Cambisol. Fire 2023, 6, 155. [Google Scholar] [CrossRef]
- Giovannini, G.; Lucchesi, S. Modifications Induced in Soil Physico-Chemical Parameters by Experimental Fires at Different Intensities. Soil Sci. 1997, 162, 479–486. [Google Scholar] [CrossRef]
- Giovannini, G.; Lucchesi, S.; Giachetti, M. Beneficial and Detrimental Effects of Heating on Soil Quality. In Fire in Ecosystem Dynamics; SPB Academic Publishing, The Hague: Amsterdam, The Netherlands, 1990; pp. 95–102. [Google Scholar]
- Badía-Villas, D.; Martí-Dalmau, C. Effect of Simulated Fire on Organic Matter and Selected Microbiological Properties of Two Contrasting Soils. Arid. Land Res. Manag. 2003, 17, 55–69. [Google Scholar] [CrossRef]
- Alfaro-Leránoz, A.; Badía-Villas, D.; Martí-Dalmau, C.; Escuer-Arregui, M.; Quintana-Esteras, S. The Effects of Fire Intensity on the Biochemical Properties of a Soil Under Scrub in the Pyrenean Subalpine Stage. Fire 2024, 7, 452. [Google Scholar] [CrossRef]
- Carvalho, M.L.; Maciel, V.F.; Bordonal, R.D.O.; Carvalho, J.L.N.; Ferreira, T.O.; Cerri, C.E.P.; Cherubin, M.R. Stabilization of Organic Matter in Soils: Drivers, Mechanisms, and Analytical Tools—A Literature Review. Rev. Bras. Cienc. Solo 2023, 47, e0230130. [Google Scholar] [CrossRef]
- Girona-García, A.; Ortiz-Perpiñá, O.; Badía-Villas, D.; Martí-Dalmau, C. Effects of Prescribed Burning on Soil Organic C, Aggregate Stability and Water Repellency in a Subalpine Shrubland: Variations among Sieve Fractions and Depths. Catena 2018, 166, 68–77. [Google Scholar] [CrossRef]
- Knicker, H. How Does Fire Affect the Nature and Stability of Soil Organic Nitrogen and Carbon? A Review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Neris, J.; Tejedor, M.; Fuentes, J.; Jiménez, C. Infiltration, Runoff and Soil Loss in Andisols Affected by Forest Fire (Canary Islands, Spain). Hydrol. Process. 2013, 27, 2814–2824. [Google Scholar] [CrossRef]
- Cowan, A.D.; Smith, J.E.; Fitzgerald, S.A. Recovering Lost Ground: Effects of Soil Burn Intensity on Nutrients and Ectomycorrhiza Communities of Ponderosa Pine Seedlings. For. Ecol. Manag. 2016, 378, 160–172. [Google Scholar] [CrossRef]
- Derkowski, A.; Kuligiewicz, A. Thermal Analysis and Thermal Reactions of Smectites: A Review of Methodology, Mechanisms, and Kinetics. Clays Clay Miner. 2022, 70, 946–972. [Google Scholar] [CrossRef]
- Cornell, R.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Guggenheim, S.; van Groos Koster, A.F. Baseline Studies of the Clay Minerals Society Source Clays: Thermal Analysis. Clays Clay Miner. 2001, 49, 433–443. [Google Scholar] [CrossRef]
- Negri, S.; Giannetta, B.; Till, J.; Oliveira de Souza, D.; Said-Pullicino, D.; Bonifacio, E. Fire Simulation Effects on the Transformation of Iron Minerals in Alpine Soils. Geoderma 2024, 444, 116858. [Google Scholar] [CrossRef]
- Zhu, B.; Fang, B.; Li, X. Dehydration Reactions and Kinetic Parameters of Gibbsite. Ceram. Int. 2010, 36, 2493–2498. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The Effect of Crystalline Phase (Anatase, Brookite and Rutile) and Size on the Photocatalytic Activity of Calcined Polymorphic Titanium Dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Parfitt, R.L. Allophane and Imogolite: Role in Soil Biogeochemical Processes. Clay Miner. 2009, 44, 135–155. [Google Scholar] [CrossRef]
- Fomina, M.; Skorochod, I. Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications. Minerals 2020, 10, 861. [Google Scholar] [CrossRef]
- Heckman, K.; Welty-Bernard, A.; Vazquez-Ortega, A.; Schwartz, E.; Chorover, J.; Rasmussen, C. The Influence of Goethite and Gibbsite on Soluble Nutrient Dynamics and Microbial Community Composition. Biogeochemistry 2013, 112, 179–195. [Google Scholar] [CrossRef]
- Kolář, L.; Kužel, S.; Horáček, J.; Čechová, V.; Borová-Batt, J.; Peterka, J. Labile Fractions of Soil Organic Matter, Their Quantity and Quality. Plant Soil Environ. 2009, 55, 245–251. [Google Scholar] [CrossRef]
- Fu, Z.; Hu, W.; Beare, M.H.; Müller, K.; Wallace, D.; Wai Chau, H. Contributions of Soil Organic Carbon to Soil Water Repellency Persistence: Characterization and Modelling. Geoderma 2021, 401, 115312. [Google Scholar] [CrossRef]
- Matus, F.J.; Paz-Pellat, F.; Covaleda, S.; Etchevers, J.D.; Hidalgo, C.; Báez, A. Upper Limit of Mineral-Associated Organic Carbon in Temperate and Sub-Tropical Soils: How Far Is It? Geoderma Reg. 2024, 37, e00811. [Google Scholar] [CrossRef]
- Armas-Herrera, C.M.; Mora-Hernández, J.L.; Arbelo-Rodríguez, C.D.; Rodríguez-Rodríguez, A. Labile Carbon Pools and Biological Activity in Volcanic Soils of the Canary Islands. Span. J. Soil Sci. 2013, 3, 7–27. [Google Scholar] [CrossRef]
- Knicker, H. Pyrogenic Organic Matter in Soil: Its Origin and Occurrence, Its Chemistry and Survival in Soil Environments. Quat. Int. 2011, 243, 251–263. [Google Scholar] [CrossRef]
- dos Santos, S.R.; da Costa, L.M.; Souza, C.M.M.; Leal, G.P.; de Mello, D.C.; da Silva, W.T.L.; Camêlo, D.d.L.; Schaefer, C.E.G.R. Thermodegradation of Organic Matter in Soils of Different Mineral Composition in Brazil. Geoderma Reg. 2024, 37, e00798. [Google Scholar] [CrossRef]

| Experimental Soil | ||
|---|---|---|
| Order | Inceptisol | Andisol |
| Soil subgroup ([5]) | Andic Dystrudept | Typic Hapludand |
| Location | Nahuelbuta National Park | Puyehue National Park |
| Parent material | Granodiorite | Basaltic-Andesitic-scoria |
| Main clay type | Kaolinite | Allophane |
| Textural class (USDA) | Loam | Sandy clay loam |
| pH (1:2.5) | 4.8 ± 0.1 | 4.8 ± 0.2 |
| Total Organic Matter (%) | 27.3 ± 0.4 | 21.1 ± 1.1 |
| Plant canopy | Araucaria araucana, Nothofagus pumilio | Nothofagus dombeyi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erazo, K.; Martí-Dalmau, C.; Badía-Villas, D.; Quintana-Esteras, S.; Bauluz, B.; Merino, C. The Effects of Burning Intensity on the Soil C-Related Properties and Mineralogy of Two Contrasting Forest Soils from Chilean National Parks. Fire 2025, 8, 277. https://doi.org/10.3390/fire8070277
Erazo K, Martí-Dalmau C, Badía-Villas D, Quintana-Esteras S, Bauluz B, Merino C. The Effects of Burning Intensity on the Soil C-Related Properties and Mineralogy of Two Contrasting Forest Soils from Chilean National Parks. Fire. 2025; 8(7):277. https://doi.org/10.3390/fire8070277
Chicago/Turabian StyleErazo, Karla, Clara Martí-Dalmau, David Badía-Villas, Silvia Quintana-Esteras, Blanca Bauluz, and Carolina Merino. 2025. "The Effects of Burning Intensity on the Soil C-Related Properties and Mineralogy of Two Contrasting Forest Soils from Chilean National Parks" Fire 8, no. 7: 277. https://doi.org/10.3390/fire8070277
APA StyleErazo, K., Martí-Dalmau, C., Badía-Villas, D., Quintana-Esteras, S., Bauluz, B., & Merino, C. (2025). The Effects of Burning Intensity on the Soil C-Related Properties and Mineralogy of Two Contrasting Forest Soils from Chilean National Parks. Fire, 8(7), 277. https://doi.org/10.3390/fire8070277







