The Effect of Chemical Modification by Synthetic and Natural Fire-Retardants on Burning and Chemical Characteristics of Structural Fir (Abies alba L.) Wood
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Application of the Retardants
2.3. Chemical Analysis
2.3.1. Chemical Analysis of Primary Raw Material
2.3.2. FTIR Analysis
2.3.3. DSC Analysis
2.4. Analysis of Combustion Heat Using an Isoperibol Calorimeter
2.5. Limiting Oxygen Index
2.6. Scanning Electron Microscopy (SEM)
3. Results
3.1. Chemical Analysis of Primary Raw Material
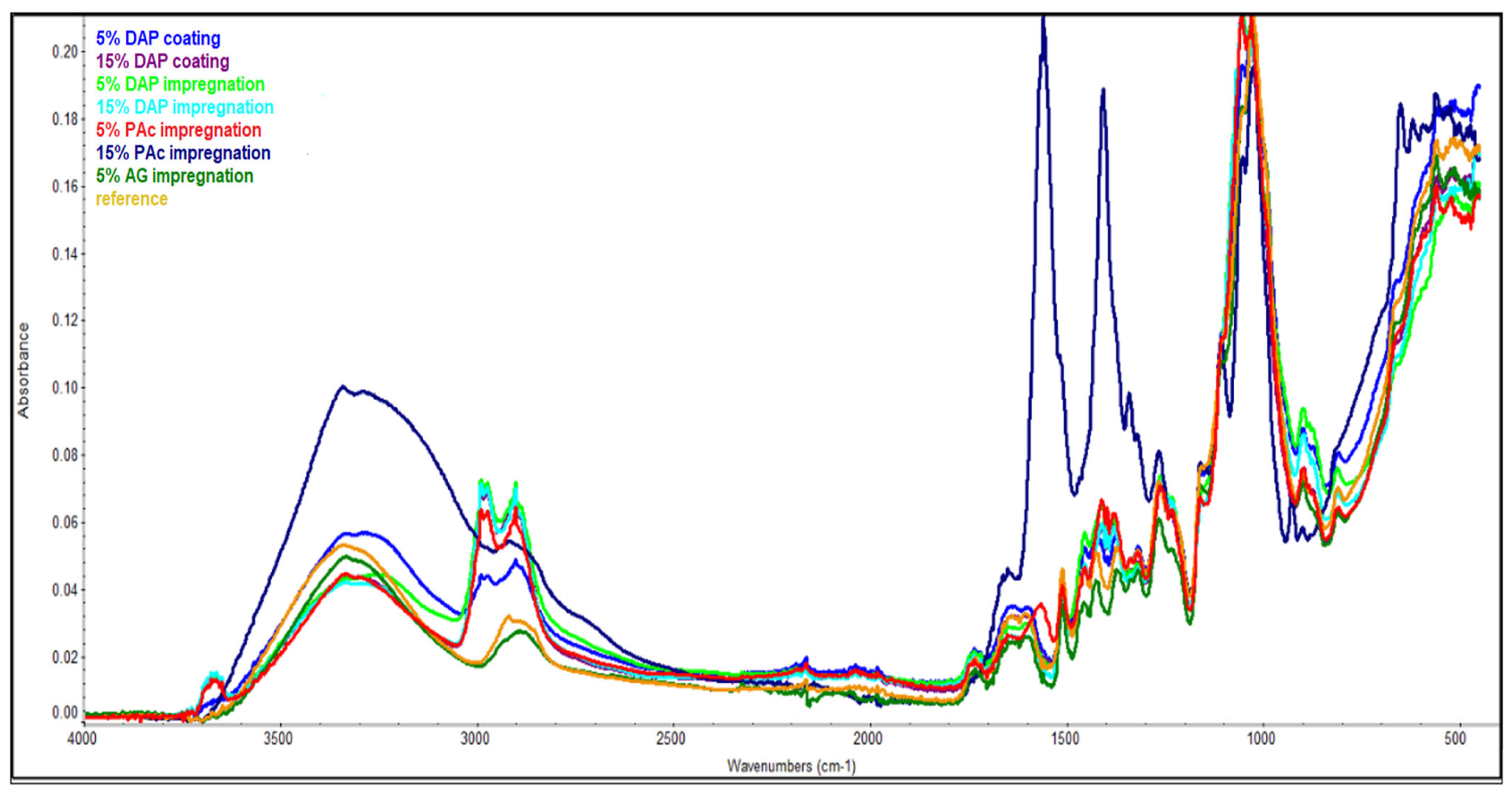
3.2. FTIR Spectrophotometry
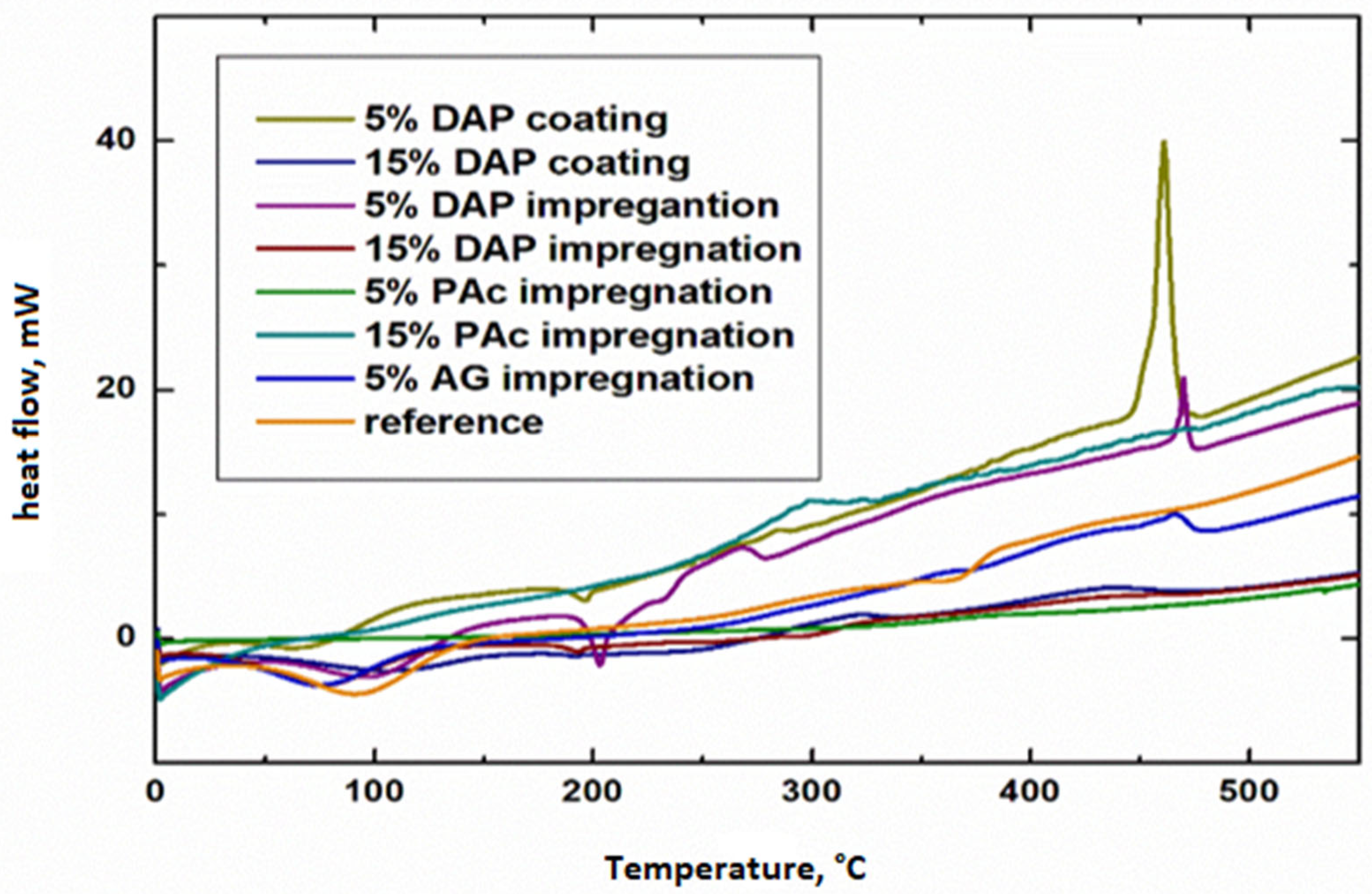
3.3. DSC Thermograms
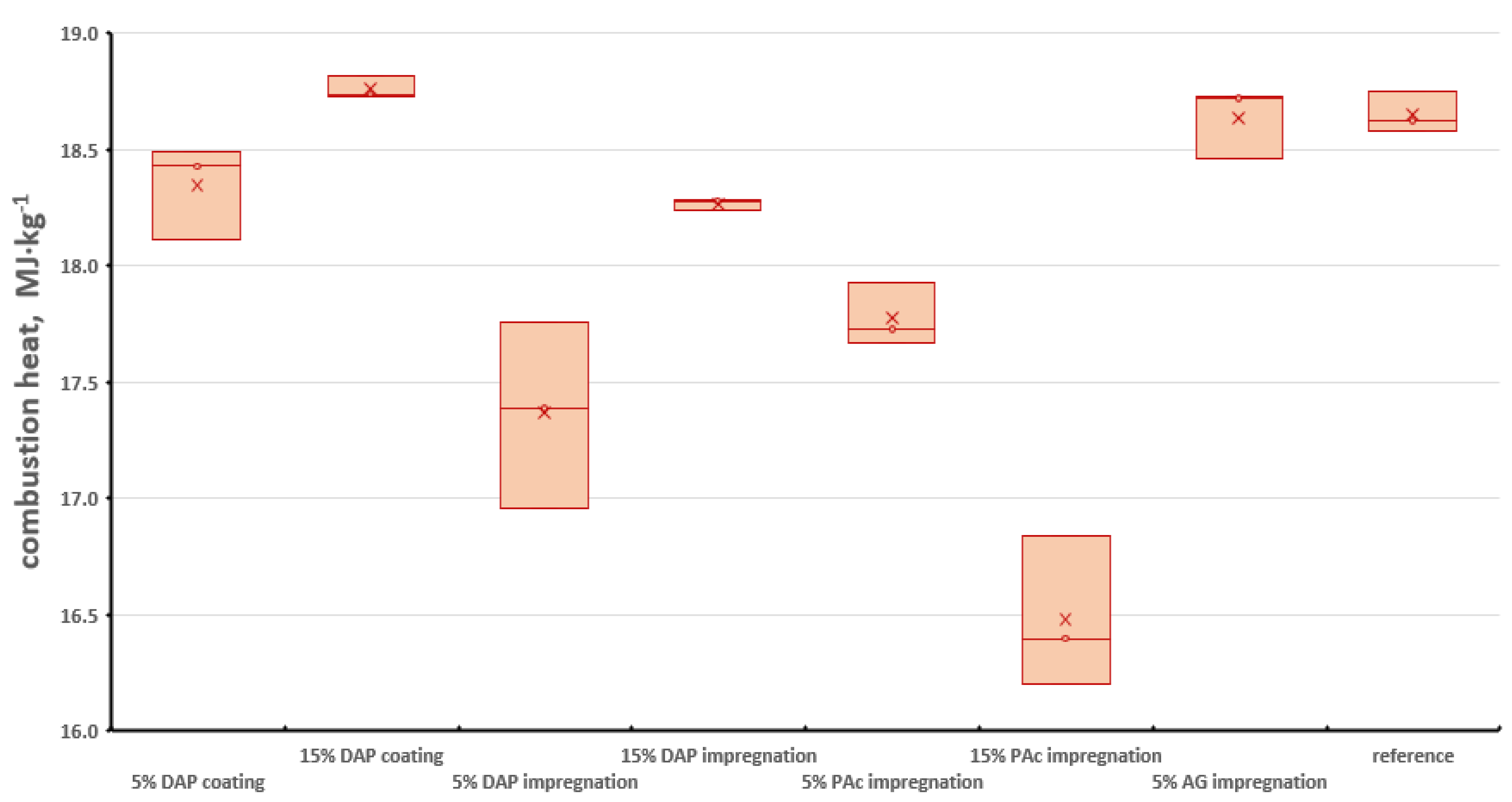
3.4. Combustion Heat Analysis
3.5. Limiting Oxygen Index
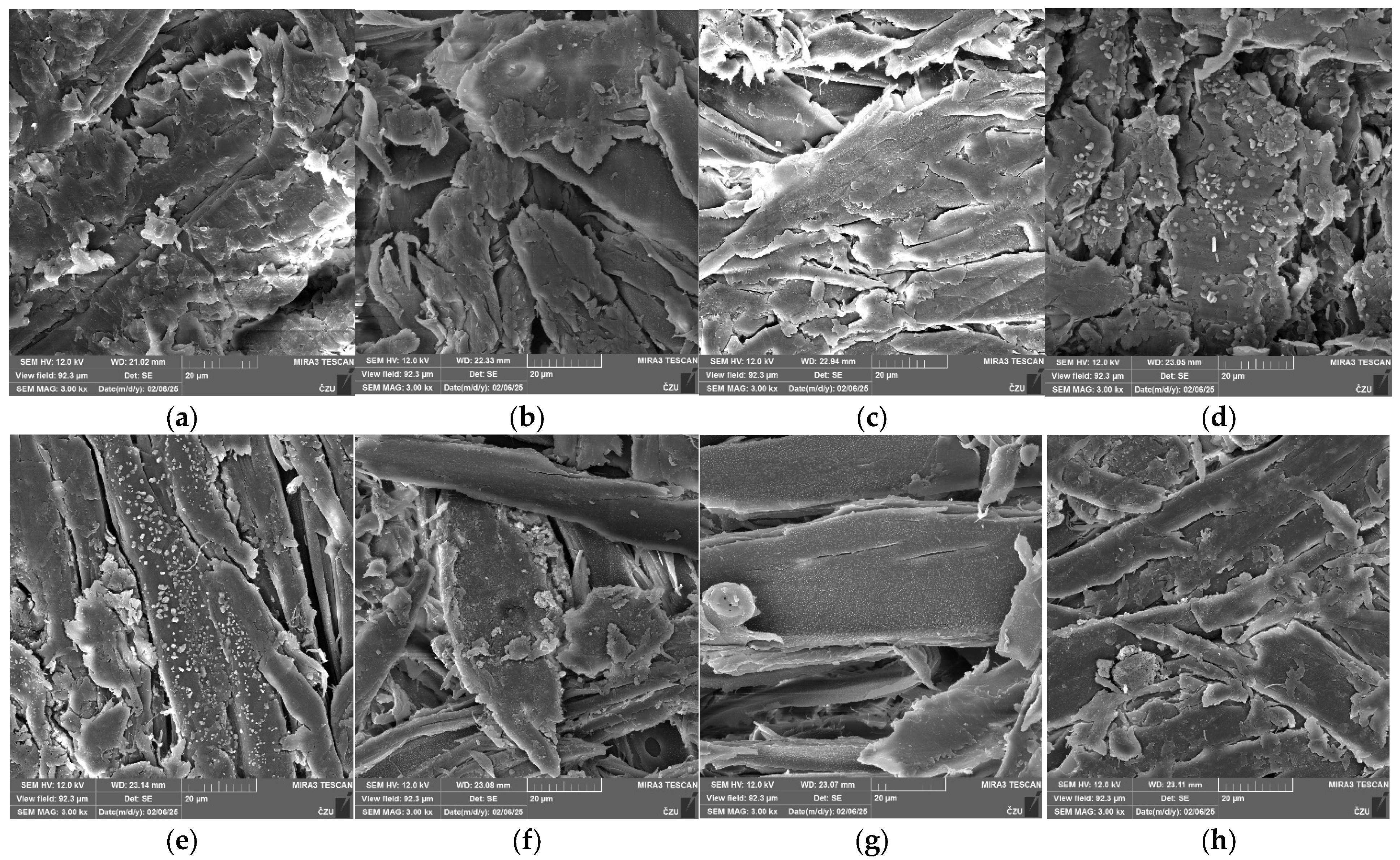
3.6. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, Z.; Huang, Q.; Zhang, Q. Life cycle assessment of mass timber construction: A review. Build. Environ. 2022, 221, 109320. [Google Scholar] [CrossRef]
- Omer, M.A.B.; Noguchi, T. A conceptual framework for understanding the contribution of building materials in the achievement of Sustainable Development Goals (SDGs). Sustain. Cities Soc. 2020, 52, 101869. [Google Scholar] [CrossRef]
- Ramagea, M.H.; Burridgeb, H.; Busse-Wicherc, M.; Feredaya, G.; Reynoldsa, T.; Shaha, D.U.; Wud, G.; Yuc, L.; Fleminga, P.; Densley-Tingleye, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Stokes, V.J.; Jinks, R.; Kerr, G. An analysis of conifer experiments in Britain to identify productive alternatives to Sitka spruce Forestry. Int. J. For. Res. 2023, 96, 170–187. [Google Scholar]
- Hilmers, T.; Biber, P.; Knoke, T.; Pretzsch, H. Assessing transformation scenarios from pure Norway spruce to mixed uneven-aged forests in mountain areas. Eur. J. For. Res. 2020, 139, 567–584. [Google Scholar] [CrossRef]
- Huber, C.; Langmaier, M.; Stadlmann, A.; Hochbichler, E.; Grabner, M.; Teischinger, A.; Konnerth, J.; Grabner, M.; Müller, U.; Pramreiter, M. Potential alternatives for Norway spruce wood: A selection based on defect-free wood properties. Ann. For. Sci. 2023, 80, 41. [Google Scholar] [CrossRef]
- Wilson, S.M.; Cameron, A.D. Alternative models for productive upland forestry. Model 2: Sitka spruce mixtures with alternative conifers. Scott. For. 2015, 69, 26–31. [Google Scholar]
- Fu, Y.; Zhang, Y.; Wang, Y. Improvement of flame-retardant and antistatic property of wood using a spray-assisted layer-by-layer self-assembly technique. Wood Mater. Sci. Eng. 2023, 18, 1553–1561. [Google Scholar] [CrossRef]
- Öhrn, O.; Sykam, K.; Gawusu, S.; Mensah, R.A.; Försth, M.; Shanmugam, V.; Babu, N.B.K.; Sas, G.; Jiang, L.; Xu, Q.; et al. Surface coated ZnO powder as flame retardant for wood: A short communication. Sci. Total Environ. 2023, 897, 165290. [Google Scholar] [CrossRef]
- Bednarek, Z.; Kaliszuk-Wietecka, A. Analysis of the fire-protection impregnation influence on wood strength. J. Civ. Eng. Manag. 2007, 13, 79–85. [Google Scholar] [CrossRef]
- Wen, M.Y.; Kang, C.W.; Park, H.J. Impregnation and mechanical properties of three softwoods treated with a new fire retardant chemical. J. Wood Sci. 2014, 60, 367–375. [Google Scholar] [CrossRef]
- Yorur, H.; Kayahan, K. Improving impregnation and penetration properties of refractory woods through cryogenic treatment. BioResources 2018, 13, 1829–1842. [Google Scholar] [CrossRef]
- Marozau, A.; Mielcarek, M.; Krok, G.; Paluch, R.; Chiliński, K. European silver fir—An alternative for the dying Norway spruce in Białowieza Forest? Folia For. Pol. Ser. A—For. 2021, 63, 150–166. [Google Scholar] [CrossRef]
- Keržič, E.; Humar, M. Studies on the material resistance and moisture dynamics of wood after artificial and natural weathering, Wood Mater. Sci. Eng. 2022, 17, 551–557. [Google Scholar]
- Marais, B.N.; Brischke, C.; Militz, H. Wood durability in terrestrial and aquatic environments—A review of biotic and abiotic influence factors. Wood Mater. Sci. Eng. 2022, 17, 82–105. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jiang, L.; Renner, J.S.; Xu, Q. Characterisation of the fire behaviour of wood: From pyrolysis to fire retardant mechanisms. J. Therm. Anal. Calorim. 2023, 148, 1407–1422. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Chen, H. Research on fire resistance and material model development of CLT components based on OpenSees. J. Build. Eng. 2022, 45, 103670. [Google Scholar] [CrossRef]
- Augustina, S.; Darmawan, T.; Sudarmanto; Narto, N.; Bahanawan, A.; Danang, S.A.; Triwibowo, D.; Amin, Y.; Sofianto, I.A.; Sejati, P.S.; et al. Effects of succinic acid impregnation on physical properties of sapwood and heartwood from plantation-grown short-rotation teak. South. For. J. For. Sci. 2023, 85, 201–211. [Google Scholar] [CrossRef]
- Lin, C.; Karlsson, O.; Das, O.; Mensah, R.A.; Mantanis, G.I.; Jones, D.; Antzutkin, O.N.; Försth, M.; Sandberg, D. High leach-resistant fire-retardant modified pine wood (Pinus sylvestris L.) by in situ phosphorylation and carbamylation. ACS Omega 2023, 8, 11381–11396. [Google Scholar] [CrossRef]
- Elrhayam, Y.; Elharf, A. 3D-QSAR studies of the chemical modification of hydroxyl groups of biomass (cellulose, hemicelluloses and lignin) using quantum chemical descriptor. Heliyon 2019, 5, e02173. [Google Scholar] [CrossRef]
- Augustina, S.; Dwianto, W.; Wahyudi, I.; Syafii, W.; Gérardin, P.; Marbun, S.D. Wood impregnation in relation to its mechanisms and properties enhancement. BioResources 2023, 18, 4332–4372. [Google Scholar] [CrossRef]
- Awais, M.; Altgen, M.; Belt, T.; Teräväinen, V.; Mäkelä, M.; Altgen, D.; Nopens, M.; Rautkari, L. Wood-water relations affected by anhydride and formaldehyde modification of wood. ASC Omega 2022, 7, 42199–42207. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.S.; Jones, D.; Strickland, G.; Cetin, N.S. Kinetic and mechanistic aspects of the acetylation of wood with acetic anhydride. Holzforschung 1998, 52, 623–629. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Hill, C.A.S. The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora puteana. Holz Als Roh Werkst. 2002, 60, 329–332. [Google Scholar] [CrossRef]
- Roussel, C.; Marchetti, V.; Lemor, A.; Wozniak, E.; Loubinoux, B.; Gérardin, P. Chemical modification of wood by polyglycerol/maleic anhydride treatment. Holzforschung 2005, 55, 57–62. [Google Scholar] [CrossRef]
- Teacǎ, C.A.; Tanasǎ, F. Wood surface modification—Classic and modern approaches in wood chemical treatment by esterification reactions. Coatings 2020, 10, 629. [Google Scholar] [CrossRef]
- Nazari, M.; Jebrane, M.; Terziev, N. Solid wood impregnated with a bio-based phase change material for low temperature energy storage in building application. J. Therm. Anal. Calorim. 2022, 147, 10677–10692. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, Z.; Chen, C.; Guo, C.; Wang, X.; Zhou, Q.; Tu, D. Effective strategy for fabricating surface impregnated and unilaterally densified wood with furfuryl alcohol/flame retardants for enhanced mechanical performance and flame retardancy. Eur. J. Wood Wood Prod. 2024, 82, 731–745. [Google Scholar] [CrossRef]
- Liu, J.; Kong, X.; Wang, C.; Yang, X. Permeability of wood impregnated with polyethylene wax emulsion in vacuum. Polymers 2023, 281, 126123. [Google Scholar] [CrossRef]
- Tondi, G.; Thevenon, M.F.; Mies, B.; Standfest, G.; Petutschnigg, A.; Wieland, S. Impregnation of Scots pine and beech with tannin solutions: Effect of viscosity and wood anatomy in wood infiltration. Wood Sci. Technol. 2013, 47, 615–626. [Google Scholar] [CrossRef]
- Özcan, C.; Kurt, Ş.; Esen, R.; Korkmaz, M. The determinated combustion properties of fir wood impregnated with fire-retardants. Online J. Sci. Technol. 2016, 6, 77–82. [Google Scholar]
- Brahmia, F.Z.; Zsolt, K.; Horváth, P.G.; Alpár, T.L. Comparative study on fire retardancy of various wood species treated with PEG 400, phosphorus, and boron compounds for use in cement-bonded wood-based products. Surf. Interfaces 2020, 21, 100736. [Google Scholar] [CrossRef]
- Stevens, R.; Es, D.S.; Bezemer, R.; Kranenbarg, A. The structure–activity relationship of fire retardant phosphorus compounds in wood. Polym. Degrad. Stab. 2006, 91, 832–841. [Google Scholar] [CrossRef]
- Liang, Y.; Jian, H.; Deng, C.; Xu, J.; Liu, Y.; Park, H.; Wen, M.; Sun, Y. Research and application of biomass-based wood flame retardants: A review. Polymers 2023, 15, 950. [Google Scholar] [CrossRef]
- Yi, X.; Cao, S.; Hao, X.; Wang, Z.; Huang, Y.; Li, L.; Guo, C. Dimensionally stable, flame-retardant, and leach-resistant furfurylated wood prepared by incorporating ammonium polyphosphate and nano-silica. Polym. Adv. Technol. 2023, 34, 2501–2514. [Google Scholar] [CrossRef]
- Lin, C.F.; Karlsson, O.; Kim, I.; Myronycheva, O.; Mensah, R.A.; Försth, M.; Das, O.; Mantanis, G.I.; Jones, D.; Sandberg, D. Fire retardancy and leaching resistance of furfurylated pine wood (Pinus sylvestris L.) treated with guanyl-urea phosphate. Polymers 2022, 14, 1829. [Google Scholar] [CrossRef]
- Hazlewood, F.J.; Rhodes, E.; Ubbelohde, A.R. Melting mechanisms and melt properties of alkali acetates. Trans. Faraday Soc. 1966, 62, 3101–3113. [Google Scholar] [CrossRef]
- Hosseinashrafi, S.K.; Hosseinihashemi, S.K.; Gorji, P.; Akhtari, M. Environment-friendly waterborne fire retardants for protection of wood and bark against fire flames. BioResources 2023, 18, 7681–7699. [Google Scholar] [CrossRef]
- Leong, W.I.; Lo, O.L.I.; Cheng, F.T.; Cheong, W.M.; Seak, L.C.U. Using recombinant adhesive proteins as durable and green flame-retardant coatings. Synth. Syst. Biotechnol. 2021, 6, 369–376. [Google Scholar] [CrossRef]
- Gaff, M.; Kačík, F.; Gašparík, M.; Torado, L.; Jones, D.; Corleto, R.; Makovická Osvaldivá, L.; Čekovská, H. The effect of synthetic and natural fire-retardants on burning and chemical characteristics of thermally modified teak (Tectona grandis L. f.) wood. Constr. Build. Mater. 2019, 200, 551–558. [Google Scholar] [CrossRef]
- Gaff, M.; Čekovská, H.; Bouček, J.; Kačíková, D.; Kubovský, I.; Tribulová, T.; Zhang, L.; Marino, S.; Kačík, F. Flammability Characteristics of Thermally Modified Meranti Wood Treated with Natural and Synthetic Fire Retardants. Polymers 2021, 13, 2160. [Google Scholar] [CrossRef] [PubMed]
- Maake, T.; Asante, J.K.O.; Mhike, W.; Mwakikunga, B. Fire-Retardant Wood Polymer Composite to Be Used as Building Materials for South African Formal and Informal Dwellings—A Review. Fire 2025, 8, 81. [Google Scholar] [CrossRef]
- Hiremath, V.S.; Reddy, D.M.; Mutra, R.R.; Sanjeev, A.; Dhilipkumar, T.; Naveen, J. Thermal degradation and fire retardant behaviour of natural fibre reinforced polymeric composites—A comprehensive review. J. Mater. Res. Technol. 2024, 30, 4053–4063. [Google Scholar] [CrossRef]
- Bifulco, A.; Climaco, I.; Casciello, A.; Passaro, J.; Battegazzore, D.; Nebbioso, V.; Russo, P.; Imparato, C.; Aronne, A.; Malucelli, G. Prediction and validation of fire parameters for a self-extinguishing and smoke suppressant electrospun PVP-based multilayer material through machine learning models. J. Mater. Sci. 2023, 60, 1019–1040. [Google Scholar] [CrossRef]
- Huo, S.; Guo, Y.; Yang, Q.; Wang, H.; Song, P. Two-dimensional nanomaterials for flame-retardant polymer composites: A mini review. Adv. Nanocompos. 2024, 1, 240–247. [Google Scholar] [CrossRef]
- Čabalová, I.; Zachar, M.; Bélik, M.; Balážová, Ž. Resistance of spruce wood (Picea abies L.) treated with a flame retardants after the radiant heat exposure. Acta Fac. Xylologiae 2021, 63, 103–116. [Google Scholar]
- Heejun, P.; Mingyu-Wen, M.W.; Cheon, S.H.; Hwang, J.W.; Oh, S.W. Flame Retardant Performance of Wood Treated with Flame Retardant Chemicals. J. Korean Wood Sci. Technol. 2012, 40, 311–318. [Google Scholar]
- Kmeťová, E.; Kačíková, D.; Kačík, F. The Progressive Test Method for Assessing the Thermal Resistance of Spruce Wood Treated with Flame Retardants. In Wood & Fire Safety; Springer: Cham, Switzerland, 2024; pp. 146–153. [Google Scholar]
- Luptáková, J.; Kačík, F.; Mitterová, I.; Zachar, M. Influence of temperature of thermal modification on the fire-technical characteristics of spruce wood. BioResources 2019, 14, 3795–3807. [Google Scholar] [CrossRef]
- Wu, M.; Emmerich, L.; Kukowiak, K.; Militz, H. Fire resistance of pine wood treated with phenol-formaldehyde resin and phosphate-based flame retardant. Wood Mater. Sci. Eng. 2023, 18, 1933–1939. [Google Scholar] [CrossRef]
- Baysal, E.; Altinok, M.; Colak, M.; Ozaki, S.K.; Toker, H. Fire resistance of Douglas fir (Pseudotsuga menzieesi) treated with borates and natural extractives. Bioresour. Technol. 2007, 98, 1101–1105. [Google Scholar] [CrossRef]
- Marino, S.; Gaff, M.; Sethy, A.K.; Kamboj, G.; Rezaei, F.; Kačík, F.; Hosseini, S.B.; Li, H.; Hui, D. Enhancing the fire resistance properties of thermally modified Robinia pseudoacacia wood with natural and synthetic flame retardants: Chemical characterisation and fire behaviour. Eur. J. Wood Wood Prod. 2024, 82, 1145–1157. [Google Scholar] [CrossRef]
- Liu, C.; Wei, L.; Ma, B.; Zhang, Y.; Jiad, X. Effect of diammonium hydrogen phosphate coated with silica on flame retardancy of epoxy resin. RSC Adv. 2024, 14, 28965–28975. [Google Scholar] [CrossRef]
- Suardana, N.P.G.; Ku, M.S.; Lim, J.K. Effects of diammonium phosphate on the flammability and mechanical properties of bio-composites. Mater. Des. 2011, 32, 1990–1999. [Google Scholar] [CrossRef]
- Gaan, S.; Sun, G. Effect of phosphorus flame retardants on thermo-oxidative decomposition of cotton. Polym. Degrad. Stab. 2007, 92, 968–974. [Google Scholar] [CrossRef]
- Terzi, E.; Kartal, S.N.; White, R.; Shinoda, K.; Imamura, Y. Fire performance and decay resistance of solid wood and plywood treated with quaternary ammonia compounds and common fire retardants. Eur. J. Wood Prod. 2011, 69, 41–51. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Ma, L.; Ge, H.; Zhu, G.; Zhu, Z. Full bio-based flame retardant towards multifunctional polylactic acid: Crystallization, flame retardant, antibacterial and enhanced mechanical properties. Int. J. Biol. Macromol. 2024, 280, 135891. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Hiziroglu, S.; Waisurasingha, C.; Kasemsiri, P. Properties of wood flour/expanded polystyrene waste composites modified with diammonium phosphate flame retardant. Polym. Compos. 2014, 36, 604–612. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal, and Other Processes; John Eiley and Sons: Hoboken, NJ, USA, 2006; p. 260. [Google Scholar]
- Li, M.Y.; Cheng, S.C.; Li, D.; Wang, S.N.; Huang, A.M.; Sun, S.Q. Structural characterization of steam-heat treated Tectona grandis wood analyzed by FT-IR and 2D-IR correlation spectroscopy. Chin. Chem. Lett. 2015, 26, 221–225. [Google Scholar] [CrossRef]
- Lopes, J.Q.; Garcia, R.A.; Dias, S.N. Infrared spectroscopy of the surface of thermally-modified teak juvenile wood. Maderas. Cienc. Tecnol. 2018, 20, 737–746. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100–168. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Liu, L.; Ma, C.; Zhang, H.; Zhang, Y.; Song, R.; Fang, Z.; Song, P. A lysine-derived flame retardant for improved flame retardancy, crystallinity, and aqueous-phase degradation of polylactide. Chem. Eng. J. 2023, 462, 142189. [Google Scholar] [CrossRef]
- Tappi T 280 wd-06; Acetone Extractives of Wood and Pulp. Tappi Test Methods: Atlanta, GA, USA, 2015.
- Tappi T 6 wd-73; Alcohol-Benzene Solubility of Wood. Tappi Test Methods: Atlanta, GA, USA, 2015.
- Tappi T 222 om-11; Acid-Insoluble Lignin in Wood and Pulp. Tappi Test Methods: Atlanta, GA, USA, 2006.
- Tappi T 13 wd-74; Lignin in Wood. Tappi Test Methods: Atlanta, GA, USA, 2015.
- Seifert, K. Uber ein neues Verfahren zur Schnellbestimmung Der Rein-Cellulose. Das Pap. 1956, 10, 301–306. [Google Scholar]
- Wright, P.J.; Wallis, A.F.A. Rapid determination of cellulose in plantation eucalypt woods to predict kraft pulp yields. Tappi J. 1998, 81, 26–30. [Google Scholar]
- Tappi T 203 cm-09; Alpha-, Beta- and Gamma-Cellulose in Pulp. Tappi Test Methods: Atlanta, GA, USA, 2009.
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Tribulová, T.; Kačík, F.; Evtuguin, D.V.; Čabalová, I.; Ďurkovič, J. The effects of transition metal sulfates on cellulose crystallinity during accelerated aging of silver fir wood. Cellulose 2019, 26, 2625–2638. [Google Scholar] [CrossRef]
- BS 2782-0; Methods of Testing Plastic—Introduction. The British Standards Institution: London, UK, 2011.
- ISO 4589-2; Plastics—Determination of burning Behaviour—Oxygen Index Method. International Organization for Standardization: London, UK, 2017.
- Yuan, J.M.; Feng, Y.R.; He, L.P. Effect of thermal treatment on properties of ramie fibers. Polym. Degrad. Stab. 2016, 133, 303–311. [Google Scholar] [CrossRef]
- Červenka, E.; Král, Z.; Tomis, B. Chemistry of Wood and Cellulose I–III; University of Pardubice: Pardubice, Czech Republic, 1980; p. 228. (In Czech) [Google Scholar]
- Kučerová, V.; Lagaňa, R.; Výbohová, E.; Hýrošová, T. The effect of chemical changes during heat treatment on the color and mechanical properties of fir wood. BioResources 2016, 11, 9079–9094. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, A.; Wang, S.; Zhang, Q. Effect of different heat treatment temperatures on the chemical composition and structure of Chinese fir wood. BioResources 2016, 11, 4006–4016. [Google Scholar] [CrossRef]
- Hrčka, R.; Kučerová, V.; Hýrošová, T.; Hönig, V. Cell wall saturation limit and selected properties of thermally modified oak wood and cellulose. Forests 2020, 11, 640. [Google Scholar] [CrossRef]
- Kačík, F.; Šmíra, P.; Kačíková, D.; Veľková, V.; Nasswettrová, A.; Vacek, V. Chemical alterations of pine wood saccharides during heat sterilisation. Carbohydr. Polym. 2015, 117, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, G.; Zhang, M.; Chen, M.; Li, D.; Min, F.; Chen, M.; Zhang, S.; Ren, Z.; Yan, Y. A comparative study of thermolysis characteristics and kinetics of seaweeds and fir wood. Process Biochem. 2006, 41, 1883–1886. [Google Scholar] [CrossRef]
- Pappa, A.A.; Tzamtzis, N.E.; Statheropoulos, M.K.; Parissakis, G.K. Thermal analysis of Pinus halepensis pine needles and their main components in the presence of (NH4)2HPO4 and (NH4)2SO4. Thermochim Acta 1995, 261, 165–173. [Google Scholar] [CrossRef]
- Szabó, P.; Várhegyi, G.; Till, F.; Faix, O. Thermogravimetric/mass spectrometric characterization of two energy crops, Arundo donax and Miscanthus sinensis. J. Anal. Appl. Pyrolysis 1996, 36, 179–190. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Z.; Feng, A.; Hao, X.; Ou, R.; Sun, L.; Liu, T.; Wang, Q. Flame retardant modification of poplar wood based on sustainable impregnation solution with high biomass content. Ind. Crops Prod. 2024, 215, 118616. [Google Scholar] [CrossRef]
- Strehler, A. Technologies of wood combustion. Ecol. Eng. 2000, 16, S25–S40. [Google Scholar] [CrossRef]
- Paredes, R.; Castells, B.; Tascón, A. Thermogravimetric assessment of biomass: Unravelling kinetic, chemical composition and combustion profiles. Fire 2024, 7, 396. [Google Scholar] [CrossRef]
- Aniszewska, M.; Gendek, A. Comparison of heat pf combustion and calorific value of the cones and wood of selected forest trees species. For. Res. Pap. 2014, 75, 231–236. [Google Scholar]
- Zhuikov, A.; Pyanykh, T.; Grishina, I.; Chicherin, S.; Zhuikova, Y. Thermogravimetric analysis of combustion of semi-coke obtained from coniferous wood and mixtures on their basis. Fire 2024, 7, 385. [Google Scholar] [CrossRef]
- Cavdar, A.D. Effect of various wood preservatives on limiting oxygen index levels of fir wood. Measurement 2014, 50, 279–284. [Google Scholar] [CrossRef]

| Retardants/Properties of Retardants | Diammonium Hydrogen Phosphate (DAP) | Potassium Acetate (PAc) | Arabinogalactan (AG) |
|---|---|---|---|
| Chemical formula | (NH4)2HPO4 | CH3CO2K | C20H36O14 |
| Characterization | inorganic substances | organic substances | natural polymer |
| Molar mass [g∙mol−1] | 132.06 | 98.15 | 285.29 |
| Viscosity [mPa∙s] | 0.0012–0.0018 | 0.0030–0.0034 | 0.0100–0.0120 |
| Density [kg∙m−3] | 1619 | 1570 | 1200 |
| Melting point (decomposes) [°C] | 155 ± 5 | 292 ± 5 | 181 ± 30 |
| Appearance | colorless monoclinic crystals | white crystals | white powder |
| Application of Retardants | Retention, % |
|---|---|
| Coating 5% DAP | 0.20 (0.04) |
| Coating 15% DAP | 0.33 (0.06) |
| Impregnation 5% DAP | 1.36 (0.11) |
| Impregnation 15% DAP | 2.32 (0.16) |
| Impregnation 5% PAc | 2.31 (0.08) |
| Impregnation 15% PAc | 3.98 (0.19) |
| Impregnation 5% AG | 1.80 (0.15) |
| Component | Extractives Acetone, % | Extractives Ethanol- Toluene, % | Lignin, % | Cellulose, % | Alpha- Cellulose, % | Beta- Cellulose, % | Gamma- Cellulose, % |
|---|---|---|---|---|---|---|---|
| Fir wood | 2.61 (0.08) | 1.21 (0.03) | 26.84 (0.78) | 47.67 (1.18) | 38.86 (0.96) | 19.96 (2.37) | 9.58 (1.36) |
| Type and Treatment with Retardant | TCI | ILO | HBI |
|---|---|---|---|
| Reference | 1.7855 (0.126) | 0.5728 (0.056) | 1.0387 (0.121) |
| Coating 5% DAP | 1.0268 (0.008) | 1.0636 (0.007) | 1.0075 (0.002) |
| Coating 15% DAP | 1.0173 (0.005) | 1.0798 (0.011) | 1.0082 (0.003) |
| Impregnation 5% DAP | 0.9957 (0.016) | 1.0848 (0.002) | 1.0010 (0.010) |
| Impregnation 15% DAP | 1.0208 (0.002) | 1.1107 (0.024) | 1.0167 (0.010) |
| Impregnation 5% PAc | 0.9922 (0.007) | 1.0688 (0.013) | 1.0145 (0.001) |
| Impregnation 15% PAc | 0.9637 (0.016) | 0.9713 (0.014) | 1.0032 (0.016) |
| Impregnation 5% AG | 1.0163 (0.007) | 1.0434 (0.030) | 0.9827 (0.010) |
| Component | Reference | Coating | Impregnation | |||||
|---|---|---|---|---|---|---|---|---|
| 5% DAP | 15% DAP | 5% DAP | 15% DAP | 5% PAc | 15% PAc | 5% AG | ||
| Fir wood | 20.75 | 22.58 | 23.49 | 23.55 | 27.75 | 24.40 | 28.70 | 21.88 |
| (0.25) | (0.08) | (0.11) | (0.15) | (0.05) | (0.40) | (0.30) | (0.08) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hájková, K.; Šedivka, P.; Holeček, T.; Berčák, R.; Sahula, L. The Effect of Chemical Modification by Synthetic and Natural Fire-Retardants on Burning and Chemical Characteristics of Structural Fir (Abies alba L.) Wood. Fire 2025, 8, 116. https://doi.org/10.3390/fire8030116
Hájková K, Šedivka P, Holeček T, Berčák R, Sahula L. The Effect of Chemical Modification by Synthetic and Natural Fire-Retardants on Burning and Chemical Characteristics of Structural Fir (Abies alba L.) Wood. Fire. 2025; 8(3):116. https://doi.org/10.3390/fire8030116
Chicago/Turabian StyleHájková, Kateřina, Přemysl Šedivka, Tomáš Holeček, Roman Berčák, and Lukáš Sahula. 2025. "The Effect of Chemical Modification by Synthetic and Natural Fire-Retardants on Burning and Chemical Characteristics of Structural Fir (Abies alba L.) Wood" Fire 8, no. 3: 116. https://doi.org/10.3390/fire8030116
APA StyleHájková, K., Šedivka, P., Holeček, T., Berčák, R., & Sahula, L. (2025). The Effect of Chemical Modification by Synthetic and Natural Fire-Retardants on Burning and Chemical Characteristics of Structural Fir (Abies alba L.) Wood. Fire, 8(3), 116. https://doi.org/10.3390/fire8030116






