Abstract
Fire is a combustion reaction where fuel reacts with oxygen in the presence of heat, releasing energy as light, heat, and flames. The main components of fire are fuel, oxygen, and heat. All three components must be present to cause a fire. Fire is a significant threat to residential and commercial buildings, often intensified by high fuel content in building materials such as wood and synthetics. This paper summarizes fire types and damages, loss of property and life, fuel content in building materials, and a method to reduce fire risk by minimizing the building material’s fuel content. This method uses minerals (coal combustion residual (CCR)), primarily inorganic oxides bonded with a small percentage of polyurethane binder, to manufacture a composite material moldable into multiple building products. The composite was tested as per the ASTM for mechanical, thermal, and fire safety performance. ASTM D635-based fire testing showed self-extinguishing behavior with significantly reduced burn rate and lengths (1–2 mm). A low calorific value of 6.6 MJ/kg was determined separately. The test results demonstrate that CCR-based mineral composites offer a fire-resistant, structurally sound, and eco-friendly alternative to wood products. This research supports recycling inorganic minerals into fire-resistant building products that enhance safety.
1. Introduction
Fire is a rapid chemical reaction known as combustion, where fuel reacts with oxygen in the presence of heat to release energy in the form of light, heat, and often flames. The main components of fire are the following (See Figure 1):
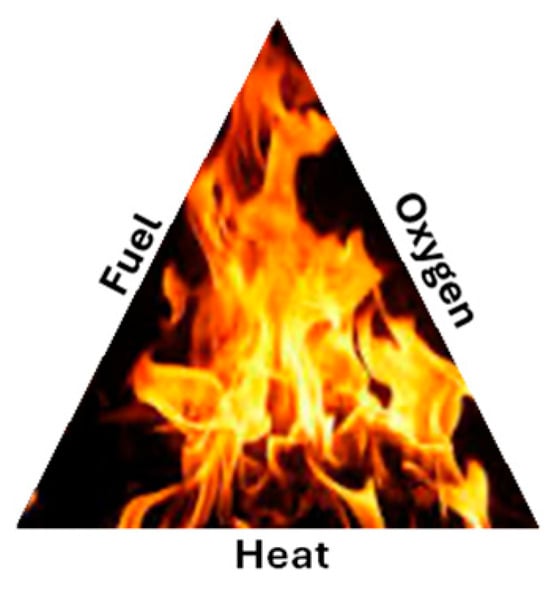
Figure 1.
Fuel, oxygen, and heat are the three components of fire.
- Fuel: A combustible material (e.g., wood, gasoline, volatiles).
- Oxygen: An essential element for combustion.
- Heat: The initial energy required to start the reaction and continue.
All three components must exist for a fire to ignite and continue to burn. If any of these components is absent, a fire cannot start and continue to burn. Air contains about 21% oxygen, which sustains fire. The availability of oxygen depends on the environment around the fire. For example, a forest fire could be exacerbated by high winds. Figure 2a,b shows a typical forest fire and a home caught fire from the forest fire, respectively. Figure 3a shows the damage after a forest fire, and Figure 3b shows a home being destroyed while another survives as the firefighters extinguish the fire. Note the destruction.

Figure 2.
(a) During a typical forest fire [1], (b) a home caught fire from a forest fire [1].

Figure 3.
(a) Destruction after a forest fire, [2], (b) one home destroyed, while the other survived, [3].
In addition to Oxygen, the other two items required are fuel and heat release during the fire. If the heat release is enough to sustain and ignite the fuel, the fire will continue. Therefore, the fuel content or the energy content and heat release rate of the product or the building materials are important factors in igniting a component and sustaining a fire. If the fuel content and the heat release rate are low, then it is difficult to ignite and sustain a fire. The concept of reducing the fuel content was used in developing structural core material for sandwich composites for ship-building applications [4,5]. Further, it was recently used in developing mineral (ash) composites for building products [6,7].
1.1. Types of Fire
Typically, there are three types of fire: forest fire, residential fire, and industrial fire.
1.1.1. Forest Fire
Based on cause or source, we can broadly classify it into three types, namely: natural fires, accidental fires, and arson.
- Natural Fires
Wildfires are caused by lightning strikes or volcanic activity [8]. They play a role in the ecosystem but can become destructive.
- Accidental Fires
A dropped cigarette, campfires left unattended, debris burning, equipment use, and faulty wiring cause a spark [9]. These fires can be prevented with proper safety measures.
- Arson
Deliberately setting fires, either maliciously or unintentionally [10]. There is a need to emphasize awareness and prevention.
1.1.2. Residential Fires
They are classified into cooking-related fires, which are a leading cause of house fires. Often occurs when food or grease overheats and ignites; Heating Equipment Fires caused by space heaters, fireplaces, or furnaces; Electrical Fires caused by faults in electrical systems or devices; Smoking-Related Fire—fires caused by cigarettes, cigars, or other smoking materials; Open flames from candles can ignite nearby flammable objects; Children Playing with Fire—Curious children playing with matches, lighters, or other flammable items; Faulty Appliances—Fires from malfunctioning or old appliances; Flammable Liquids wherein fires caused by improper storage or use of flammable liquids (e.g., gasoline, paint thinner); Holiday Decorations—Seasonal decorations like Christmas trees, lights, or candles can ignite fires; arson, etc. and other causes [11].
1.1.3. Industrial Fires
Industrial fires can occur in facilities such as factories, warehouses, chemical plants, and manufacturing units. These fires are often severe due to the presence of flammable materials, high-energy systems, and complex machinery. Common causes of industrial fires are electrical failures, combustible materials, hot work, mechanical failures, human error or negligence, chemical reactions, static electricity, arson or sabotage, and external factors [12]. Some of these can be prevented and/or mitigated by various methods.
1.2. Fire Damage and Loss
According to the US National Fire Protection Association (NFPA), in 2023, fires caused a total of 23 billion dollars in property damages for both residential and non-residential buildings. Deaths and injuries amounted to 3670 and 13,350, respectively [13]. More than one-third of all fires (34%) occurred in or on structures and were majorly responsible for the fire-related losses, including 3070 civilian deaths (84%), 11,790 civilian injuries (88%), and $14.7 billion in direct property damage (64%). Roughly one-quarter of fires (24%) occurred in residential properties—including one- or two-family homes and multifamily housing—yet they accounted for over three-quarters of civilian fire deaths (79%) and injuries (77%). Specifically, one- or two-family homes were the site of 18% of all fires but caused 68% of the civilian deaths and 56% of the injuries. Although only 6% of fires occurred in apartments, they still resulted in 11% of the civilian deaths and 21% of the injuries [13].
The most common building materials in buildings that fuel fire are synthetic materials, medium-density fiberboard (MDF), and wood. According to the US NFPA, efforts to improve safety, building codes, and public awareness are critical in reducing these losses [14]. However, less progress has been made in preventing these losses. Thus, more work needs to be conducted to reduce fire incidence and improve life safety, particularly regarding home fires. Here is an example of the fire damage and the loss incurred during the Eaton Fire in Los Angeles County, California, beginning on 7 January 2025, which lasted 24 days. This wildfire led to the tragic loss of 17 lives, 8 injuries, and the evacuation of more than 100,000 residents. The devastating fire destroyed over 9000 buildings, making it one of the most destructive fires in California’s history [15].
1.3. Fire Behavior and Fuel Content of Residential Construction Materials
Table 1 summarizes the fuel content (energy content), heat release, and the fire behavior of commonly used materials in residential construction. Wood products have high fuel content (HFC). Medium-density fiberboard (MDF), an engineered wood product made from wood fibers, resin, and wax, has HFC similar to wood. Similarly, chipboard, a mix of wood particles with epoxy resin and pressed together with intense heat and pressure to produce rigid, smooth surface boards, also exhibits HFC comparable to wood. On the other hand, note the high fuel content and heat release of synthetic materials (used for building products) compared to the wood products. These HFC materials contribute to rapid fire propagation. To minimize the impact of fire, one must find ways of reducing fuel in wood/synthetic products. The total fuel content in wood and wood-based products is 18–20 MJ/kg [16], and synthetic products are about 20 MJ to 40 MJ/kg [16]. It is preferable to avoid synthetic products.

Table 1.
Fuel content of wood and synthetics used in building materials.
The objective of this paper is to show how easily one can replace wood/synthetic products with a mineral product that can be produced from inorganic minerals that are less expensive and safer. The demonstration is made by coal combustion residual (CCR) produced by coal-burning electric power plants. Two types of CCR are considered fly ash, from currently running power plants, and legacy ash (pond ash, ash collected in ponds as a mixture of fly ash, bottom ash, and other waste).
2. Measurement of Fire Properties
It is essential to understand the construction material’s fire properties to assess its performance and safety in buildings. The key fire performance properties of a material are measured by:
- Ignition time in seconds at different heat fluxes: 25, 50, 75, and 100 kW/m2. Measured by cone calorimeter according to ASTM E1354 [17].
- Burn rate, in/s or cm/s.
- Burn-off time, in seconds, is the time required to put out the fire once the fire source is removed.
- Fuel content (Joules/unit weight). Measured by thermogravimetric test as per ASTM E1131 [18]. Also called Calorific value.
- Heat release rate (rate at which the material releases energy during combustion, influencing fire severity) at different heat fluxes (25, 50, 75, and 100 kW/m2) for building materials. For residential building products, 25 kW/m2 heat flux data are used.
3. Fire Prevention/Mitigation with Mineral Composites
The primary focus on fire prevention/mitigation strategies involves reducing the fire ignition, slowing down the fire spread, and minimizing the damage. This can be achieved by reducing the fuel content in the building materials, removing easily ignitable elements, and minimizing synthetic materials. One effective approach is the use of inorganic, mineral-based composite materials, which offer superior fire resistance relative to wood, wood-based products, and synthetic materials.
3.1. Mineral Composites
The inorganic minerals used in these composites are the coal combustion residuals (CCR) from electric power plants. These CCRs may be in the form of freshly produced ash called fly ash or processed ash (STAR processing, a thermal process that reduces the amount of unburnt carbon in the ash, transforming it into a high-quality product for use in the construction) from harvested ash. The use of these minerals offers a sustainable and fire-resistant alternative to traditional building materials. The mineral content of these CCRs is primarily oxides of Alumina (Al), Silica (Si), Iron (Fe), and Calcium (Ca). When these oxides are finely distributed within the binder (polymer or inorganic). The amount of heat released is insufficient to continue the fire. This concept is well adapted here.
3.1.1. Why Mineral Composites?
Mineral composites, particularly from CCRs like fly ash, offer cost-effectiveness, fire-resistance, and sustainability when used as building materials. In addition to freshly produced ash, utilizing the harvested ash reduces landfills and supports the circular economy. Through the formulations, design of experiments, processing, and performance characterization, we have demonstrated that these mineral composites offer lightweight, high fire resistance, enhanced thermal stability, and mechanical strength, making them suitable for building products. Overall, these specific inorganic mineral composite building products are environmentally friendly and efficient solutions for building safer, more resilient, and fire-resistant infrastructure [7].
3.1.2. Fly Ash Production
Fly ash is a byproduct of coal-burning electric power plants. Figure 4 shows how the coal is burned in the superheater, generating electricity for power generation. During the combustion process, fine particles, including fly ash, are carried with the flue gases. This fly ash is then captured by electrostatic precipitators (ESP) or fabric filters (FF) before the flue gases are introduced into flue gas desulfurization (FGD) units. The collected fly ash, as shown in Figure 4, consists of silica, alumina, and unburned carbon and is utilized in processing mineral composites to make the required building products (see Figure 5 for the block diagram).
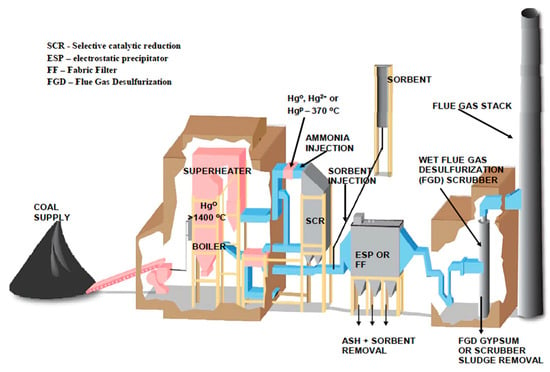
Figure 4.
Fly ash production from a power plant.

Figure 5.
Processing of mineral composite using powerplant fly ash and specific building products.
3.1.3. Harvesting of Legacy Ash for Processing of Mineral Composites
Legacy ash is stored in engineered ponds or landfills, as shown in Figure 6a. The process of harvesting the legacy ash begins with the site assessment, groundwater condition evaluation, and ash composition. Heavy machineries (See Figure 6b) are required to excavate by removing the mixture of stored fly ash, bottom ash, and any other waste from ponds and landfills. This ash is STAR processed [19] in a reactor and screened to utilize in the processing of mineral composites to make the required building products (See Figure 7 for the block diagram).
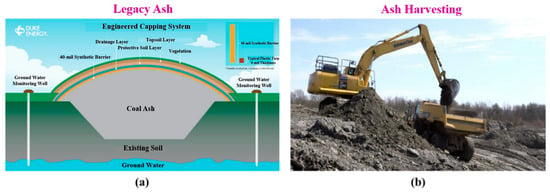
Figure 6.
(a) Legacy ash, (b) harvesting of legacy ash.
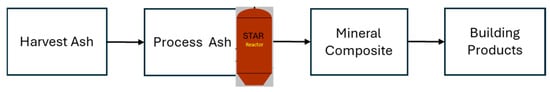
Figure 7.
Utilization of STAR-processed harvested ash for mineral composite building products.
3.1.4. Comparison of Fresh Fly Ash and Processed Ash Mineralogy
Fresh powerplant fly ash contains a mix of coarse (crystalline) and fine (amorphous) particles with varying amounts of unburned carbon, whereas the processed fly ash may undergo changes such as the reduction of unburned carbon content and an increase in the concentration of reactive silica and alumina phases. However, the fresh fly ash and processed ash share a similar base composition, as shown in Table 2. This particular ash is from Appalachian mountain coal and was originally provided by Southeast Fly Ash (SEFA), which is now known as Heidelberg Materials. The mineralogy data were provided to us by SEFA.

Table 2.
Comparison of the mineralogy of fly ash and processed ash.
3.1.5. Processing of Mineral Composite Using Polyurethane Binder
The processing method of the mineral composites using fly ash is simple. We mixed the liquid polymer ingredients (30% by weight), additives, and fly ash (70% by weight), with no solvents, using a lab-sized high-speed electric mixer with a high-shear blade @ 1400 Rpm for 4 to 5 min until the mix temperature reached 35 °C (95 °F), and then the mix was poured into a rubber mold of a certain size. Then, we pressed the mix in the mold with a pressure between 25 and 65 psi (170 and 440 kPa), depending on the required density of the composite. The two-part polyurethane in the mix reacted, got warm, and cured on its own. The total processing time was about 30 to 45 min, depending on how much and the type of curing agent we used. After taking the piece out of the mold, it was post-cured either by allowing it to sit at room temperature for a week or by putting it in an oven at 80 °C (176 °F) for about 30 min. We changed the rubber mold to make different pieces of different sizes and shapes [7]. Our processing method is flexible and can be adapted to make different building products by modifying the mix formulation. The processing details have been reported in the reference [7]; we are reiterating them in this work for the completeness of the paper.
3.1.6. Mineral Composite: Building and Other Structural Products
Figure 8 shows the different mineral composite building products that we have fabricated. We have successfully demonstrated the fabrication of a wide range of high-performance building products. These include chair molds, baseboards, decorative trims, structural elements such as door sills, door jambs, siding, deck panels, wall boards, stone-veneered tabletops, and stone-veneered tiles—all backed with durable mineral composite. Additionally, laminated wall and floor tiles, electric utility box covers, and electrical pole cross arms have been produced using this material. These mineral composite-based products can make a significant impact with fire resistance, enhancing safety, high mechanical strength for structural integrity, water resistance, ensuring long-term durability, mold and fungus resistance, promoting healthier indoor environments, thermal and electrical insulation, and bringing in multifunctionality relative to traditional building materials. Further, we have fabricated a 7ft school sign board (Figure 8), which was environmentally field tested for 7 years with no structural damage. Furthermore, we can make 2″ × 4″ mineral composite frames and 4″ × 8″ lightweight core material for fire-resistant doors. All the products can be made using continuous manufacturing.

Figure 8.
Mineral composite; building and other structural products.
Mineral composites made using 70% to 80% filler by weight in 20% to 30% polyurethane are extremely safe due to their low fuel content. For example, 100% polyurethane will have a fuel content or calorific value of about 33 MJ/kg (Table 1), whereas the mineral composite with 70% ash loading will have 10 MJ/kg, and for 80% ash loading, it is 6.6 MJ/kg, which is significantly low. Figure 9 shows the encapsulated ash particles (dispersed) by polyurethane, wherein the particles are in point-to-point contact with a very low amount of polymer in between the particles. This technique of making the material will not allow the material to ignite, spread the flame, or continue the fire; instead, the fire is cut off. This innovation demonstrates the potential of ash-based mineral composites by delivering sustainable, safe, and long-lasting alternatives to fire-prone conventional building products.
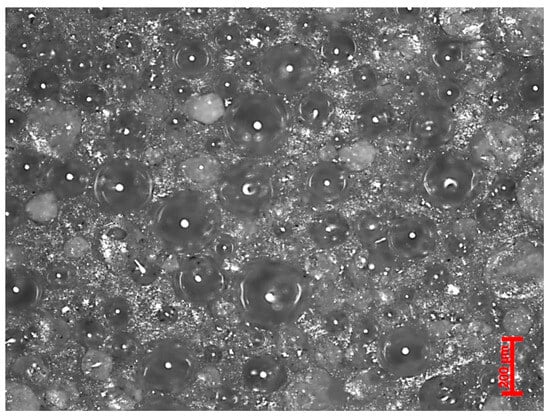
Figure 9.
Encapsulated ash particles by polymer.
3.1.7. Leachate Analysis and Material Properties of Mineral Composites
We conducted two different leaching tests: the EPA standard M1313 tumbling test [20] for fly ash and an in-house-built continuous water circulation tank test that imitates river flow, similar to EPA M1315. In the M1313 test, we used distilled water with a neutral pH of 7 to validate our encapsulation idea. All the test details are described in the reference [7].
The leachates from fly ash, pond ash (harvested ash), and coal powder were measured against EPA limits. Elements such as antimony, arsenic, selenium, and vanadium exceeded the Maximum Contamination Limit (MCL) in the leachates from fly ash and pond ash, while the coal powder leachates were much lower than the MCL in most cases and even below the instrument limit [7]. The data are shown in Table 3.

Table 3.
Leachates from coal powder, fly ash, pond ash, and ash-composite blocks, µg/L.
On the other hand, we made the mineral composite blocks and subjected them to testing. The leachates from the blocks taken at 1, 6, and 14 months were significantly lower than those from the fly ash or pond ash in powder form, with many values less than 1/3rd the MCL and some undetectable (See Table 3). These results demonstrate the safety and benefits of mineral composites for environmental and safety concerns.
The mechanical tests were conducted: (1) compression and (2) flexure according to ASTM D695 [21] and D790 [22], respectively. All the test details are reported in the reference [10]. Table 4 presents a summary comparison of the density, compression, and flexure strength of mineral composites and two commercially available materials: 1. Polyethylene Composite (Commercial 1) and 2. Cement Fiber Board (Commercial 2). For nearly the same density, mineral composites exhibit higher compression and flexural strengths compared to commercial materials.

Table 4.
Mechanical properties of mineral composites and commercial materials.
The electrical and thermal insulation properties were measured according to ASTM standards and are reported in the reference [7]. Also, the material was tested for termites and insects for 3 months and found to be unaffected. Alternatively, the termite groups died during this test period. This paper focuses mainly on the fire performance of the mineral composites relative to wood.
3.1.8. Fire Test and Results
Fire testing was performed according to ASTM D635 [23] to measure the burning time or rate of burning for wood and mineral composites in a horizontal position. In this method, a specimen is supported horizontally at one end, and the other end is exposed to fire for 30 s. The specimen geometry was 4.92 in (125 mm) in length, 0.5 in (13 mm) in width, and 0.12 in (3 mm) thick. However, some mineral-composite specimens were 1.97 in (50 mm) in length due to expected limited burning. Each specimen was marked with two lines perpendicular to the longitudinal axis at 1 in (25 mm) and 4 in (100 mm) from the end that is to be ignited. A burner was placed under the specimen at a 45° angle so that the test flame impinges on the free end of the test specimen to a depth of approximately 6 mm. Then, the flame was applied for 30 s without changing its position and then removed. Burning time and length of burning were measured. An average burning rate was calculated for specimens that burned to the 4 in (100 mm) mark. Figure 10a,b shows the fire test setup, and the tested samples are also shown in Figure 10b. This has been reported in the reference [7]; we are reiterating it in this work for the completeness of the paper.
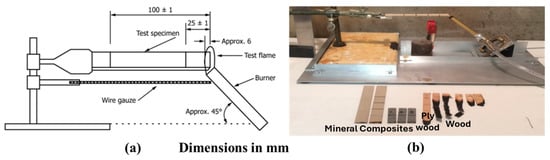
Figure 10.
(a) Test setup (picture taken from ASTM D635) [23], (b) test setup used for fire test.
We performed the fire tests for three types of wood (Red Oak, Poplar, and plywood) and mineral composite with fly ash loading at 70% by weight (wt). The results of the test are listed in Table 5. Definitions of the terms used in Table 5 are tI = Ignition time, s, t25 = Time to reach for the flame to reach the 1 in (25.4 mm) mark, s, tE = Extinguishing time, s, and ltb = Total burn length, in (mm). Both Red Oak and Poplar specimens caught fire and continued to burn without self-extinguishing, but the plywood continued to burn for about 25 s after removing the flame. The total burn length varied from 0.2 to 0.4 in (5 to 10 mm). The mineral composite started to ignite in the form of flickering and extinguished immediately after removing the flame. The total burn length varied from only 0.04 to 0.08 in (1 to 2 mm). This demonstrates the high resistance of the mineral composites to fire.

Table 5.
Fire test of wood and ash composites (0.12 in thick and 0.51 in wide).
4. Concluding Remarks
Fire is a rapid chemical reaction known as combustion, where fuel reacts with oxygen in the presence of heat to release energy in the form of light, heat, and often flames. The main components of fire are Fuel, Oxygen, and heat. The availability of air/oxygen (21% of air) is difficult to control, whereas the fuel content and heat (to ignite and sustain) in a material can be managed/controlled through the proper selection of material and design. The paper demonstrated a practical, scalable solution to mitigate fire hazards through the development of low-fuel, mineral-based composite materials derived from coal combustion residuals (CCR), including both fresh fly ash and processed legacy ash. Here, fire resistance (reduced calorific values and a strong self-extinguishing behavior) was achieved through the use of as little polymer (synthetic material) as possible and dispersing the polymer within a large volume (70–80%) of inorganic minerals. Fire testing confirms that the material resists ignition, limits flame spread, and offers enhanced safety over conventional wood products. Additionally, leaching studies confirm its environmental safety, while mechanical testing shows superior strength compared to existing commercial materials. Beyond structural integrity, no or insignificant leaching, and fire resistance, they also provide thermal and electrical insulation and resistance to moisture, termites, and insects, and are antifungal. Several building products were fabricated and demonstrated. This technology offers a way of recycling mineral waste and reducing the use of virgin minerals. The adoption of such a technological solution for making building products, including structural members, represents a significant step forward in mitigating construction-related fire risks and simultaneously aiding waste management.
Author Contributions
Conceptualization, K.S.; methodology, K.S.; software, B.K. and D.S.; validation, K.S., B.K. and D.S.; formal analysis, B.K. and D.S.; investigation, K.S., B.K. and D.S.; resources, K.S., B.K. and D.S.; data curation, K.S. and B.K.; writing—original draft preparation, K.S., B.K. and D.S.; writing—review and editing, K.S., B.K. and D.S.; visualization, K.S.; supervision, K.S.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
Partial financial support by the North Carolina Department of Environmental Quality (NC DEQ), Raleigh, NC, USA, in conducting the mineral composite work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors acknowledge the partial financial support of the North Carolina Department of Environmental Quality (NC DEQ) in conducting the mineral composite work and the support of the NC DEQ chemistry laboratory for conducting the chemical analysis of the leachate test samples. Furthermore, the support and encouragement of the NC Legislature, Trudy Wade, and John Hardister are acknowledged.
Conflicts of Interest
Authors Bharath Kenchappa and Dhruva Shivakumar are employed by the company ARIS Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- California Governor Says Pacific Palisades Wildfire has Destroyed Many Structures as Winds Kick Up. Available online: https://apnews.com/article/wildfire-southern-california-santa-ana-winds-c48661615061eb631784b666cddfa4ac (accessed on 10 March 2025).
- See the Devastation Left Behind by the Deadly Wildfires Across Southern California: Photos. Available online: https://nypost.com/2025/01/08/us-news/see-the-devastation-left-behind-by-the-palisades-fire-in-los-angeles-photos/ (accessed on 10 March 2025).
- AP PHOTOS: Amid Charred Neighborhoods, a Handful of L.A. Homes Remain Untouched. Available online: https://apnews.com/article/california-wildfires-last-remaining-untouched-homes-photo-gallery-5adfbac6e9d52ffaed5126b7d7469733 (accessed on 10 March 2025).
- Argade, S.; Shivakumar, K.N.; Sadler, R.L.; Sharpe, M.M.; Swaminathan, G.; Sorathia, U. Mechanical Fire Resistance Properties of a Core Material; Long Beach Convention Center: Long Beach, CA, USA, 2004. [Google Scholar]
- Shivakumar, K.N.; Chen, H. Structural Performance of Eco-Core Sandwich Panels. In Major Accomplishments in Composite Materials and Sandwich Structures; Daniel, I.M., Gdoutos, E.E., Rajapakse, Y.D.S., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 381–406. Available online: http://link.springer.com/10.1007/978-90-481-3141-9_15 (accessed on 10 March 2025).
- Shivakumar, K.N.; Brown, W.H.; Imran, K.A. Fly ash composites, a step toward pond ash composites. Coal Combust. Gasif. Prod. 2019, 11, 66–74. [Google Scholar]
- Shivakumar, K.N.; Kenchappa, B.; Imran, K.A. Mechanical, Fire, and Electrical Insulation Properties of Polyurethane Fly Ash Composites. Polymers 2024, 16, 1507. [Google Scholar] [CrossRef] [PubMed]
- Wild Fire Causes and Evaluations. Available online: https://www.nps.gov/articles/wildfire-causes-and-evaluation.htm (accessed on 14 April 2025).
- Forest Service, U.S. Department of Agriculture. Fire. Available online: https://www.fs.usda.gov/visit/know-before-you-go/fire (accessed on 14 April 2025).
- U.S. Fire Administration. Arson Awareness Week. Available online: https://www.usfa.fema.gov/about/usfa-events/2024-05-05-aaw/?utm_source (accessed on 14 April 2025).
- U.S. Fire Administration. Residential Fire Estimate Summaries. Available online: https://www.usfa.fema.gov/statistics/residential-fires/ (accessed on 14 April 2025).
- U.S. Fire Administration. Fire in Industrial or Manufacturing Properties. Available online: https://www.nfpa.org/education-and-research/research/nfpa-research/fire-statistical-reports/fires-in-us-industrial-or-manufacturing-properties (accessed on 14 April 2025).
- Shelby Hall. NFPA Research Fire loss in the United States. 2024. Available online: https://www.nfpa.org/education-and-research/research/nfpa-research/fire-statistical-reports/fire-loss-in-the-united-states (accessed on 10 March 2025).
- National Fire Protection Association. Available online: https://en.wikipedia.org/wiki/National_Fire_Protection_Association?utm_source (accessed on 10 March 2025).
- Eaton Fire. Available online: https://en.wikipedia.org/wiki/Eaton_Fire?utm_source (accessed on 10 March 2025).
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- ASTM E1354-23; Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products Using an Oxygen Consumption Calorimeter. Annual Book of ASTM Standards, 04.07. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM E1131-20; Standard Test Method for Compositional Analysis by Thermogravimetry. Annual Book of ASTM Standards, 14.01. ASTM: West Conshohocken, PA, USA, 2020.
- STAR Technology. Available online: https://www.heidelbergmaterials.us/home/services/benefication/star-technology (accessed on 14 April 2025).
- LEAF (Leaching Environmental Assessment Framework). Vol. 2018. Available online: https://www.epa.gov/hw-sw846/leaching-environmental-assessment-framework-leaf-methods-and-guidance#:~:text=The%20Leaching%20Environmental%20Assessment%20Framework,of%20inorganic%20constituents%20of%20potential (accessed on 22 April 2024).
- ASTM D695-15; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM: West Conshohocken, PA, USA, 2015.
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM: West Conshohocken, PA, USA, 2017. Available online: https://cdn.standards.iteh.ai/samples/97762/3bfa4bc9fbf54c68ad0a721a86682dff/ASTM-D790-17.pdf (accessed on 10 March 2025).
- ASTM D 635-22; Standard Test Method for Rate of Burning and/or Extent and Time of Burning of Plastics in a Horizontal Position. ASTM: West Conshohocken, PA, USA, 2022.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).