Relationship Between Post-Fire Vegetation Recovery and Soil Temperature in the Mediterranean Forest
Abstract
1. Introduction
2. Materials and Methods
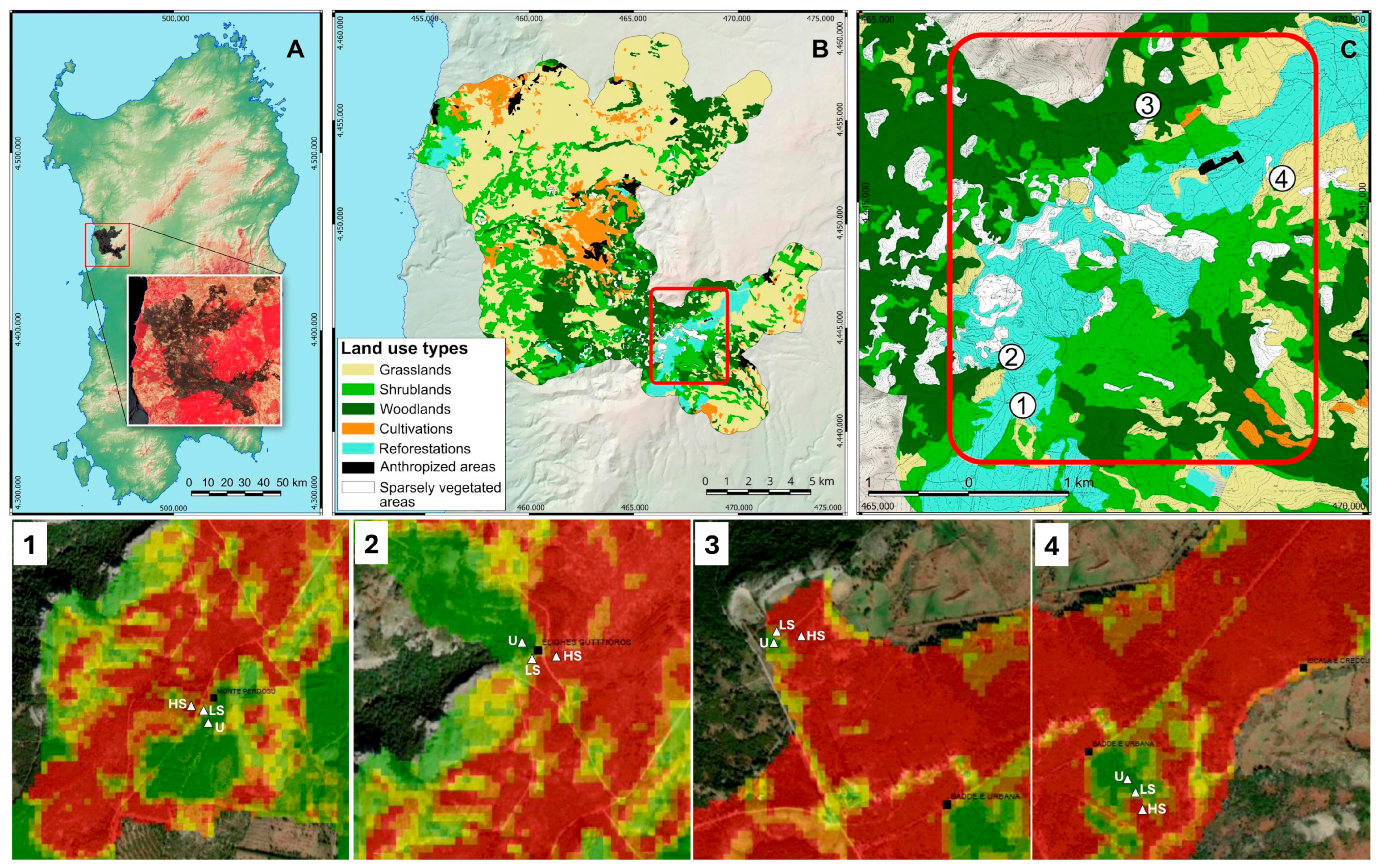
2.1. Study Area
2.2. Experimental Protocol and Data Collection
2.3. Data Analysis
- (1)
- Structural plants, including all characteristic or differential species of the association (Saniculo europaeae-Quercetum ilicis), alliance (Fraxino orni-Quercion ilicis), and vegetation class (Quercetea ilicis);
- (2)
- Other species not included in the above-mentioned syntaxa.
3. Results
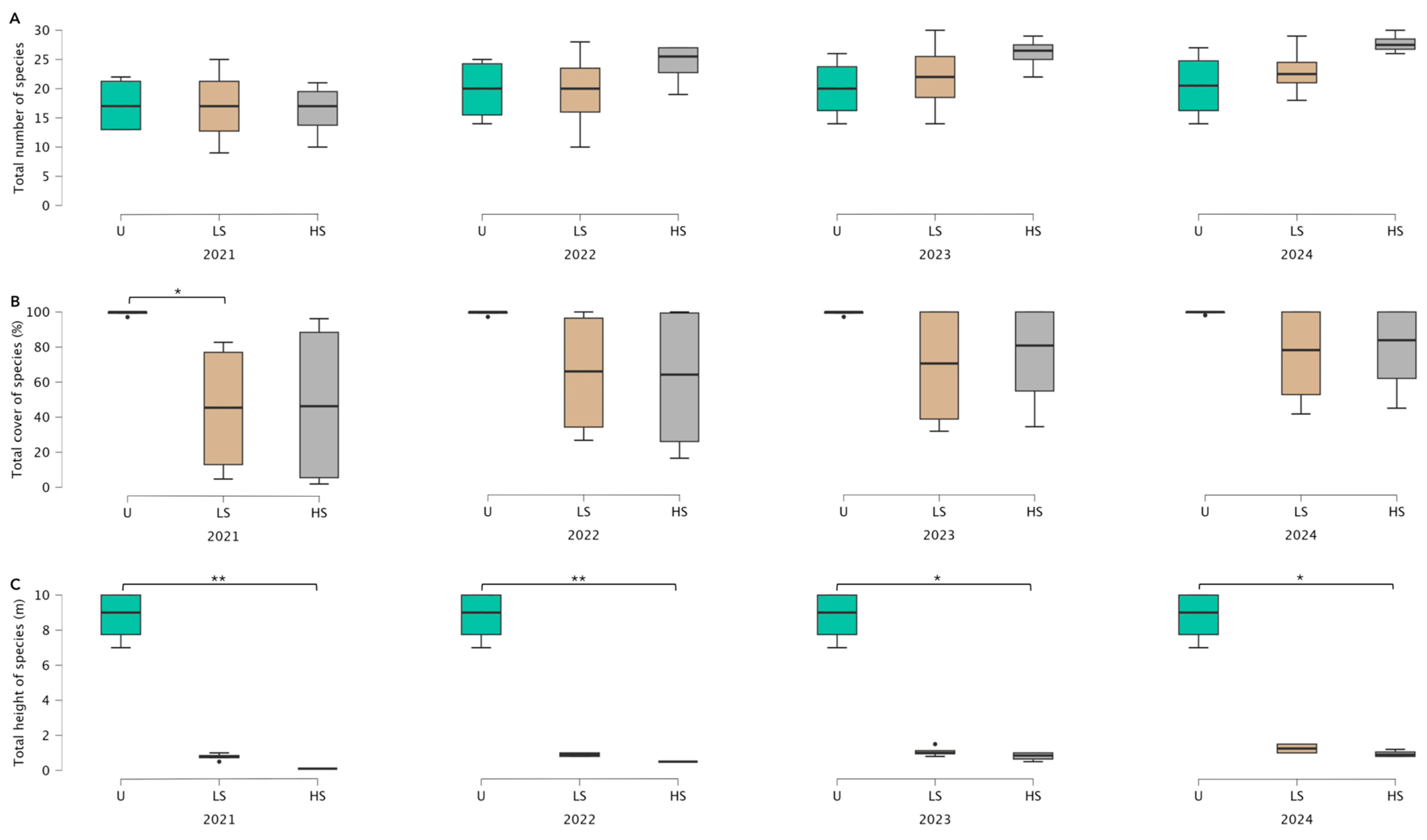
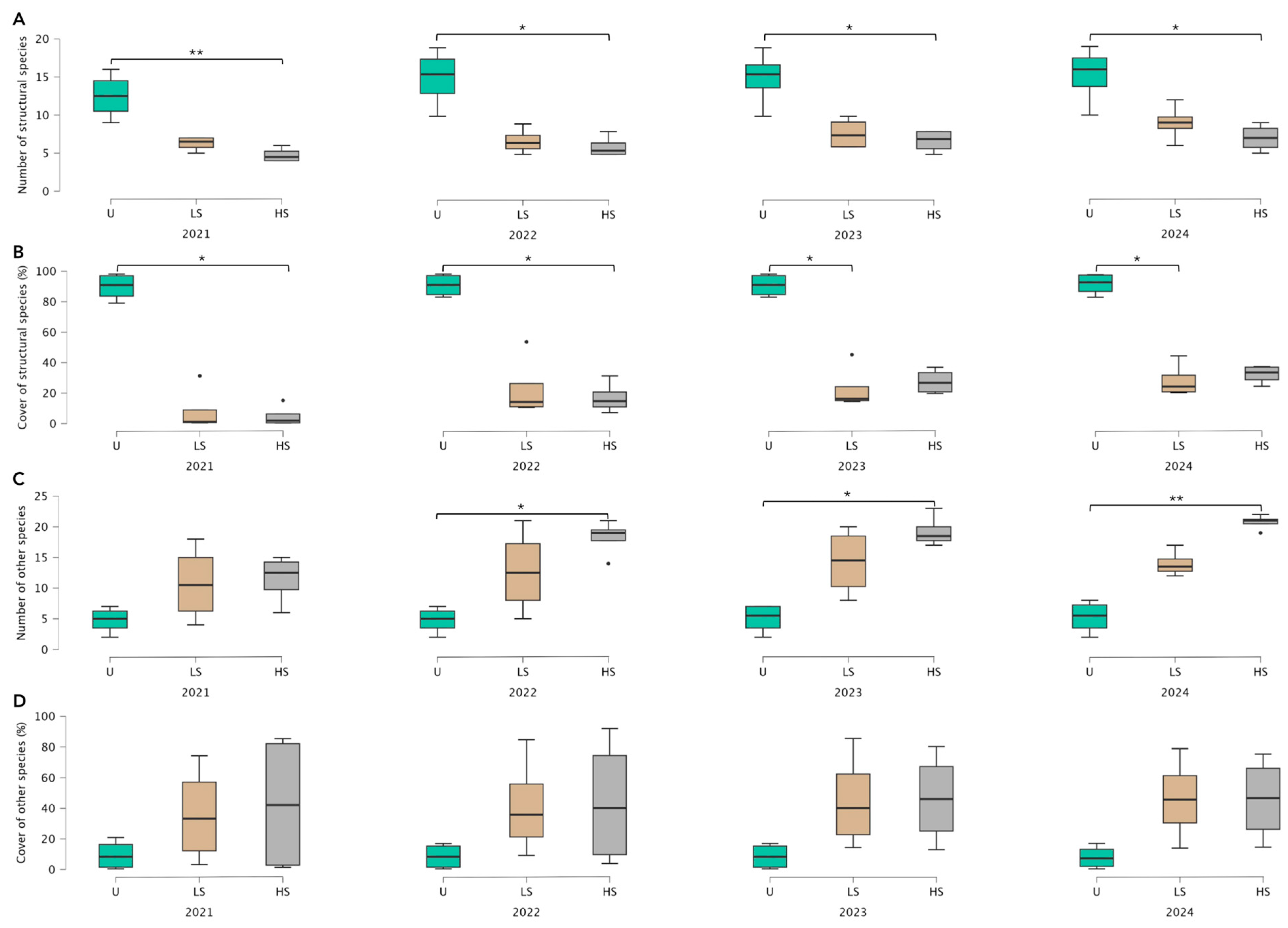
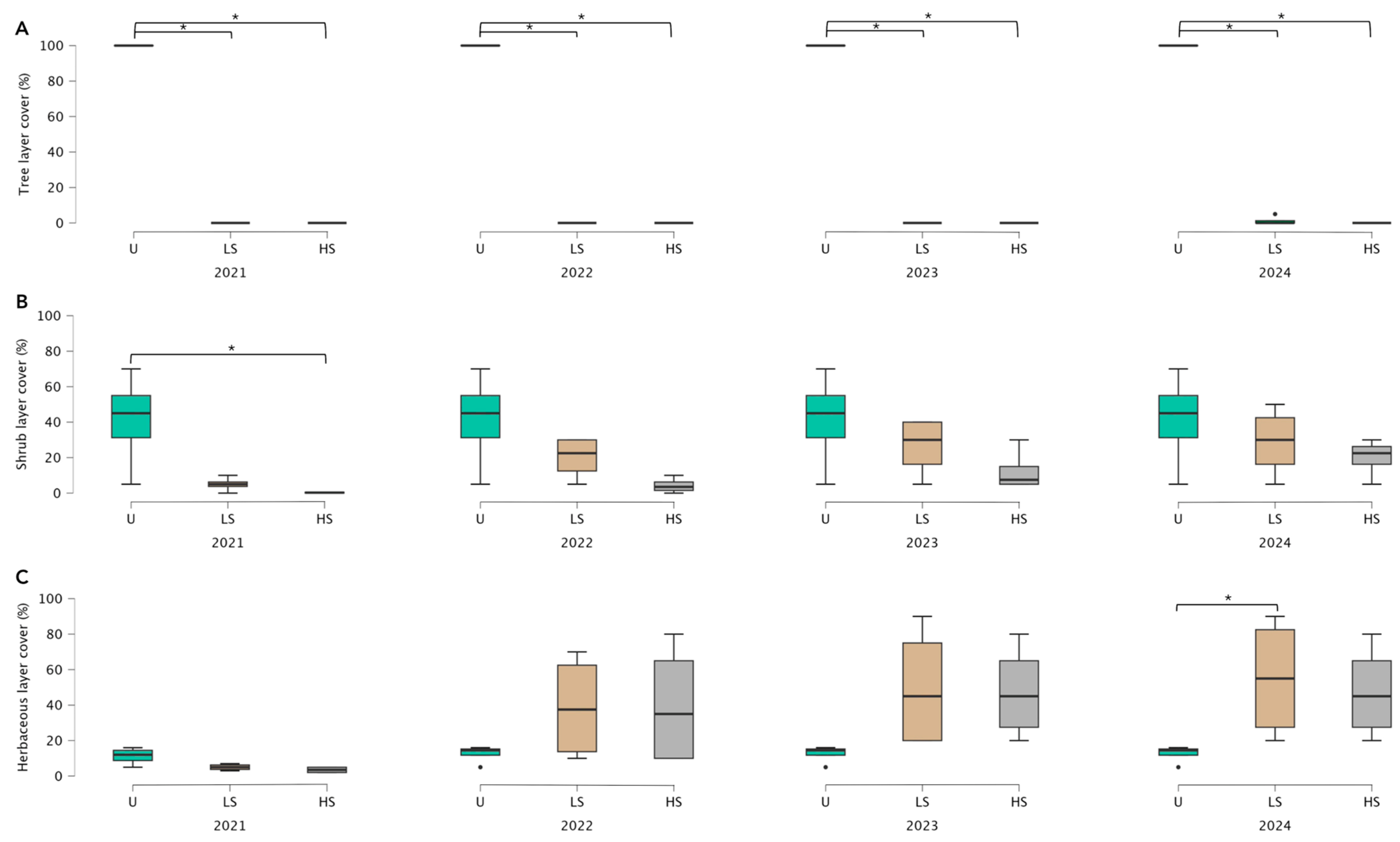
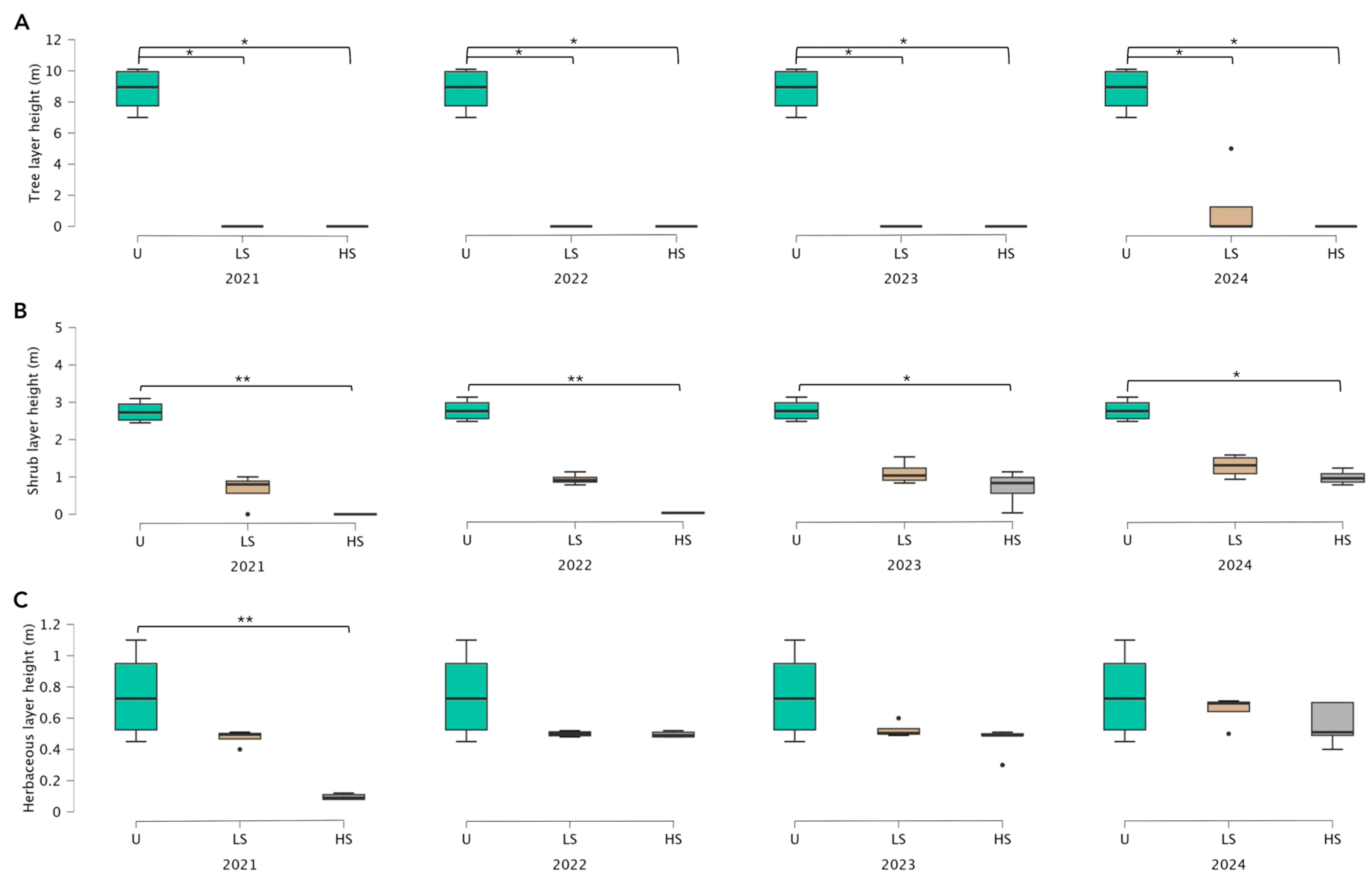
3.1. Vegetation Recovery Dynamics Following Different Levels of Fire Severity
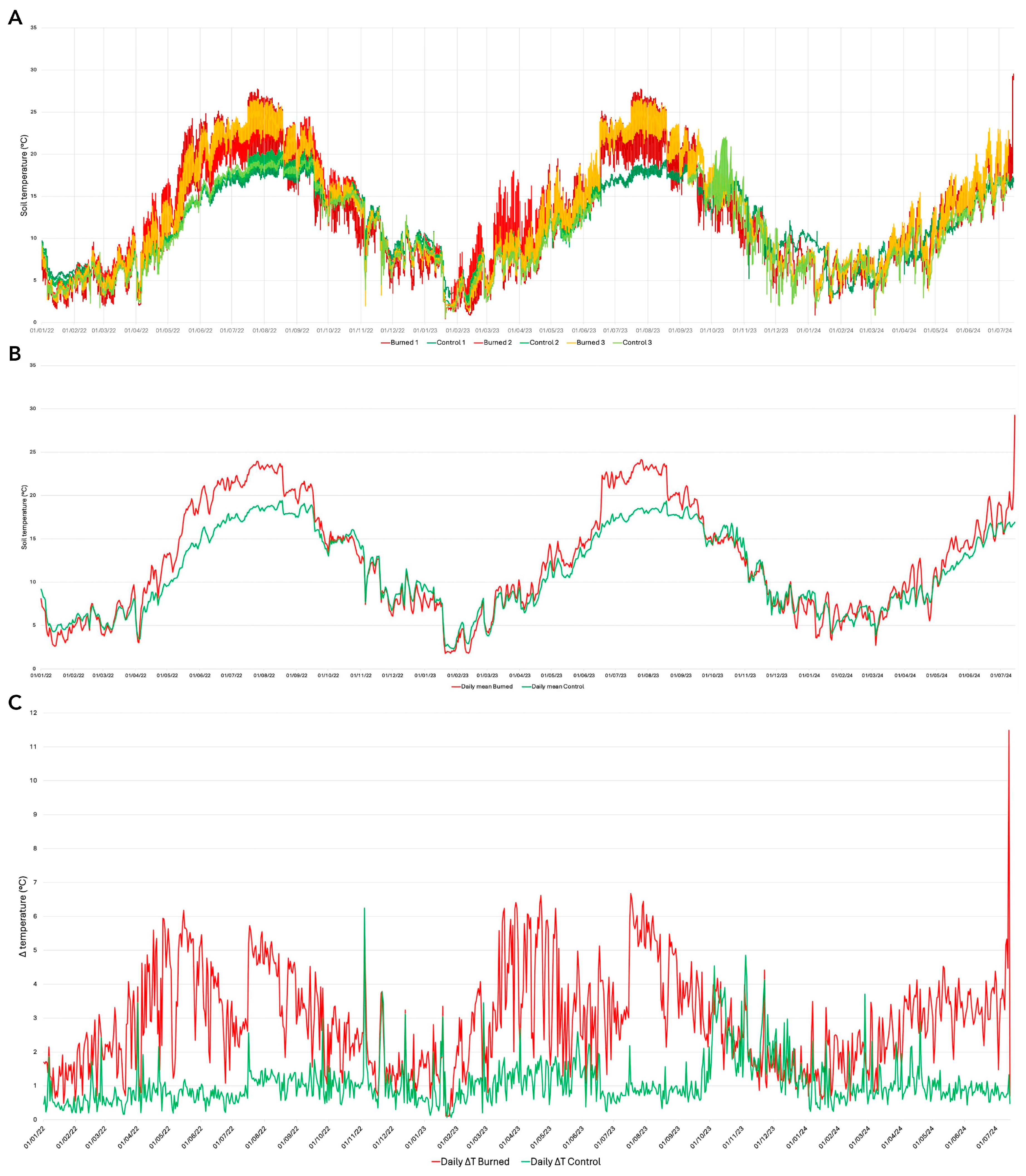
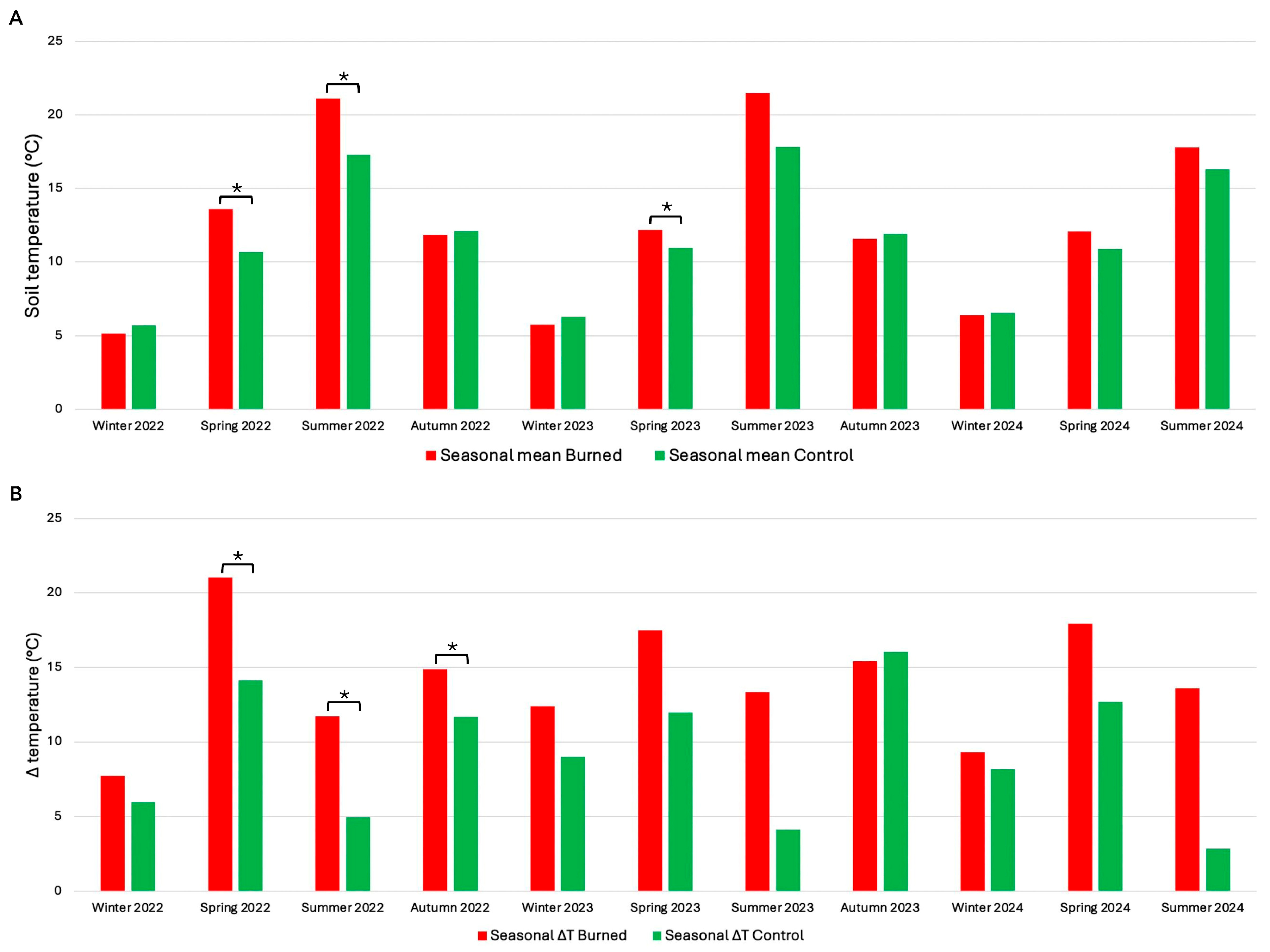
3.2. Soil Temperature Regimes Following a Megafire and Relationships with Post-Fire Vegetation Recovery Patterns
4. Discussion
4.1. Vegetation Recovery Dynamics Following Different Levels of Fire Severity
4.2. Soil Temperature Regimes Following a Megafire and Relationships with Post-Fire Vegetation Recovery Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.Q.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology—Variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Geiger, R.; Aron, R.H.; Todhunter, P. The Climate Near the Ground; Rowman and Little Field Publishers, Inc.: Lanham, MD, USA, 2003. [Google Scholar]
- De Frenne, P.; Zellweger, F.; Rodriguez-Sanchez, F.; Scheffers, B.R.; Hylander, K.; Luoto, M.; Vellend, M.; Verheyen, K.; Lenoir, J. Global buffering of temperatures under forest canopies. Nat. Ecol. Evol. 2019, 3, 744–749. [Google Scholar] [CrossRef]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K. Forest microclimates and climate change: Importance, drivers and future research agenda. Global Change Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- De Frenne, P.; Rodriguez-Sanchez, F.; Coomes, D.A.; Baeten, L.; Verstraeten, G.; Vellend, M.; Bernhardt-Romermann, M.; Brown, C.D.; Brunet, J.; Cornelis, J.; et al. Microclimate moderates plant responses to macroclimate warming. Proc. Natl. Acad. Sci. USA 2013, 110, 18561–18565. [Google Scholar] [CrossRef]
- Xu, X.; Huang, A.; Belle, E.; De Frenne, P.; Jia, G. Protected areas provide thermal buffer against climate change. Sci. Adv. 2022, 8, eabo0119. [Google Scholar] [CrossRef]
- Ulrey, C.; Quintana-Ascencio, P.F.; Kauffman, G.; Smith, A.B.; Menges, E.S. Life at the top: Long-term demography, microclimatic refugia, and responses to climate change for a high-elevation southern Appalachian endemic plant. Biol. Conserv. 2016, 200, 80–92. [Google Scholar] [CrossRef]
- Ewers, R.M.; Banks-Leite, C. Fragmentation impairs the micro- climate buffering effect of tropical forests. PLoS ONE 2013, 8, e58093. [Google Scholar] [CrossRef] [PubMed]
- von Arx, G.; Graf Pannatier, E.; Thimonier, A.; Rebetez, M.; Gilliam, F. Microclimate in forests with varying leaf area index and soil moisture: Potential implications for seedling establishment in a changing climate. J. Ecol. 2013, 101, 1201–1213. [Google Scholar] [CrossRef]
- Dobrowski, S.Z. A climatic basis for microrefugia: The influence of terrain on climate. Glob. Change Biol. 2011, 17, 1022–1035. [Google Scholar] [CrossRef]
- Jucker, T.; Hardwick, S.R.; Both, S.; Elias, D.M.O.M.; Ewers, R.M.; Milodowski, D.T.; Swinfield, T.; Coomes, D.A. Canopy structure and topography jointly constrain the microclimate of human-modified tropical landscapes. Glob. Change Biol. 2018, 24, 5243–5258. [Google Scholar] [CrossRef]
- McLaughlin, B.C.; Ackerly, D.D.; Zion Klos, P.; Natali, J.; Dawson, T.E.; Thompson, S.E. Hydrologic refugia, plants, and climate change. Glob. Change Biol. 2017, 23, 2941–2961. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.T.; Dobrowski, S.Z.; Holden, Z.A.; Higuera, P.E.; Abatzoglou, J.T. Microclimatic buffering in forests of the future: The role of local water balance. Ecography 2019, 42, 1–11. [Google Scholar] [CrossRef]
- Pleim, J.E.; Gilliam, R. An indirect data assimilation scheme for deep soil temperature in the Pleim-Xiu land surface model. JAMC 2009, 48, 1362–1376. [Google Scholar] [CrossRef]
- Portillo-Estrada, M.; Pihlatie, M.; Korhonen, J.F.J.; Levula, J.; Frumau, A.K.F.; Ibrom, A.; Lembrechts, J.J.; Morillas, L.; Horvath, L.; Jones, S.K.; et al. Climatic controls on leaf litter decom- position across European forests and grasslands revealed by reciprocal litter transplantation experiments. Biogeosciences 2016, 13, 1621–1633. [Google Scholar] [CrossRef]
- Hursh, A.; Ballantyne, A.; Cooper, L.; Maneta, M.; Kimball, J.; Watts, J. The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale. Glob. Change Biol. 2017, 23, 2090–2103. [Google Scholar] [CrossRef]
- Gottschall, F.; Davids, S.; Newiger-Dous, T.E.; Auge, H.; Cesarz, S.; Eisenhauer, N. Tree species identity determines wood de- composition via microclimatic effects. Ecol. Evol. 2019, 9, 12113–12127. [Google Scholar] [CrossRef]
- Lembrechts, J.J.; Ashcroft, M.B.; Frenne, P.D.; Kemppinen, J.; Kopecký, M.; Luoto, M.; Maclean, I.M.D.; Crowther, T.W.; Bailey, J.J.; Haesen, S.; et al. Global Maps of Soil Temperature. Glob. Change Biol. 2022, 28, 3110–3144. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Smethurst, P.J.; O’Connell, A.M.; Carlyle, C.J.; Khanna, P.K. Soil temperature under forests: A simple model for predicting soil temperature under a range of forest types. Agric. For. Meteorol. 2004, 121, 167–182. [Google Scholar] [CrossRef]
- García-Llamas, P.; Suárez-Seoane, S.; Fernández-Guisuraga, J.M.; Fernández-García, V.; Fernández-Manso, A.; Quintano, C.; Taboada, A.; Marcos, E.; Calvo, L. Evaluation and comparison of Landsat 8, Sentinel-2 and Deimos-1 remote sensing indices for assessing burn severity in Mediterranean fire-prone ecosystems. Int. J. Appl. Earth Observ. Geoinf. 2019, 80, 137–144. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H. Fire effects on soils: The human dimension. Philos. Trans. R. Soc. B 2016, 371, 20150171. [Google Scholar] [CrossRef]
- Memoli, V.; Santorufo, L.; Santini, G.; Ruggiero, A.G.; Giarra, A.; Ranieri, P.; Di Natale, G.; Ceccherini, M.T.; Trifuoggi, M.; Barile, R.; et al. The combined role of plant cover and fire occurrence on soil properties reveals response to wildfire in the Mediterranean basin. Eur. J. Soil Biol. 2022, 112, 103430. [Google Scholar] [CrossRef]
- González-Pelayo, O.; Andreu, V.; Gimeno-García, E.; Campo, J.; Rubio, J.L. Effects of fire and vegetation cover on hydrological characteristics of a Mediterranean shrubland soil. Hydrobiol. Proc. 2010, 24, 1504–1513. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Huerta, S.; Calvo, L. Soil-vegetation relationships in Mediterranean forests after fire. For. Ecosyst. 2021, 8, 18. [Google Scholar] [CrossRef]
- Caon, L.; Vallejo, V.R.; Ritsema, C.J.; Geissen, V. Effects of Wildfire on Soil Nutrients in Mediterranean Ecosystems. Earth Sci. Rev. 2014, 139, 47–58. [Google Scholar] [CrossRef]
- Megremi, I.; Stathopoulou, E.; Vorris, E.; Kostakis, M.; Karavoltsos, S.; Thomaidis, N.; Vasilatos, C. Mediterranean Wildfires’ Effect on Soil Quality and Properties: A Case from Northern Euboea, Greece. Land 2024, 13, 325. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Blasi, C.; Biondi, E.; Bovio, G.; Camia, A.; Capogna, F.; Corona, P.; Cullotta, S.; Esposito, A.; Fabiani, M.L.; et al. Incendi e Complessità Ecosistemica. Dalla Pianificazione Forestale Al Recupero Ambientale; Blasi, C., Bovio, G., Corona, P.M., Marchetti, M., Maturani, A., Eds.; Ministero Dell’ambiente e della Tutela del Territorio; SBI, 2004; Available online: https://iris.uniroma1.it/handle/11573/64313 (accessed on 13 February 2025).
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Koutsias, N.; Karteris, M. Classification analyses of vegetation for delineating forest fire fuel complexes in a Mediterranean test site using satellite remote sensing and GIS. Int. J. Remote Sens. 2002, 24, 3093–3104. [Google Scholar] [CrossRef]
- Xofis, P.; Buckley, P.G.; Takos, I.; Mitchley, J. Long Term Post-Fire Vegetation Dynamics in North-East Mediterranean Ecosystems. The Case of Mount Athos Greece. Fire 2021, 4, 92. [Google Scholar] [CrossRef]
- Nolè, A.; Rita, A.; Spatola, M.F.; Borghetti, M. Biogeographic variability in wildfire severity and post-fire vegetation recovery across the European forests via remote sensing-derived spectral metrics. Sci. Total Environ. 2022, 823, 153807. [Google Scholar] [CrossRef] [PubMed]
- Heim, R.J.; Bucharova, A.; Brodt, L.; Kamp, J.; Rieker, D.; Soromotin, A.V.; Yurtaev, A.; Holzel, N. Post-fire vegetation succession in the Siberian subarctic tundra over 45 years. Sci. Total Environ. 2021, 760, 143425. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, F.; Coomes, D.; Lenoir, J.; Depauw, L.; Maes, S.L.; Wulf, M.; Kirby, K.J.; Brunet, J.; Kopecký, M.; Máliš, F.; et al. Seasonal drivers of understorey temperature buffering in temperate deciduous forests across Europe. Glob. Ecol. Biogeogr. 2019, 28, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Cramer, W.; Carnicer, J.; Georgopoulou, E.; Hilmi, N.J.M.; Le Cozannet, G.; Lionello, P. Cross-chapter paper 4: Mediterranean Region. In Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 2233–2272. [Google Scholar] [CrossRef]
- de Rigo, D.; Libertà, G.; Houston Durrant, T.; Artés Vivancos, T.; San-Miguel-Ayanz, J. Forest Fire Danger Extremes in Europe Under Climate Change: Variability and Uncertainty; Publication Office of the European Union: Luxemburg, 2017. [Google Scholar]
- Huerta, S.; Marcos, E.; Fernández-García, V.; Calvo, L. Resilience of Mediterranean communities to fire depends on burn severity and type of ecosystem. Fire Ecol. 2022, 18, 28. [Google Scholar] [CrossRef]
- Gibson, R.K.; White, L.A.; Hislop, S.; Nolan, R.H.; Dorrough, J. The post-fire stability index; a new approach to monitoring post-fire recovery by satellite imagery. Remote Sens. Environ. 2022, 280, 113151. [Google Scholar] [CrossRef]
- Liu, Z. Effects of climate and fire on short-term vegetation recovery in the boreal larch forests of Northeastern China. Sci. Rep. 2016, 6, 37572. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, C.; Li, R. Comparison of vegetation regeneration after wildfire between Mediterranean and tundra ecosystems by using landsat images. Ann. GIS 2018, 24, 99–112. [Google Scholar] [CrossRef]
- Arrogante-Funes, F.; Mouillot, F.; Moreira, B.; Aguado, I.; Chuvieco, E. Mapping and assessment of ecological vulnerability to wildfires in Europe. Fire Ecol. 2024, 20, 98. [Google Scholar] [CrossRef]
- Bastos, A.; Gouveia, C.M.; DaCamara, C.C.; Trigo, R.M. Modelling post-fire vegetation recovery in Portugal. Biogeosciences 2011, 8, 3593–3607. [Google Scholar] [CrossRef]
- Bright, B.C.; Hudak, A.T.; Kennedy, R.E.; Braaten, J.D.; Henareh Khalyani, A. Examining post-fire vegetation recovery with landsat time series analysis in three western north american forest types. Fire Ecol. 2019, 15, 8. [Google Scholar] [CrossRef]
- Cerdá, A.; Doerr, S.H. Influence of vegetation recovery on soil hydrology and erodibility following fire: An 11-year investigation. Int. J. Wildl. Fire 2015, 14, 423–437. [Google Scholar] [CrossRef]
- Gouveia, C.; DaCamara, C.C.; Trigo, R.M. Post-fire vegetation recovery in Portugal based on spot/vegetation data. Nat. Hazards Earth Syst. Sci. 2010, 10, 673–684. [Google Scholar] [CrossRef]
- Viana-Soto, A.; Aguado, I.; Salas, J.; García, M. Identifying post-fire recovery trajectories and driving factors using landsat time series in fire-prone mediterranean pine forests. Remote Sens. 2020, 12, 1499. [Google Scholar] [CrossRef]
- Ermitão, T.; Gouveia, C.M.; Bastos, A.; Russo, A.C. Recovery Following Recurrent Fires Across Mediterranean Ecosystems. Glob. Change Biol. 2024, 30, e70013. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Calderisi, G.; Cogoni, D.; Fenu, G. Post-Fire Vegetation (Non-)Recovery across the Edges of a Wildfire: An Unexplored Theme. Fire 2024, 7, 250. [Google Scholar] [CrossRef]
- de Villalobos, A.E.; Peláez, D.V.; Bóo, R.M.; Mayor, M.D.; Elia, O.R. Effect of high temperatures on seed germination of Prosopis caldenia Burk. J. Arid Environ. 2002, 52, 371–378. [Google Scholar] [CrossRef]
- Cruz, Ó.; García-Duro, J.; Riveiro, S.F.; García-García, C.; Casal, M.; Reyes, O. Fire Severity Drives the Natural Regeneration of Cytisus scoparius L. (Link) and Salix atrocinerea Brot. Communities and the Germinative Behaviour of These Species. Forests 2020, 11, 124. [Google Scholar] [CrossRef]
- Rossetti, I.; Cogoni, D.; Calderisi, G.; Fenu, G. Short-Term Effects and Vegetation Response after a Megafire in a Mediterranean Area. Land 2022, 11, 2328. [Google Scholar] [CrossRef]
- Lawes, M.J.; Clarke, P.J. Ecology of Plant Resprouting: Populations to Community Responses in Fire-Prone Ecosystems. Plant Ecol. 2011, 212, 1937–1943. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a Key Functional Trait: How Buds, Protection and Resources Drive Persistence After Fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Pausas, J.G. Bark Thickness and Fire Regime. Funct. Ecol. 2015, 29, 315–327. [Google Scholar] [CrossRef]
- Harrison, S.P.; Prentice, I.C.; Bloomfield, K.J.; Dong, N.; Forkel, M.; Forrest, M.; Ningthoujam, R.K.; Pellegrini, A.; Shen, Y.; Baudena, M.; et al. Understanding and Modelling Wildfire Regimes: An Ecological Perspective. Environ. Res. Lett. 2021, 16, 125008. [Google Scholar] [CrossRef]
- Nolan, R.H.; Collins, L.; Leigh, A.; Ooi, M.K.J.; Curran, T.J.; Fairman, T.A.; de Dios, V.R.; Bradstock, R. Limits to Post-Fire Vegetation Recovery Under Climate Change. Plant Cell Environ. 2021, 44, 3471–3489. [Google Scholar] [CrossRef]
- Wundram, D.; Pape, R.; Löffler, J. Alpine soil temperature variability at multiple scales. Arct. Antarct. Alp. Res. 2010, 42, 117–128. [Google Scholar] [CrossRef]
- Liancourt, P.; Sharkhuu, A.; Ariuntsetseg, L.; Boldgiv, B.; Helliker, B.R.; Plante, A.F.; Petraits, P.S.; Casper, B.B. Temporal and spatial variation in how vegetation alters the soil moisture response to climate manipulation. Plant Soil 2012, 351, 249–261. [Google Scholar] [CrossRef]
- Legates, D.R.; Mahmood, R.; Levia, D.F.; DeLiberty, T.L.; Quiring, S.M.; Houser, C.; Nelson, F.E. Soil moisture: A central and unifying theme in physical geography. Prog. Phys. Geogr. 2010, 35, 65–86. [Google Scholar] [CrossRef]
- Gornall, J.L.; Woodin, S.J.; Jonsdottir, I.S.; van der Wal, R. Balancing positive and negative plant interactions: How mosses structure vascular plant communities. Oecologia 2011, 166, 769–782. [Google Scholar] [CrossRef]
- Monteiro, J.; Domingues, I.; Brilhante, M.; Serafim, J.; Nunes, S.; Trigo, R.; Branquinho, C. Changes in bryophyte functional composition during post-fire succession. Sci. Total Environ. 2024, 925, 171592. [Google Scholar] [CrossRef]
- Sapkota, D.; Edwards, D.; Massam, M.; Evans, K. A Pantropical Analysis of Fire Impacts and Post-Fire Species Recovery of Plant Life Forms. Ecol. Evol. 2025, 15, e71018. [Google Scholar] [CrossRef] [PubMed]
- Carmignani, L.; Oggiano, G.; Funedda, A.; Conti, P.; Pasci, S. The geological map of Sardinia (Italy) at 1: 250,000 scale. J. Maps 2016, 12, 826–835. [Google Scholar] [CrossRef]
- Regione Autonoma della Sardegna (RAS). Piano Forestale Ambientale Regionale: Distretto 12—Montiferru. 2007. Available online: https://www.regione.sardegna.it/documenti/1_5_20080214173430.pdf (accessed on 10 December 2024).
- Rivas-Martínez, S.; Rivas-Saenz, S. Worldwide bioclimatic classification system. Glob. Geobot. 2011, 1, 1–634. [Google Scholar]
- Linley, G.D.; Jolly, C.J.; Doherty, T.S.; Geary, W.L.; Armenteras, D.; Belcher, C.M.; Bliege Bird, R.; Duane, A.; Fletcher, M.-S.; Giorgis, M.A.; et al. What do you mean, ‘megafire’? Glob. Ecol. Biogeogr. 2022, 31, 1906–1922. [Google Scholar] [CrossRef]
- De Frenne, P.; Beugnon, R.; Klinges, D.; Lenoir, J.; Niittynen, P.; Pincebourde, S.; Senior, R.A.; Aalto, J.; Chytrý, K.; Gillingham, P.K. Ten practical guidelines for microclimate research in terrestrial ecosystems. Methods Ecol. Evol. 2024, 16, 269–294. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Blanchard, W.; Mcburney, L.; Bowd, E.; Youngentob, K.N.; Marsh, K.; Taylor, C. Stand age related differences in forest microclimate. For. Ecol. Manag. 2022, 510, 120101. [Google Scholar] [CrossRef]
- Máliš, F.; Ujházy, K.; Hederová, L.; Ujházyová, M.; Csölleová, L.; Coomes, D.A.; Zellweger, F. Microclimate variation and recovery time in managed and old-growth temperate forests. Agric. For. Meteorol. 2023, 342, 109722. [Google Scholar] [CrossRef]
- Santi, I.; Carrari, E.; De Frenne, P.; Valerio, M.; Gasperini, C.; Cabrucci, M.; Selvi, F. Impact of Coppicing on Microclimate and Understorey Vegetation Diversity in an Ancient Mediterranean Oak Forest. Sci. Total Environ. 2024, 918, 170531. [Google Scholar] [CrossRef]
- JASP Team. JASP [Computer Software], Version 0.19.1. 2024. Available online: https://jasp-stats.org (accessed on 22 February 2025).
- StatSoft, Inc. STATISTICA [Computer Software], Version 8.0; StatSoft, Inc.: Tulsa, OK, USA, 2007.
- Capitanio, R.; Carcaillet, C. Post-fire Mediterranean vegetation dynamics and diversity: A discussion of succession models. For. Ecol. Manag. 2008, 255, 431–439. [Google Scholar] [CrossRef]
- Calvo, L.; Santalla, S.; Marcos, E.; Valbuena, L.; Tárrega, R.; Luis, E. Regeneration after wildfire in communities dominated by Pinus pinaster, an obligate seeder, and in others dominated by Quercus pyrenaica, a typical resprouter. For. Ecol. Manag. 2003, 184, 209–223. [Google Scholar] [CrossRef]
- Malkinson, D.; Wittenberg, L.; Beeri, O.; Barzilai, R. Effects of repeated fires on the structure, composition, and dynamics of Mediterranean maquis: Short- and long-term perspectives. Ecosystems 2011, 14, 478–488. [Google Scholar] [CrossRef]
- Schaffhauser, A.; Curt, T.; Tatoni, T. Fire-vegetation interplay in a mosaic structure of Quercus suber woodlands and Mediterranean maquis under recurrent fires. For. Ecol. Manag. 2011, 262, 730–738. [Google Scholar] [CrossRef]
- Schaffhauser, A.; Curt, T.; Véla, E.; Tatoni, T. Recurrent fires and environment shape the vegetation in Quercus suber L. woodlands and maquis. Comptes Rendus Biol. 2012, 335, 424–434. [Google Scholar] [CrossRef]
- Tessler, N.; Wittenberg, L.; Greenbaum, N. Vegetation cover and species richness after recurrent forest fires in the Eastern Mediterranean ecosystem of Mount Carmel, Israel. Sci. Total Environ. 2016, 572, 1395–1402. [Google Scholar] [CrossRef]
- Bates, J.D.; Davies, K.W.; Bournoville, J.; Boyd, C.; O’Connor, R.; Svejcar, T.J. Herbaceous biomass response to prescribed fire in juniper-encroached sagebrush steppe. Rangel. Ecol. Manag. 2019, 72, 28–35. [Google Scholar] [CrossRef]
- Bashirzadeh, M.; Abedi, M.; Shefferson, R.P.; Farzam, M. Post-Fire Recovery of Plant Biodiversity Changes Depending on Time Intervals since Last Fire in Semiarid Shrublands. Fire 2023, 6, 103. [Google Scholar] [CrossRef]
- Hanes, T.L. Succession after fire in Chaparral of southern California. Ecol. Monogr. 1971, 41, 27–52. [Google Scholar] [CrossRef]
- Lavorel, S. Ecological diversity and resilience of Mediterranean vegetation to disturbance. Divers. Distrib. 1999, 5, 3–13. [Google Scholar] [CrossRef]
- Pausas, J.G.; Vallejo, V.R. The role of fire in European Mediterranean ecosystems. In Remote Sensing of Large Wildfire in the European Mediterranean Basin; Chuvieco, E., Ed.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Buhk, C.; Gotzenberger, L.; Wesche, K.; Gomez, P.S.; Hensen, I. Post-fire regeneration in a Mediterranean pine forest with historically low fire frequency. Acta Oecol. 2006, 30, 288–298. [Google Scholar] [CrossRef]
- Castillo, M.S.; Plaza, V.Á.; Garfias, S.R. A recent review of fire behavior and fire effects on native vegetation in Central Chile. Glob. Ecol. Conserv. 2020, 24, e01210. [Google Scholar] [CrossRef]
- Trabaud, L.; Lepart, J. Diversity and stability in garrigue ecosystems after fire. Vegetatio 1980, 43, 49–57. [Google Scholar] [CrossRef]
- Trabaud, L. Dynamics after fire of sclerophyllous plant communities in the mediterranean basin. Ecol. Mediterr. 1987, 13, 25–37. [Google Scholar] [CrossRef]
- Thompson, J.D. Plant Evolution in the Mediterranean, Insights for Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2020; pp. 1–352. [Google Scholar]
- Keeley, J.E. Fire management impacts on invasive plant species in the western United States. Conserv. Biol. 2006, 20, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.R.; Raposo, M.A.M.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.A. Fire as a selection agent for the dissemination of invasive species: Case study on the evolution of forest coverage. Environments 2020, 7, 57. [Google Scholar] [CrossRef]
- Grupenhoff, A.R.; Safford, H.D. High fire frequency in California chaparral reduces postfire shrub regeneration and native plant diversity. Ecosphere 2024, 15, e70128. [Google Scholar] [CrossRef]
- Shakesby, R.A.; Doerr, S.H. Wildfire as a hydrological and geomorphological agent. Earth Sci. Rev. 2006, 74, 269–307. [Google Scholar] [CrossRef]
- Zavala, L.M.; de Celis, R.; López, A.J. How wildfires affect soil properties. A brief review. Cuad. Investig. Geogr. 2014, 40, 311–331. [Google Scholar] [CrossRef]
- Wagenbrenner, J.W.; Ebel, B.A.; Bladon, K.D.; Kinoshita, A.M. Post-wildfire hydrologic recovery in Mediterranean climates: A systematic review and case study to identify current knowledge and opportunities. J. Hydrol. 2021, 602, 126772. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Plaza-Álvarez, P.A.; Yáñez, M.D.C.; Miralles, I.; Ortega, R.; Soria, R.; Zema, D.A. Long-term evaluation of soil functionality in Mediterranean forests after a wildfire and post-fire hillslope stabilisation. For. Ecol. Manag. 2024, 555, 121715. [Google Scholar] [CrossRef]
- Uribe, C.; Inclán, R.; Sánchez, D.M.; Clavero, M.A.; Fernández, A.M.; Morante, R.; Cardeña, A.; Blanco, A.; Van Miegroet, H. Effect of wildfires on soil respiration in three typical Mediterranean forest ecosystems in Madrid, Spain. Plant Soil 2013, 369, 403–420. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderisi, G.; Salaris, E.; Cogoni, D.; Rossetti, I.; Murtas, F.; Fenu, G. Relationship Between Post-Fire Vegetation Recovery and Soil Temperature in the Mediterranean Forest. Fire 2025, 8, 91. https://doi.org/10.3390/fire8030091
Calderisi G, Salaris E, Cogoni D, Rossetti I, Murtas F, Fenu G. Relationship Between Post-Fire Vegetation Recovery and Soil Temperature in the Mediterranean Forest. Fire. 2025; 8(3):91. https://doi.org/10.3390/fire8030091
Chicago/Turabian StyleCalderisi, Giulia, Enrico Salaris, Donatella Cogoni, Ivo Rossetti, Filippo Murtas, and Giuseppe Fenu. 2025. "Relationship Between Post-Fire Vegetation Recovery and Soil Temperature in the Mediterranean Forest" Fire 8, no. 3: 91. https://doi.org/10.3390/fire8030091
APA StyleCalderisi, G., Salaris, E., Cogoni, D., Rossetti, I., Murtas, F., & Fenu, G. (2025). Relationship Between Post-Fire Vegetation Recovery and Soil Temperature in the Mediterranean Forest. Fire, 8(3), 91. https://doi.org/10.3390/fire8030091






