1. Introduction
In 2015, during a rock concert, a fire broke out in the artistic venue, the “Colectiv” club, located in a building in Bucharest, an event that resulted in the death of several people and the bodily injury of others (64 individuals and caused injuries to 185 others) [
1,
2,
3]. One of the particularities of the event was the presence and direct involvement in the fire of polyurethane foam, used as a material for acoustic sound-absorbing of the interior space, an aspect found, moreover, in a series of other fires in clubs located on various meridians of the globe (USA, Russia, Brazil, Thailand), events that received intense media coverage worldwide, especially for the rapid development of the fire and the large number of losses (material, human) that resulted.
Identifying the origin of a fire is a key aspect of forensic fire investigations. During the conduct of a fire investigation, these operations are based on scientific approaches and involve finding the origin of the fire, which includes figuring out what ignited first, where the fire started, and the events that preceded the fire. In some cases, determining the fire’s cause is impossible if its origin remains unknown. Moreover, if the origin is identified incorrectly, the conclusions regarding the cause will, in turn, be inaccurate. Each fire incident is unique and influenced by various factors affecting its spread and intensity. Nonetheless, the scientific method makes it possible to pinpoint broad rules and guidelines that guarantee the repeatability of events. Understanding heat transmission, combustion principles, and fire spread dynamics enables investigators to determine the source of a fire. In many cases, existing data are reanalyzed, and new information is sought through physical experiments in the laboratory, computer simulations, and numerical modeling of the event. These methods are used when, in the process of formulating conclusions, an acceptable correlation is not achieved between the results of the analysis and the clear evidence presented by the investigator.
Flexible polyurethane foam is commonly used as a sound-absorbing material in rooms or halls where musical artistic activities are carried out, with a dual role: to avoid reverberations of certain frequencies of musical instruments or voices, and to reduce the noise level outside these rooms. Although this sound-absorbing method is very easy and effective, flexible polyurethane foam presents a high risk of flammability, the thermal decomposition of the solid material into isocyanates (flammable and toxic gases) determines the rapid propagation of flames, making it difficult to extinguish and evacuate people caught in a fire event [
4,
5]. A series of fires in which polyurethane foam played a leading role, among other factors, were highly publicized worldwide due to the rapid development of the fire and the large number of losses (material, human) resulting (
Table 1).
The event that occurred on the evening of 30 October 2015, at around 10:30 p.m., in a building located in Bucharest, during a rock concert, resulted in the death of 26 people during the event; the injury and hospitalization of a large number of people in medical facilities in Bucharest and the subsequent death of 38 people, totaling 64 deceased people and 147 injured; the destruction of the Colectiv club through: setting fire to the ceiling, thermal damage to varying degrees to the false ceiling made of wooden slat grids, the existing decorative pieces in the club (paintings, posters, banners, etc.), musical equipment (instruments, acoustic enclosures, etc.), elements for light shows (projectors, lasers along with the related power cables), furniture (sofas, chairs, tables, etc.).
With the development of technology, new materials are created, such as composite materials made of polyurethane foam with fir sawdust, which have also been demonstrated to have sound absorption properties, but it is also important to study their flammability properties [
6]. It is essential that the flammability properties of these composite materials are evaluated, in addition to their sound absorption qualities. For example, adding polyurethane foam and wood chips together can save heating and cooling costs by improving acoustic performance and acting as an effective barrier against heat transfer. In addition, it is essential to understand how fire-resistant these composite materials are, especially in the case of building codes that prioritize occupant safety. Further research on flame-retardant chemicals can lead to advancements that improve durability and compliance with strict safety standards. A comprehensive approach will be crucial as we investigate these complex qualities to create durable materials that meet a range of functional needs while simultaneously reducing environmental impact [
7].
This paper aims to describe the methods used for the analysis of flexible polyurethane foam, under the influence of thermal radiative fields generated by a pilot flame, in terms of vertical and horizontal burning rates, product emission, as well as temperatures developed inside the tested samples. The methods used in this paper were the most feasible and reliable ones, the obtained results being in good agreement with the scientific literature, thus using them provides the main characteristic parameters of combustible materials, which are necessary to be known before performing the large-scale fire experiments. Using methods like gravimetry, volumetry, spectrophotometry, and the experimental setup for the collection of the combustion gases represents the innovation of the present work, being a method that can be used with minimal resources and that can deliver trustworthy results.
2. Materials and Methods
Samples tested in the present paper included the evidence collected from the scene of the event (some of them being even thermally affected) and the following sound-absorbing polyurethane foam (sponge) that was selected to be the same model of the ones involved in the case fire (
Table 2):
For these samples, several analyses were performed, namely, tests for determining the specific flammability parameters, burning rate, and ignition temperature, and the combustion gases were also investigated.
The burning rate was performed using directions from UL 94–Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances [
13] and SR EN ISO 9773 Plastics. Determination of the fire behavior of samples placed vertically in contact with an ignition source in the form of a small flame [
14]. Samples with a length of 200 mm ± 5 mm, a width of 50 mm ± 2 mm, and a thickness depending on each type of sound-absorbing foam tested are used, being preconditioned at 25 °C. Inside the fume cupboard, the sample is fixed on a stand with a strong clamp or other similar device. The lower part of the sample must be 300 mm ± 10 mm above a cotton absorbent. By adjusting the cylinder valve, a flame with a height of 20 mm ± 1 mm was obtained. The burner flame is applied, centered on the middle of the lower section of the sample, so that the upper part of the burner is at 10 mm ± 1 mm from the lowest end of the sample and is maintained at this distance for 3 s ± 0.5 s. After applying the flame to the sample for 3 s ± 0.5 s, it is removed at a distance of at least 150 mm and, simultaneously, the time measuring device is used to start measuring the burning time of the sample (
Figure 1).
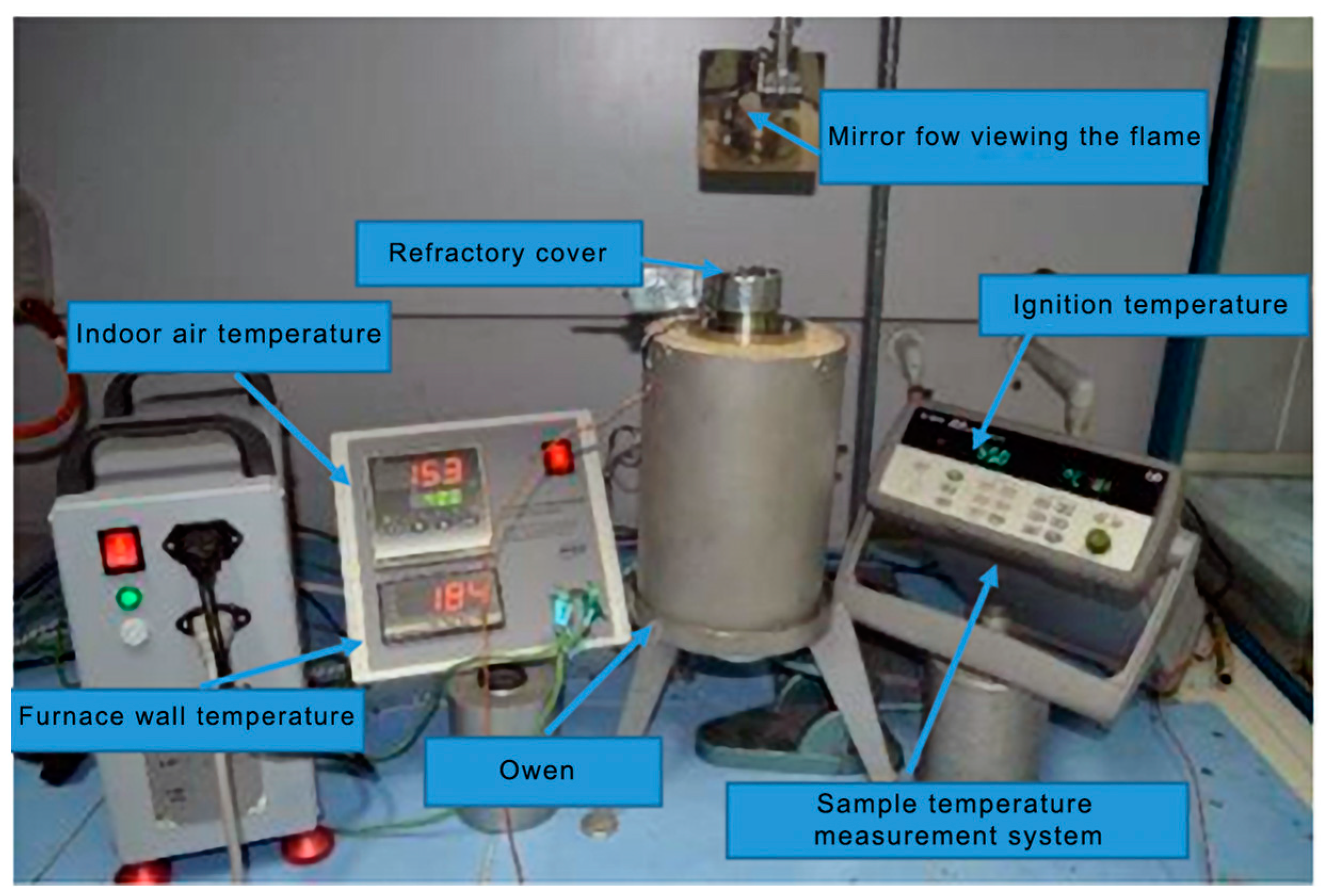
The ignition temperature of the sound-absorbing sponge (flexible polyurethane foam, respectively, of the ignition temperature of the volatile substances released during thermal decomposition was evaluated using the ASTM D1929 standard, that provides laboratory methods for determining the spontaneous ignition temperature (SIT) and the flash ignition temperature (FIT) of plastics [
15]. To determine the spontaneous ignition temperature, the sample of material, of known dimensions, is placed in the preheated furnace tube and kept in the furnace for 10 min. At the end of the 10 min, depending on the result, ignition or non-ignition, the furnace temperature is lowered or raised until the lowest temperature at which the sample ignites is obtained. The ignition temperature of the vapors is determined by using a pilot flame at the top of the furnace. The spontaneous ignition temperature is the temperature at which the self-healing properties of the sample lead to initiation. Initiation occurs under specified test conditions in the absence of an additional ignition source. The lowest temperature at which a sample burns within a period of not more than 10 min is the spontaneous ignition temperature. The vapor ignition temperature is the lowest temperature, under specified conditions, at which sufficient flammable gases evolve to ignite on contact with an external pilot flame. The lowest temperature at which ignition is observed within a period of not more than 10 min is the spontaneous ignition temperature. The experimental setup required for the determination is shown in
Figure 2.
The preparation of the analyzed samples was adapted to the method presented in the mentioned standard, considering the particularities of the analyzed material, namely its low density.
Following the study of the specialized literature regarding the type of gases resulting from the combustion of the flexible polyurethane foam, used in the manufacture of the sound-absorbing sponge, from the data available, respectively, the conditions of its use on-site, as well as the quantitative determination methods available in the laboratory, it was established that the following gases will be determined:
Hydrogen cyanide (HCN) by the spectrophotometric method;
Hydrogen chloride (HCl) by the ion-chromatographic method;
Saturated and unsaturated aliphatic hydrocarbons, carbon monoxide, and carbon dioxide by the gas-chromatographic method;
Oxygen, carbon dioxide, and carbon monoxide by the volumetric method for concentrations higher than the detection limits of the other methods.
The determinations were carried out on sound-absorbing sponge samples as follows:
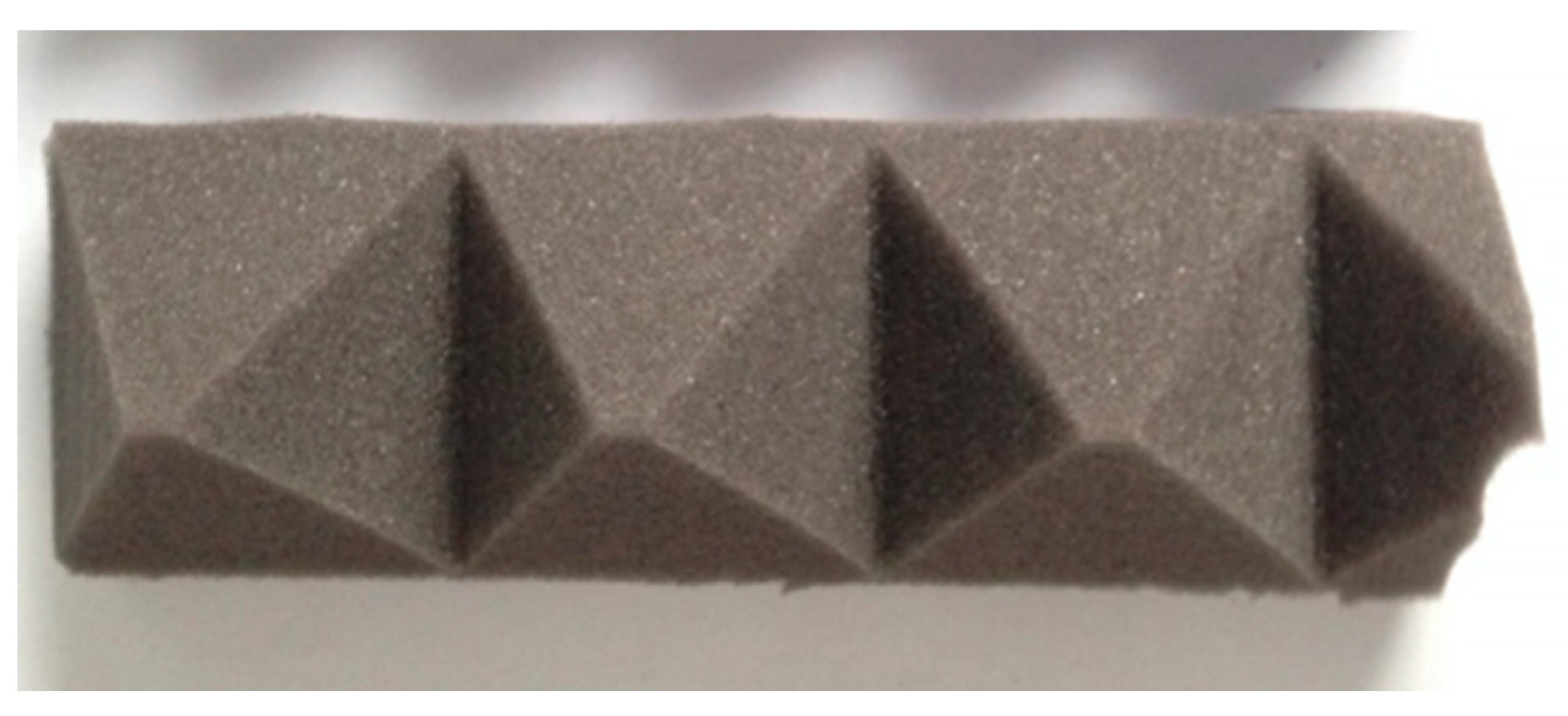
Sample A: Pyramidal type N2138 sound-absorbing sponge (
Figure 3);
Sample B: Self-adhesive pyramidal sound-absorbing sponge collected from the scene of the event (
Figure 4).
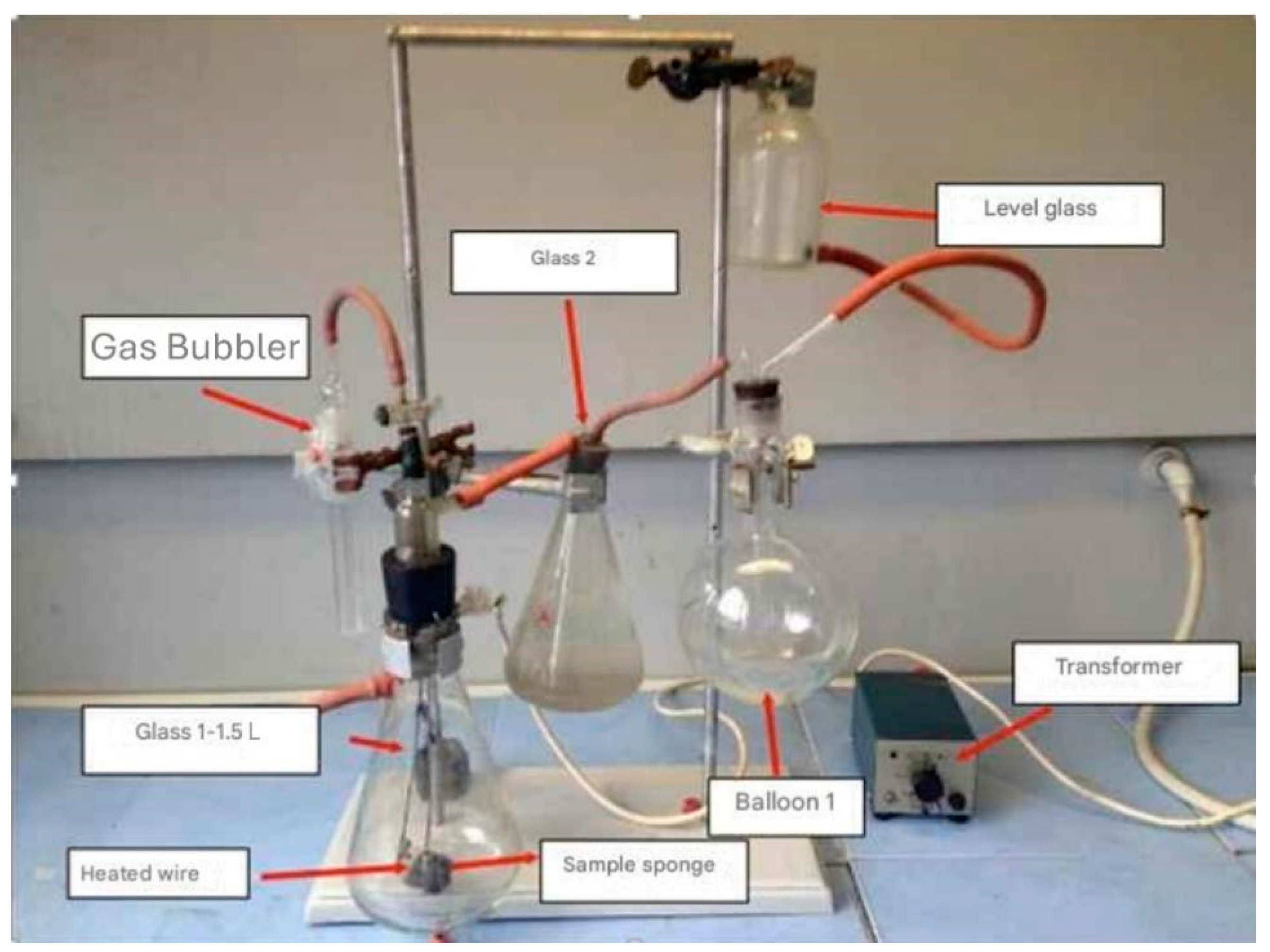
To collect the gases resulting from the combustion of the sound-absorbing sponge (flexible polyurethane foam), a system based on the principle of communicating vessels was used (
Figure 5). Thus, by burning the sample, the combustion gases are released into Erlenmeyer beaker no. 1, which, due to the increase in volume, pushes the solution into Erlenmeyer beaker no. 2, thus retaining the entire amount of combustion gases. With the help of the level glass and through flask 1 (which prevents the contamination of the combustion gases with air from the chemical niche), the combustion gases are passed through the bubbler, in which there is an absorbent NaOH solution (for the absorption of HCN and HCl). The volume of gases bubbled or sampled is equal to the amount of solution added to the level glass. The sound-absorbing sponge sample was introduced into glass 1. Glass no. 2 was filled with a saline solution of distilled water, and heated to 40 °C to avoid the solubilization of the combustion gases at the contact surface. The sponge sample was ignited using a heated wire, controlled by the transformer. The sponge quantity was burned with a flame until the oxygen in beaker 1 was consumed. The burned gases were then collected by bubbling into an absorbent solution, respectively, directly into the sampling container for subsequent analysis.
The method used to determine the concentration of hydrogen cyanide (HCN) released by burning the sound-absorbing sponge, specifically, the polyurethane foam, was the spectrophotometric method. The principle of the method is based on the reaction of the CN- ion with chlorine and the formation of cyanogen chloride, which reacts with pyridine in the presence of barbituric acid, leading to a violet compound, colorimetric at a wavelength of 588 nm. The determination method of hydrogen chloride (HCl) is the indirect method of measuring the measurement by collecting samples and subjecting them to ion-chromatographic analysis (ion-chromatograph Dionex ICS 3000, Thermo Fisher Scientific, Mundelein, IL, USA). As an analytical technique, chromatography is a physical separation technique where the parts that need to be separated are split at the boundary between two phases, one of which is stationary and does not move and the other of which does. The mobile phase flows around the immobile one; the mobile phase is liquid. Detection is performed using conductivity detectors. The automatic sampling system will inject a small amount of sample into the ion chromatograph.
The chromatographic system, comprising precolumns, analytical columns, suppressors, and conductivity detectors, will separate and measure the anions and cations of interest. For the determination of aliphatic hydrocarbons, carbon monoxide, and carbon dioxide, gas chromatography was used gas chromatography, the method consists of the separation of a gas mixture into its components at the boundary between two phases, one of which is immobile—stationary, and the second, mobile. The mobile phase flows around the stationary one. The stationary phase is a solid substance (adsorbent), and the mobile phase is gaseous. In gas-adsorbent chromatography (Clarus 500 chromatograph with catalytic reactor, Perkin Elmer, Inc., Shelton, CT, USA), the column is filled with a suitable active adsorbent. The carrier gas, together with the mixture to be separated, flows around the adsorbent. In its active centers, the molecules of the substance are fixed, and the equilibrium between the concentration in the gas phase and the concentration on the adsorbent is established. This equilibrium is represented by the respective adsorption isotherm.
This method allows the determination of the concentrations of saturated aliphatic hydrocarbons (methane, ethane, propane, butane, pentane, etc.) and unsaturated (ethylene, acetylene, etc.), hydrogen, carbon dioxide, and carbon monoxide in gas mixtures. Oxygen, carbon dioxide, and carbon monoxide concentrations were measured by volumetric method, the gas analysis method is based on the determination of carbon dioxide, oxygen, and carbon monoxide by absorption in absorbent solutions, potassium hydroxide, pyrogallol, and copper chloride, respectively, and measuring the volume of gas remaining after absorption (gas analyzer ORSAT, Globetrek Enginering Corporation, Navi Mumbai, India). The method is used for the determination of oxygen, carbon dioxide, and carbon monoxide in the measuring range of 0.2–100%.
3. Results
3.1. Combustion Test Results
Vertical and horizontal burning tests were performed on both standard samples and samples taken at the event site. The dimensions of samples were L = 200 mm; l = 50 mm; h = 20 mm for formwork sponge type N2138; h = 30 mm for flat sponge type N2138 and h = 60 mm for pyramidal sponge type N2138. The same dimensions had the samples taken at the event site. The results obtained are summarized in
Table 3.
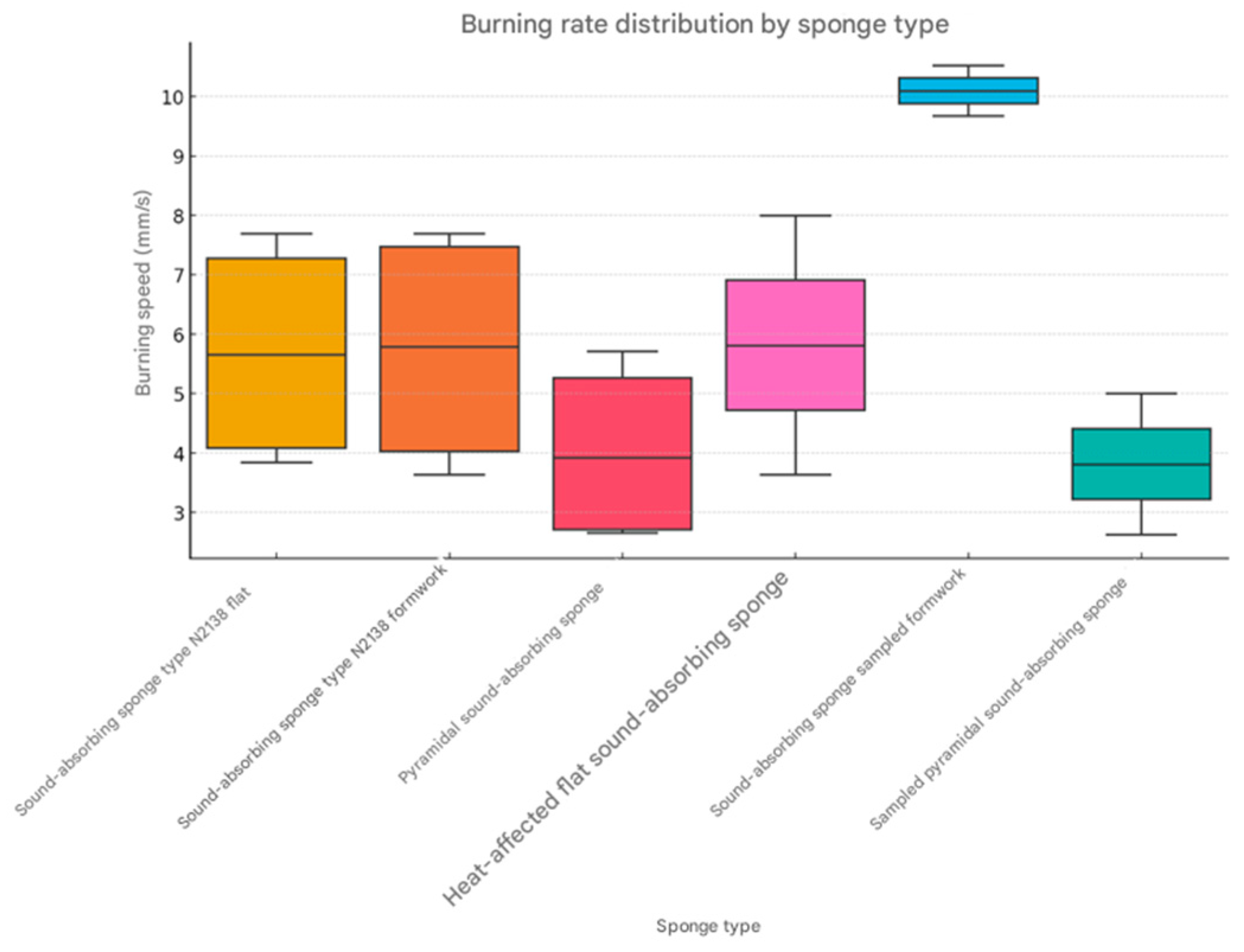
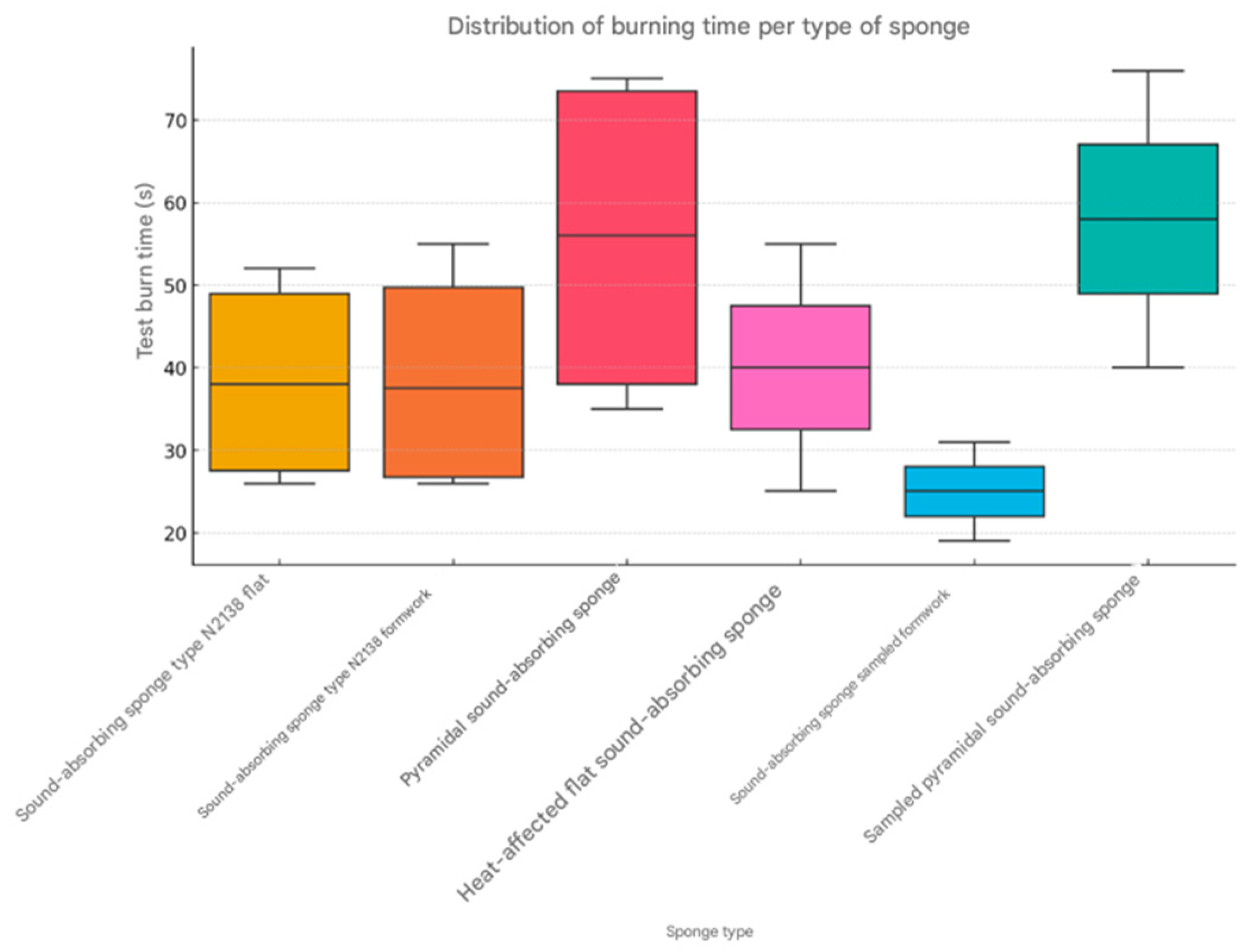
Based on the presented data, three statistical graphs were created for more detailed visual analysis: the burning rate per sponge type—it shows the distribution of the burning rate, highlighting the medians and possible extreme values (
Figure 6); for the burning duration per sponge type—it provides a visual comparison of the burning durations for each sponge type (
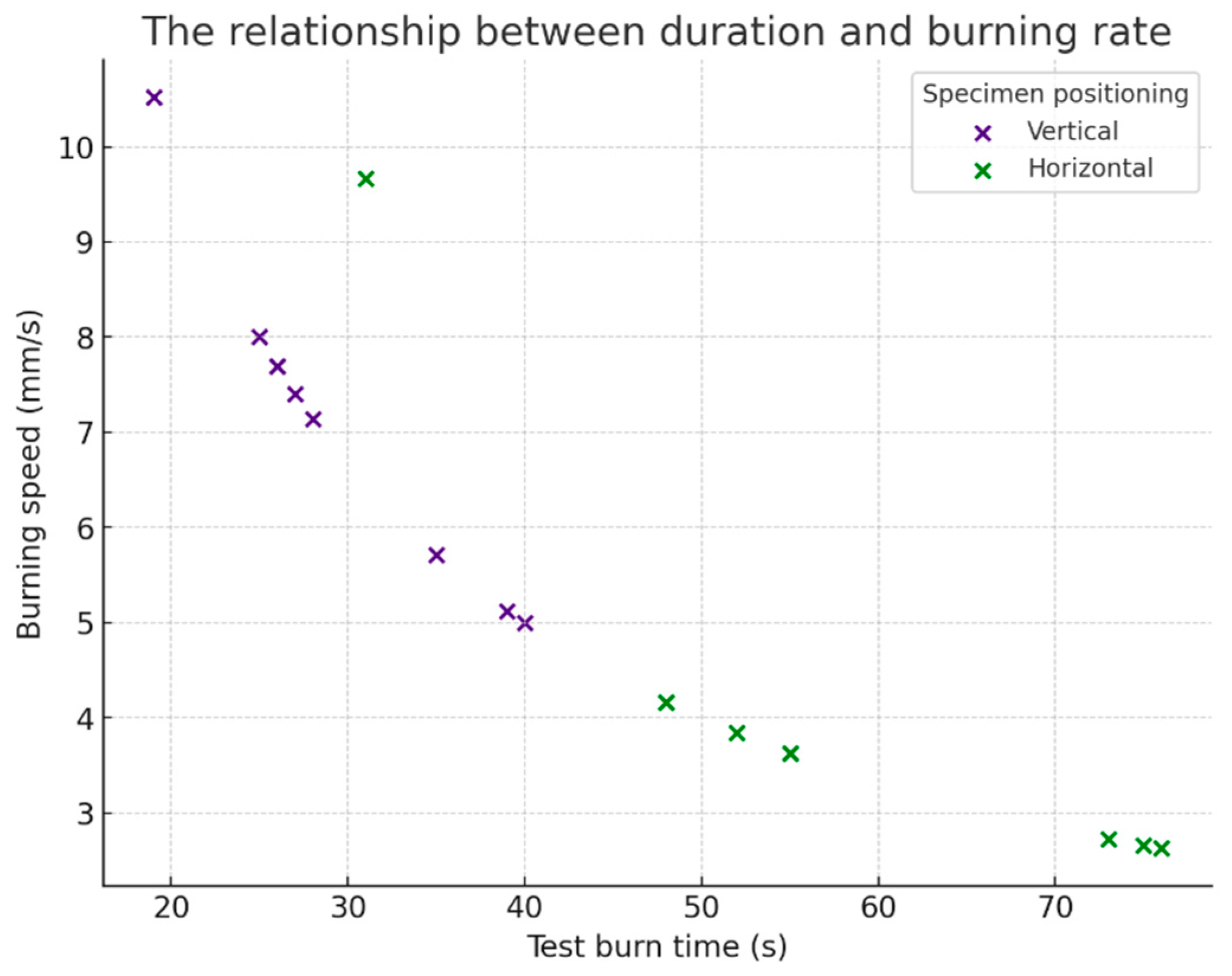
Figure 7); for the relationship between duration and burning rate—it illustrates the correlation between burning duration and burning rate, differentiated by the position of the sample (vertical/horizontal) (
Figure 8).
3.2. Ignition Temperature
The tests for ignition temperature are summarized in
Table 4.
The ignition temperatures determined for the samples collected from the event site were about 5% higher than those of the materials in their normal state. The difference is explained by the fact that the materials involved in the event were impregnated with smoke, soot, dust, etc., which slows down the self-ignition and volatilization phenomena.
Results of Determination of Quantity of Burned Sound-Absorbing Sponge
To determine the amount of sound-absorbing sponge consumed by combustion in a known volume of air, namely 1.5 L, three successive determinations were made on the same initial quantity. The sample subjected to combustion was weighed initially and after combustion on an analytical balance (
Table 5).
3.3. Results Regarding the Determination of the Quantities of Hazardous Gases Generated During the Combustion of Sponges
The results in the table represent the arithmetic mean of the three successive determinations. In this experiment, the flue gases were not collected, its purpose being to determine the quantity of sample consumed during combustion in the known volume of air, being necessary to relate the quantity of flue gases to the quantity of material consumed, through combustion.
To determine the hydrogen cyanide acid, the flue gases originating from the combustion of the sound-absorbing sponge were sampled by bubbling them in a 0.01 N NaOH solution. The absorbent solution was analyzed with a spectrophotometer to determine the CN- ion. The cyanide ion concentration, obtained by colorimetry at 588 nm, was reported to the volume of flue gases sampled by bubbling, respectively, to the quantity of material burned. The conversion factor of the CN-ion to HCN was used, namely 1.039 (
Table 6).
Analyzing the results obtained by the method described above, it is appreciated that the values of the quantities of hydrogen cyanide generated by burning polyurethane foam determined experimentally are in good correlation with the data reported in the literature [
16,
17,
18].
To verify the method for determining HCl from the sound-absorbing sponge sample, in the first stage, the sound-absorbing sponge sample type N2138 plan (Sample C) was contaminated with adhesive, which according to the technical sheet contains more than 50% methylene chloride. The thus contaminated sample was introduced into the experimental stand and subjected to combustion. The absorbent solution, NaOH 0.01 N, was analyzed according to the ion-chromatographic method. A test was also performed by burning a known amount of adhesive (Sample D). The results were reported considering the previous analysis of the absorbent solution, the conversion factor of the Cl
- ion into hydrogen chloride, and the volume of gas bubbling through the absorbent solution (
Table 7).
The results obtained for aliphatic hydrocarbons, carbon monoxide, carbon dioxide, and oxygen are presented in
Table 8.
4. Discussions
The experimental results obtained when determining the fire behavior of the samples in contact with an ignition source in the form of a small flame showed similar behavior for the pyramidal and planar sample types, both in the normal state and in the affected state (taken from the Colectiv Club), in terms of burning rate. Based on this fact, it can be appreciated that the sound-absorbing sponge existing in the club does not present contaminations with other substances that would significantly modify its burning behavior. The results indicate that the burning times, respectively, and the burning rates determined for the sound-absorbing sponge samples (used in the INSEMEX scale experiment) are comparable to those obtained for the types of sound-absorbing sponge taken from the Colectiv Club, so that the equivalent reproduction of the event is demonstrated.
The results obtained in the experimental determinations for the samples of sound-absorbing sponge type N2138, in normal conditions, are in accordance with the data reported in the material safety data sheet, these, obtained experimentally, showed similar behavior for the types of sound-absorbing sponge samples, both in normal condition and in damaged condition (taken from the Colectiv Club), in terms of ignition temperatures.
Average burning rate by type of sponge. Calculating the average burning rate for each type of sponge, we observe the following (
Table 9):
The most flammable sponge is the sampled formwork sound-absorbing sponge, with an average burning speed of 10.10 mm/s. The most resistant to burning is the sampled pyramidal sound-absorbing sponge, with an average burning speed of 3.82 mm/s.
Comparing average firing rate by position (
Table 10):
The vertical samples burned at an average rate of 72% higher than the horizontal ones. This result indicates that the flame propagates more rapidly vertically due to thermal convection that favors upward combustion.
Burning duration and correlation with the type of sponge. The longest burning duration was observed for the pyramidal sponge in the horizontal position (76 s). The shortest duration was for the formwork sponge taken in the vertical position (19 s).
The sponges taken (thermally affected or previously exposed) tend to burn faster, which suggests a degradation of the flame-retardant properties over time.
The behavior of the examined samples, both from unutilized flexible polyurethane foam and from the fire site, illustrates the combustion of flammable solid materials in open space, conforming to all principles of ignition, propagation (radiative, convective, and conductive), and the production of reaction products (high-temperature effluents and smoke). The combustion kinetics of the samples indicate that the burning rate in the horizontal plane is lower than that in the vertical plane, which is characteristic of free burning observed in flames occurring in enclosed areas during the early phase of fire spread, after which these rates invert. This similarity is illustrated by results from computer simulations of these events conducted in FDS Pyrosim. The progression of the combustion process:
The time interval for commencing the combustion process (
tig) denotes the aggregate of the solid’s pyrolysis duration (
tpy) and the induction period of the gaseous phase (
tin):
With an increase in flow rate, the heat flux on the surface rises, hence reducing the time necessary for heating and pyrolysis of the solid. There are current concerns about expressing the phenomenon of pyrolysis specific to flexible polyurethane foam through mathematical functions [
19].
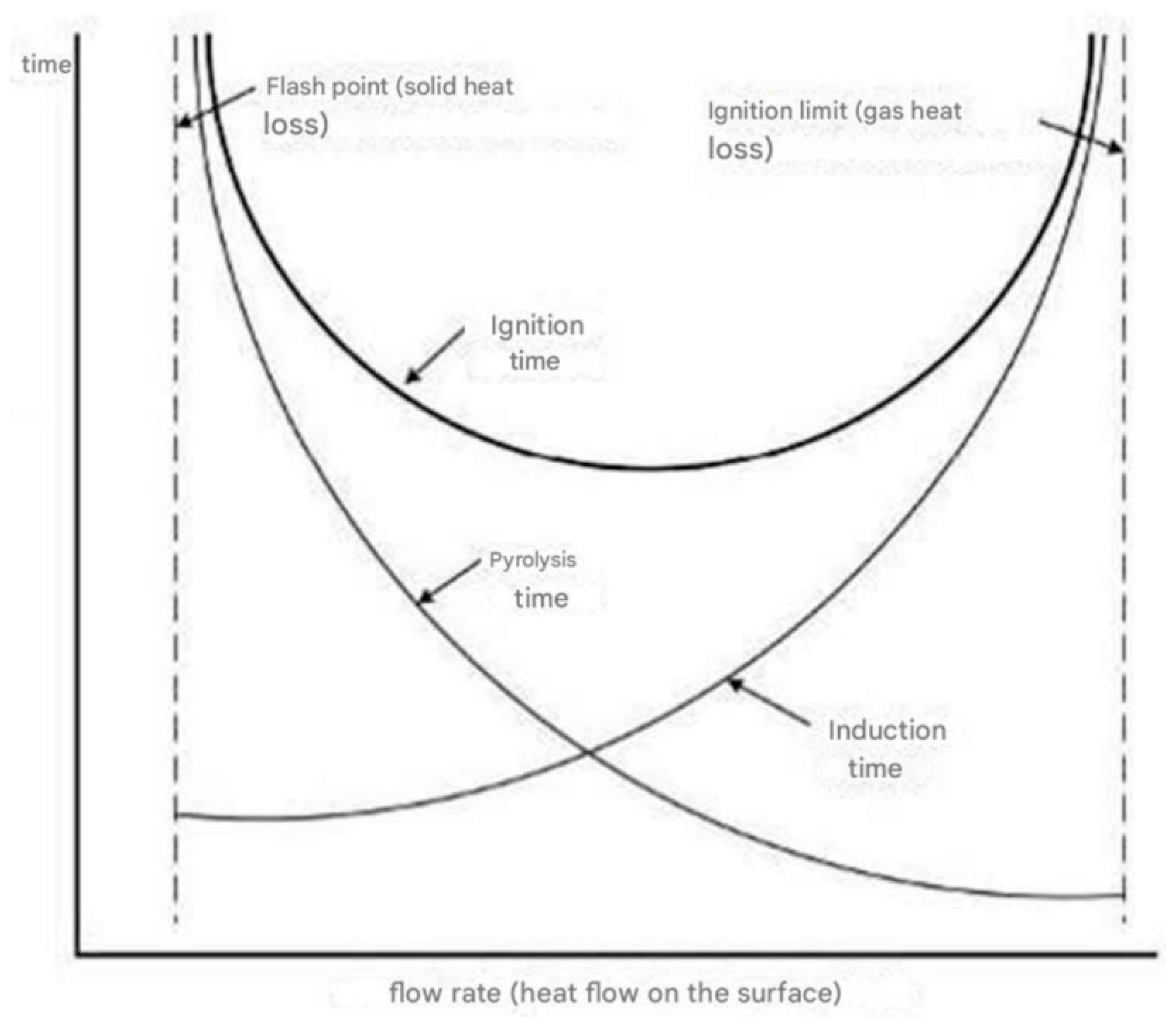
An increase in gas velocity will lead to a reduction in the residence time of the flow. This phenomenon will postpone the commencement of the chemical reaction or may inhibit its initiation. The mechanism can be illustrated graphically as follows (
Figure 9):
There are two primary types of ignitions:
- -
Piloted Ignition—initiated by an external source such as a flame or spark.
- -
Non-piloted Ignition—occurs spontaneously due to combustible substances heating.
The existence of a pilot ignition will influence the ignition process by locally diminishing the induction time. The primary mechanism in the ignition process will be the heating of the material, characterized by a modest gas flow rate and standard oxygen concentration.
For solid combustible materials, the ignition phenomenon is complex because it involves heat transfer within the material. The received energy corresponds to an incident heat flux that increases the material’s temperature until it reaches the pyrolysis or decomposition threshold, leading to the evaporation of combustible vapors. The ignition time depends on the intensity of the heat flux, thermal inertia, ignition temperature, and the moisture content of the material. To delay the ignition of combustible products, certain chemical compounds are incorporated into their structure or applied to their surface.
The material’s properties and heat transfer affect ignition time and heat release. The methods for estimating the ignition time of solid materials exposed to thermal radiation vary depending on their thickness. Unlike wood, thick materials do not immediately perceive an increase in temperature on the opposite surface.
You can estimate the ignition period using the equation from Mikkola and Wickman’s Method for Thin Materials [
20]:
where:
tig—is the ignition period (s);
Tig—is the ignition temperature (°C);
T0—is the initial temperature (°C);
—is the material density (kg/m3);
L0—is the material thickness (m);
c—is the specific heat (kJ/kgK);
qr—is the external heat flux (kW/m2);
qcrt—is the critical heat flux for ignition (kW/m2).
For thick combustible materials, the ignition period is determined by
where k is the thermal conductivity (W/mK).
- (b)
Flame propagation
The spread of flame on solid surfaces can be regarded as a continual process of regulated ignition. For flame propagation, an adequate quantity of heat must be conveyed from the flame or alternative heat sources to the leading edge of the flame. The evaporated fuel is subsequently disseminated and convectively moved away from the surface, where it amalgamates with oxidizers to create a flammable mixture in the vicinity of the flame, which will eventually ignite.
The essential equations governing flame propagation are
and
The flame propagation speed will be derived from both equations:
- (c)
Heat transfer
It is the spontaneous, irreversible process of heat transfer in space, signifying the exchange of thermal energy between bodies or portions of the same body due to a temperature gradient. Heat transfer is the transfer of energy between physicochemical systems or distinct components of the same system, occurring during a transformation in which no mechanical work is executed.
According to the Second Law of Thermodynamics, heat transport comprises three components:
- -
Radiation transfer;
- -
Conduction transfer;
- -
Convection transfer.
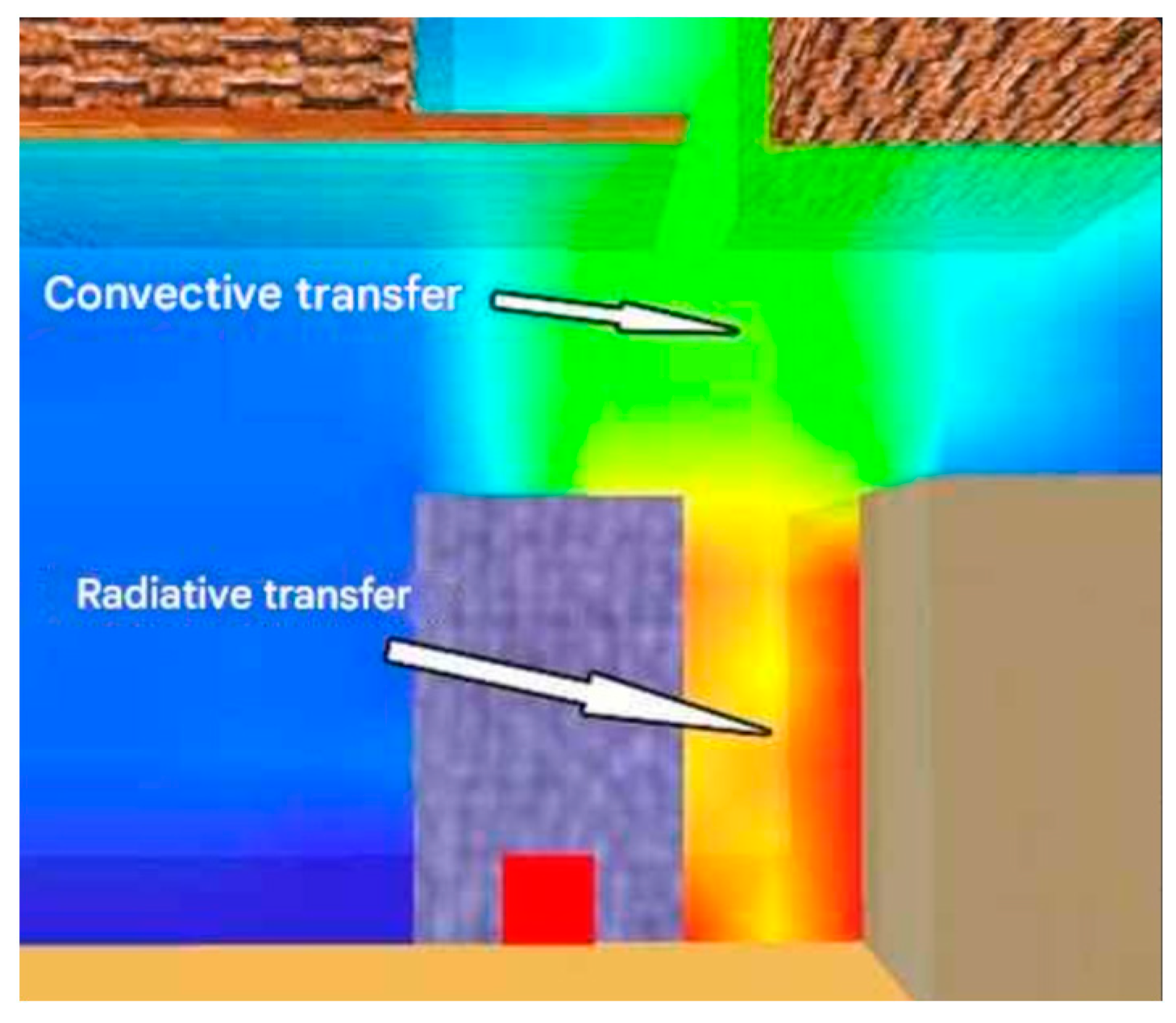
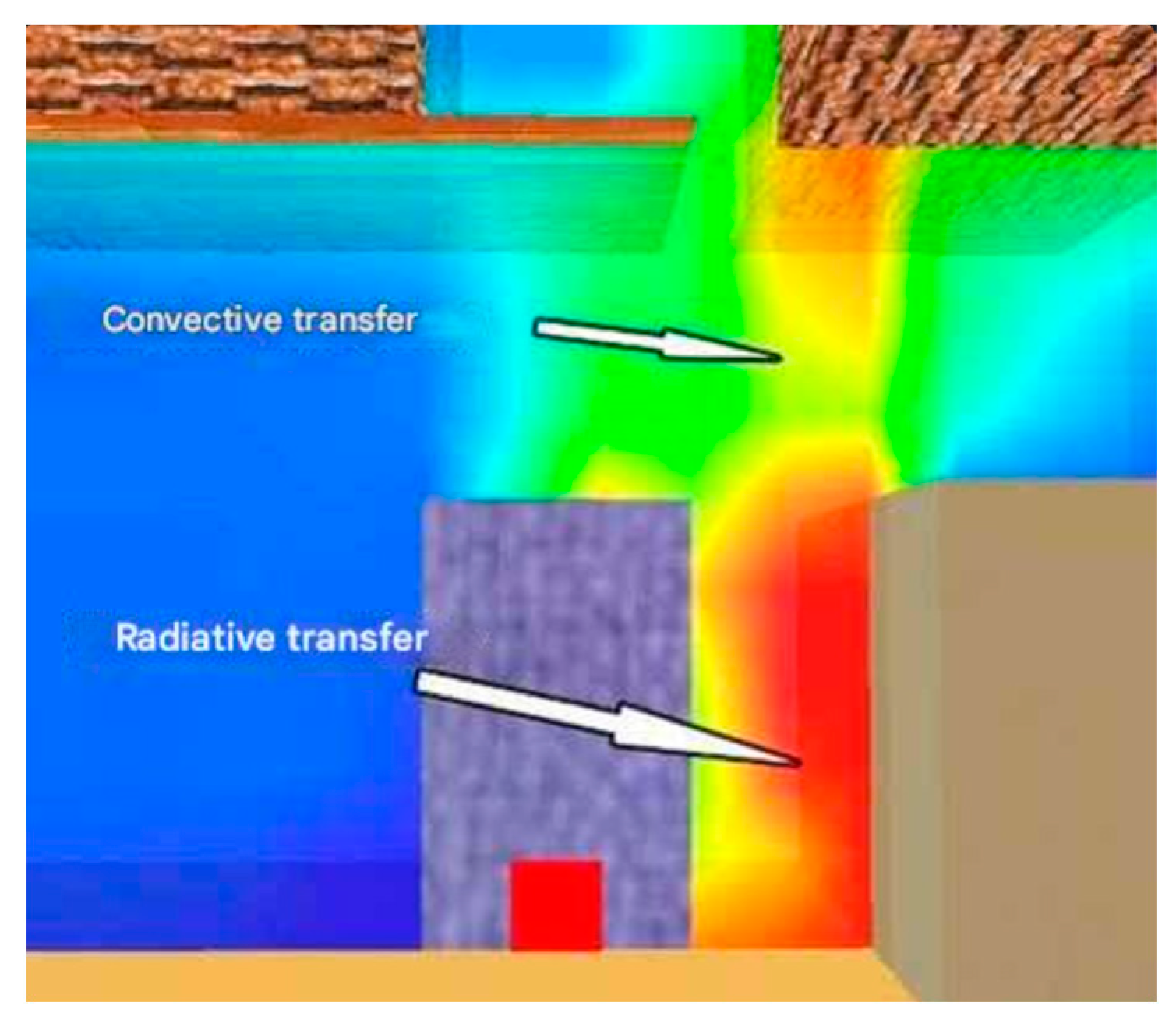
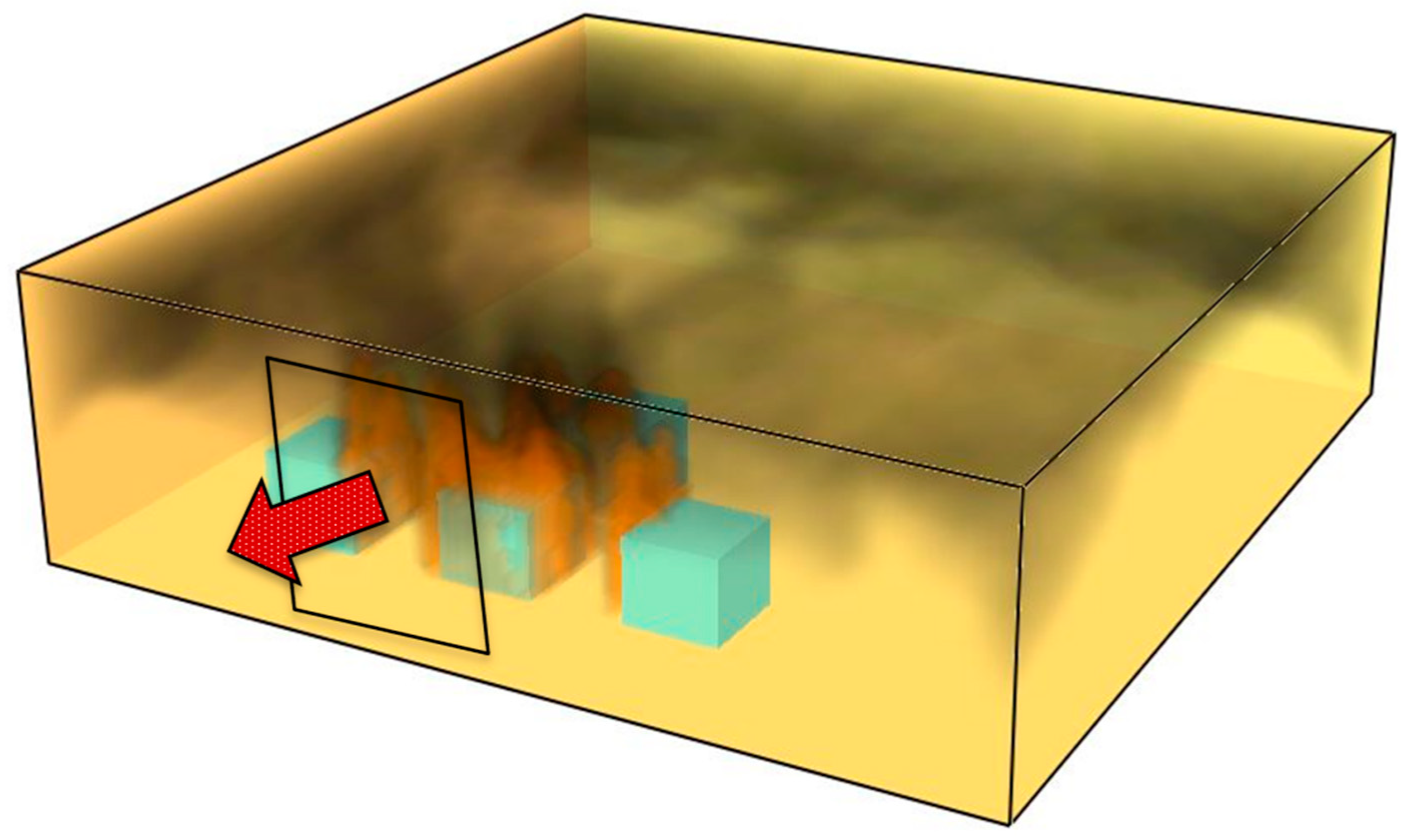
The graphics below illustrate three phases of heat transfer. The beginning stage (
Figure 10) depicts the commencement of heat transfer from the burning object to a nearby cubic-shaped combustible substance. This transfer transpires in the horizontal plane and is predominantly governed by radiative transport, a characteristic that intensifies in the subsequent stage as temperatures increase. Convective heat transfer initiates in the initial phase, facilitated by the ascent of hot gases towards flammable materials located at a higher elevation, thereby pre-heating their substance.
During the second phase, radiative heat transfer elevates the cube’s surface temperature to the threshold of uncontrolled ignition (absent a pilot flame), resulting in spontaneous ignition of the material and consequently an escalation in the volume of hot gases ascending towards the ceiling of the compartment—an enclosed space (
Figure 11).
The third phase is marked by an escalation in heat transmission to nearby objects, facilitated through convective transfer due to the ascent of hot gases (
Figure 12). The temperature of the objects attains and beyond the ignition threshold, hence sustaining the flame front within the confined compartment.
- (d)
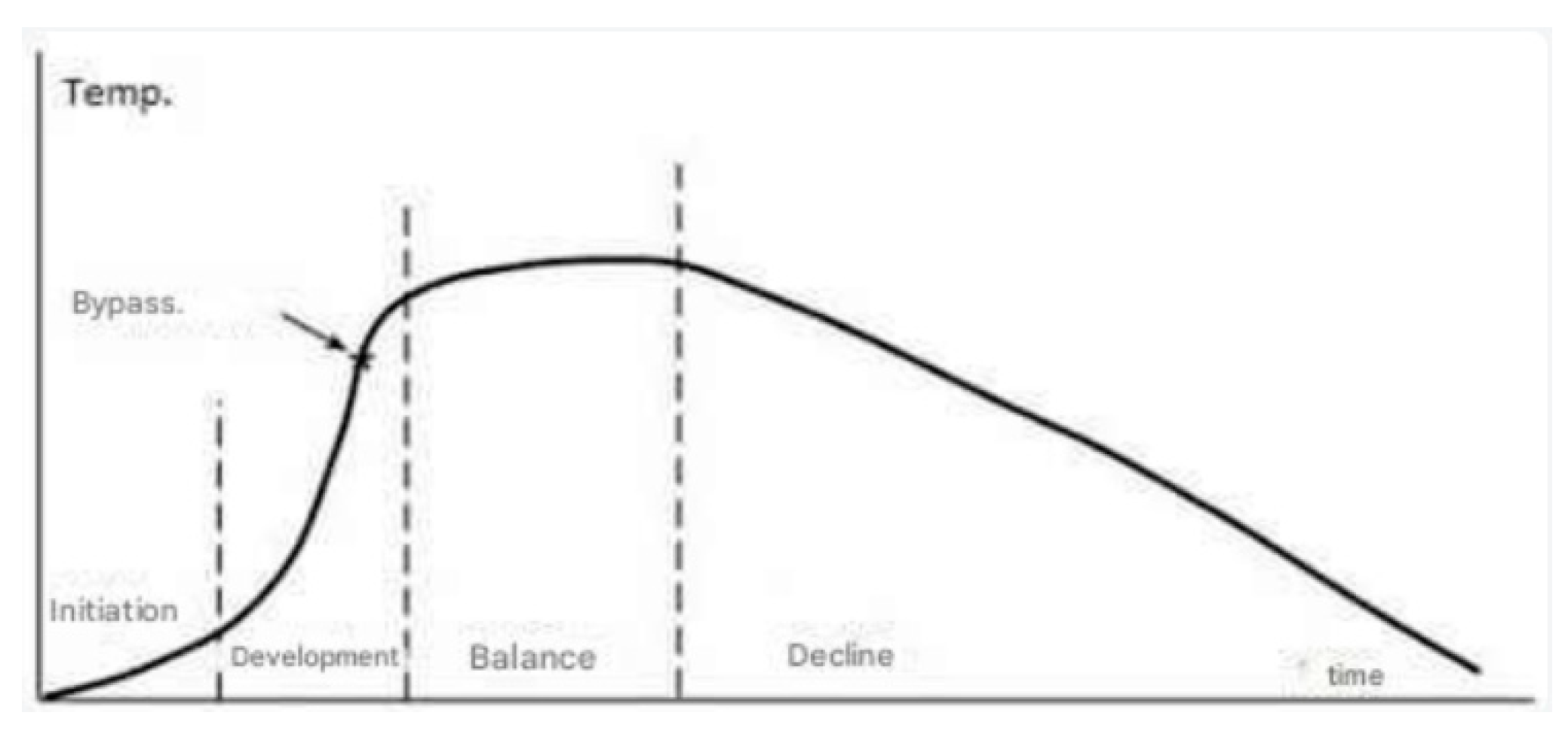
The emergence of a fire in an enclosed compartment
At first, the fire within a compartment can be regarded as an independent, unique combustion event initiated by an ignition source in an atmosphere with standard oxygen levels. The progression of the fire is contingent upon the quantity of heat emitted by the combustible material, the volume of the enclosure, its ventilation, and the amount of fuel contained within the compartment (
Figure 13).
During the initial phase of the fire, the heated gases ascend above a slender flame, generating a current of fresh air that supplies the flame with oxygen. This behavior results in a reduction of flame temperature, accompanied by an increase in the amount of smoke ascending to the roof of the compartment.
Upon the smoke volume ascending to the ceiling enveloping its entirety, the second phase of the fire commences, during which the smoke layer drops due to air entrainment and gas expansion prompted by the increasing temperature. The gas expansion often elevates the temperature of the smoke layer; however, this rise is mitigated by the ongoing influx of fresh air. The length of this second phase of the fire is contingent upon the heat emitted by the combustible material, the volume and arrangement of the compartment, the heat dissipation, and the ventilation apertures of the compartment. In a fully enclosed compartment, the smoke layer progressively rises from the roof to the floor until the space is filled or until combustion quits due to fuel exhaustion or oxygen depletion. In ventilated compartments, the volume of smoke increases until equilibrium is achieved between the quantity of mechanically or naturally ventilated air and the volume of smoke generated.
The pre-conflagration phase commences with the outward development of the smoke layer beyond the compartment, facilitated by ventilation through its open apertures.
The concluding phase, with natural or mechanical ventilation prior to flashover, is marked by the utmost amount of peril both within and outside the compartment. This phase transpires when the heat conditions within the compartment attain a threshold that causes all exposed flammable objects to ignite almost simultaneously, typically accompanied by adequate airflow to support a vigorous fire. In this phase, airflow and combustion rate within the compartment are constrained. Ventilation is constrained by the dimensions, configuration, and positioning of the apertures.
With adequate ventilation, the flame can consume the entire space, swiftly transforming the fire’s behavior from a growing stage to complete compartment involvement. This juncture is referred to as the flashover point. The development of a heated upper layer in the initial stages generates a radiative flux that affects the combustible material, thereby elevating the combustion rate and the temperature of the smoke layer.
Typically, during the smoldering phase, the fire attains its maximum developmental threshold, encompassing all combustible items. This phase is contingent upon and immediately constrained by the oxygen supply from the fresh air flow; conversely, when the oxygen supply is adequate, the limitation is determined by the quantity of flammable material.
4.1. IGNITION of Combustible Materials
4.1.1. Fire Propagation
As the fire progresses, its intensity escalates due to the successive burning of materials inside the impacted region. The progression of fire encompasses the subsequent stages:
Incipient phase—Determined by the ignition source, ventilation conditions, and temperature characteristics of the materials;
Growth phase—marked by a swift rise in temperature and propagation of flames;
Fully developed phase—All combustible items present ignite concurrently;
Decay phase—the conflagration subsides due to the exhaustion of fuel or a decrease in oxygen levels.
4.1.2. Mechanisms of Fire Propagation in Confined Spaces [21,22]
Heat transfer within an enclosure transpires via:
- -
Radiation refers to the transformation of thermal energy into electromagnetic waves, predominantly within the infrared range.
- -
Convection—circulation of heated gases within the container.
- -
Conduction—thermal transfer through walls, ceilings, and floors.
In a confined environment, the fire often advances through many phases:
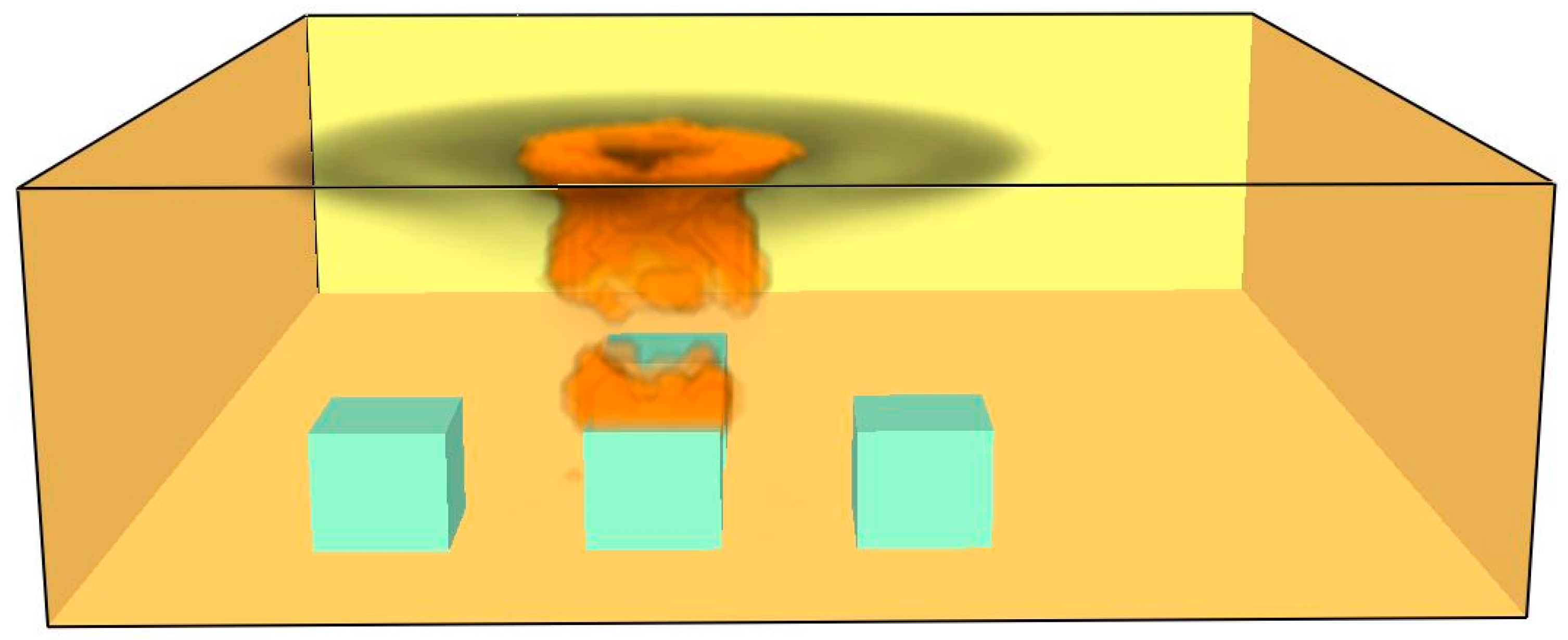
- -
In the flame column phase, heated gases ascend vertically and disperse horizontally along the ceiling in a slender jet, as illustrated in
Figure 14.
- -
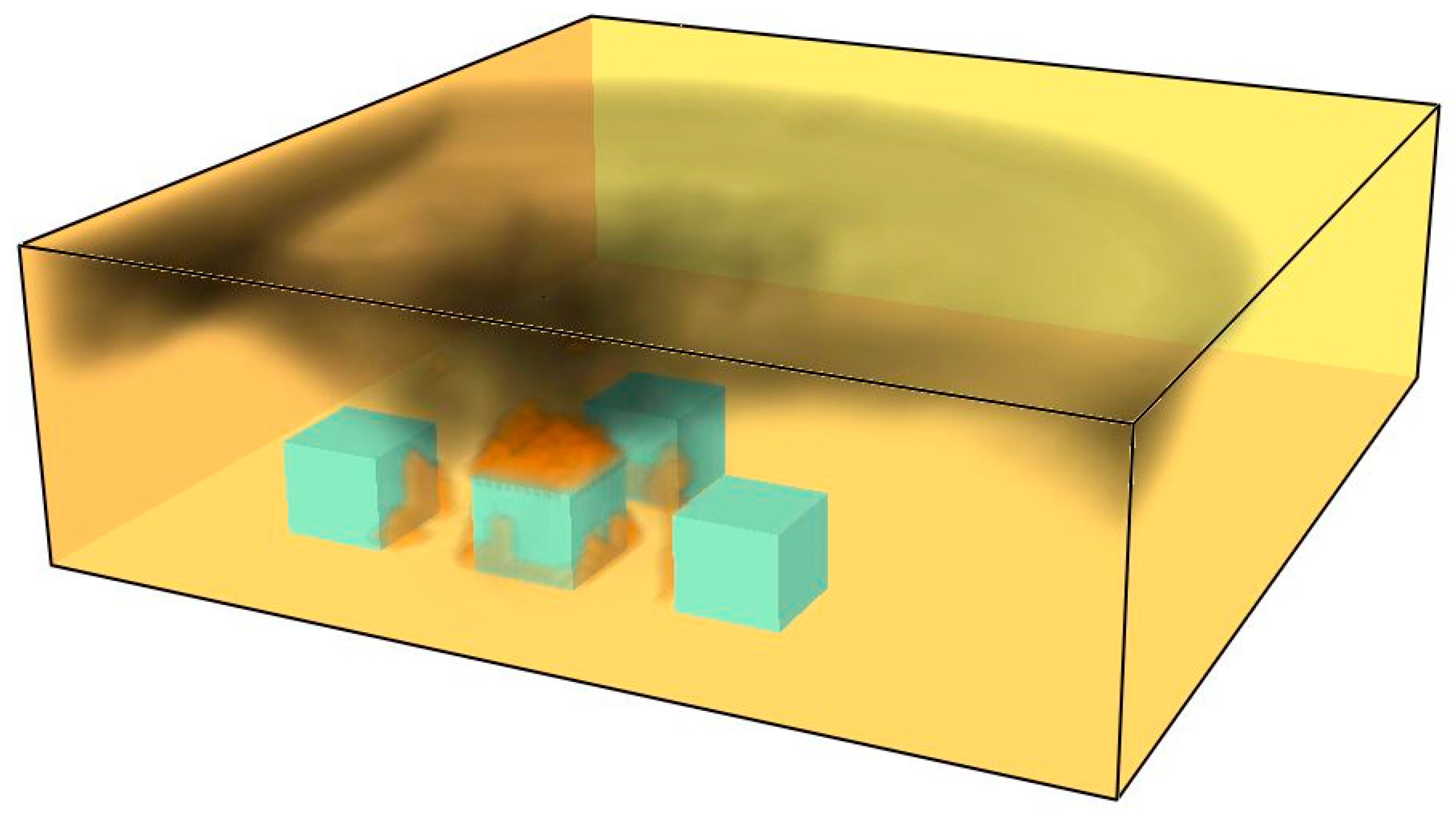
The accumulation of hot gases and smoke leads to the formation of a smoke layer, resulting in pyrolysis and the ignition of adjacent materials—see
Figure 15.
- -
Pre-flashover ventilated phase—fire escalates as smoke and hot gases begin to exit the room (
Figure 16).
In ventilated compartments, the balance between air mass flow and combustion gases dictates the propagation or suppression of fire.
By determining the amount of sound-absorbing sponge consumed by combustion, the following was obtained: In the air volume of 1.5 L, the existing oxygen is sufficient for the combustion of 0.063 g of sample A, pyramidal type N2138 sound-absorbing sponge, respectively, 0.076 g of sample B, self-adhesive pyramidal sound-absorbing sponge collected from the scene of the event. By burning a quantity of approx. 60 mg of sponge in an initial volume of 1.5 L of air, an additional volume of 0.12 L of flue gases, and an overpressure of 75 mm H2O (735.5 Pa) are generated. The results indicate that the mass loss by combustion of the analyzed sound-absorbing sponge samples is comparable.
It should be noted that, applying these proportions to the specific case in the club (surface covered with sponge approx. 400 m2, 3 cm thick, total sponge mass approx. 260 kg), an additional volume of combustion gases of approx. 520 Nm3 (gases at ambient temperature and pressure) results. The additional volume of gases was evacuated from the club premises, through all free surfaces communicating with the outside (the openings of the four fans, respectively, and the free space in the airlock at the entrance to the club where the victims were).
By determining the amount of hydrogen cyanide, it was noted that by burning a quantity of 0.063 g of sample A, in a volume of 1.5 L of air, a quantity of 0.941 mg of HCN was obtained. By burning a quantity of 0.076 g of sample B, in a volume of 1.5 L of air, a quantity of 0.844 mg of HCN was obtained. By relating the quantity of sound-absorbing sponge burned to the quantity of HCN produced, the following results were experimentally obtained by the described method: 1 g of sample A subjected to combustion results in 14.93 mg of HCN; 1 g of sample B subjected to combustion results in 11.11 mg of HCN.
The explanation for the fact that for the pyramidal sponge, taken from the site, the mass of material consumed in the combustion process was higher, related to the concentration of HCN emitted, is the presence of the self-adhesive material on the tested sample (the density of the adhesive is at least an order of magnitude higher than that of the sponge).
The purpose of the determination of hydrogen chloride was to verify the method of determination, mainly qualitative and secondarily quantitative, due to the test conditions of the Cl- ion in the combustion gases. The presence of hydrogen chloride in the gases resulting from the combustion of the sound-absorbing sponge is conditioned by the application of a quantity of adhesive on the surface of the polyurethane foam.
The concentration of HCl is significantly higher in the presence of adhesive, Sample D (containing 0.036 g of adhesive) generated 19.66 mg/L HCl, almost four times more than Sample C (4.91 mg/L). This result confirms that the presence of methylene chloride in the adhesive contributes significantly to the release of HCl during combustion.
The composition of the emitted gases indicates incomplete combustion processes. High levels of CO (carbon monoxide), with 900 ppm in Sample A and 1050 ppm in Sample B, suggest partial combustion, which can be dangerous in confined spaces. Methane and ethane are present in higher concentrations in Sample B, which shows a different combustion compared to Sample A. The increase in CO2 and decrease in O2 confirm the consumption of oxygen in the combustion process, CO2 is slightly higher in Sample B (3.2%) compared to Sample A (2.9%), which indicates a more intense conversion of carbon-to-carbon dioxide. O2 decreased from 17.3% (Sample A) to 17.0% (Sample B), confirming that the combustion consumed oxygen.
When determining aliphatic hydrocarbons, carbon monoxide and carbon dioxide by the gas-chromatography method, higher values were noted for the pyramidal sponge. The explanation for the fact that for the pyramidal sponge, taken from the site, the concentrations of gases resulting from the combustion process were higher is assumed to be the presence of the self-adhesive material on the tested sample. However, the results obtained demonstrate that for the analyzed sound-absorbing sponge samples the concentrations are comparable, so that, for the characterization of the mixture of gases resulting from combustion, the use of the sound-absorbing sponge type N2138 in the INSEMEX scale experiment allows the equivalent reproduction of the event in which the sound-absorbing sponge material existing on-site was involved.
Determination of oxygen, carbon dioxide, and carbon monoxide concentration by the volumetric method shows that in the air volume of 1.5 L, the existing oxygen is sufficient for the combustion of 0.063 g of sample A, pyramidal type N2138 sound-absorbing sponge, respectively, 0.076 g of sample B, self-adhesive pyramidal sound-absorbing sponge collected from the scene of the event.
The results reveal that the experimental levels of gases produced by the combustion of polyurethane foam show a good correlation with the specialized literature [
23].
The results indicate that the mass loss by combustion of the sound-absorbing sponge samples used is comparable. Following the combustion of the specified amounts of sound-absorbing sponge, in the air volume of 1.5 L, a total volume of flue gases of 1.62 L and an overpressure of 75 mm H2O resulted. By relating the amount of sound-absorbing sponge burned to the amount of HCN produced, the following results were obtained experimentally using the described method: 14.93 mg of HCN is obtained for 1 g of sample A subjected to combustion; 11.11 mg of HCN is obtained for 1 g of sample B subjected to combustion.
The generation of HCN (hydrogen cyanide) differs between samples: Sample A produced 0.941 mg of HCN, and Sample B produced 0.844 mg of HCN, a slightly lower value. This difference may indicate variations in the material composition or in the combustion conditions. In addition to these harmful gases, the combustion of flexible polyurethane foam also produces combustible gases such as methane, propane, and propene, which can contribute to the risk of fire spread. The analysis of the results indicates that the experimentally calculated levels of hydrogen cyanide produced by the combustion of polyurethane foam correlate well with the facts documented in the specialized literature [
16,
17,
18].
The results obtained using the methods described above demonstrate that for the sound-absorbing sponge samples analyzed, both in the normal state and in the affected state, the concentrations are similar, thus the use of the sound-absorbing sponge type N2138 in the INSEMEX scale experiment allows for the equivalent reproduction of the event.
5. Conclusions
Analysis of the gases resulting from the combustion of the sound-absorbing sponge indicates the presence of dangerous toxic compounds (HCN, CO, HCl), which can endanger human health in the event of a fire. Also, the combustion process is incomplete, generating flammable The data analyzed refer to the combustion of six types of sound-absorbing sponges, each tested in a vertical and horizontal position, where the burning time and burning rate were measured. The purpose of the analysis is to identify trends regarding the flammability of these materials and the impact of positioning on combustion.
The vertical position accelerates combustion, and in practical applications, this aspect must be considered for fire prevention. The sampled formwork sponge is the most flammable and should be avoided in applications where there is a high risk of fire.
The sampled pyramidal sponge is the most resistant to combustion, making it a safer choice. The sampled sponges tend to burn faster, indicating that exposure over time to external factors can reduce the flame-retardant properties.
Quantitative determinations of the amount of sound-absorbing sponge consumed by combustion in a controlled volume of air (1.5 L) revealed low but differentiated mass losses between the samples.
This variation suggests possible differences in the material composition, density, or internal structure of the samples. Although the losses are small, they indicate that the material is not completely burned, which may influence its behavior in real fire exposure scenarios.