Pilot Ignition of Ammonia Spray Using Dimethyl Ether Spray at Elevated Temperature: A Numerical Study
Abstract
1. Introduction
2. Numerical Approach
2.1. Schematic and Simulation Schemes
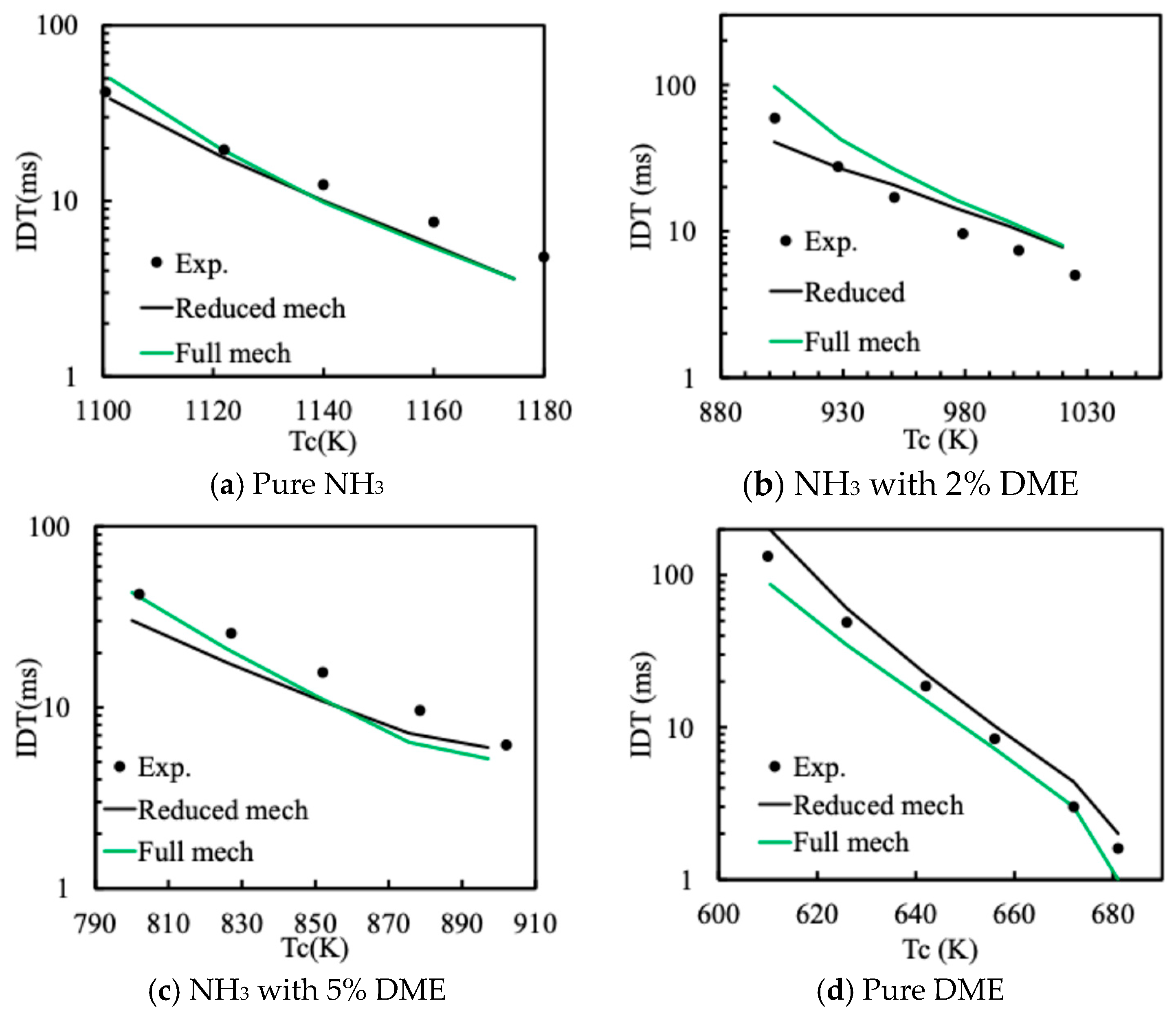
2.2. Mechanism Reduction and Validation
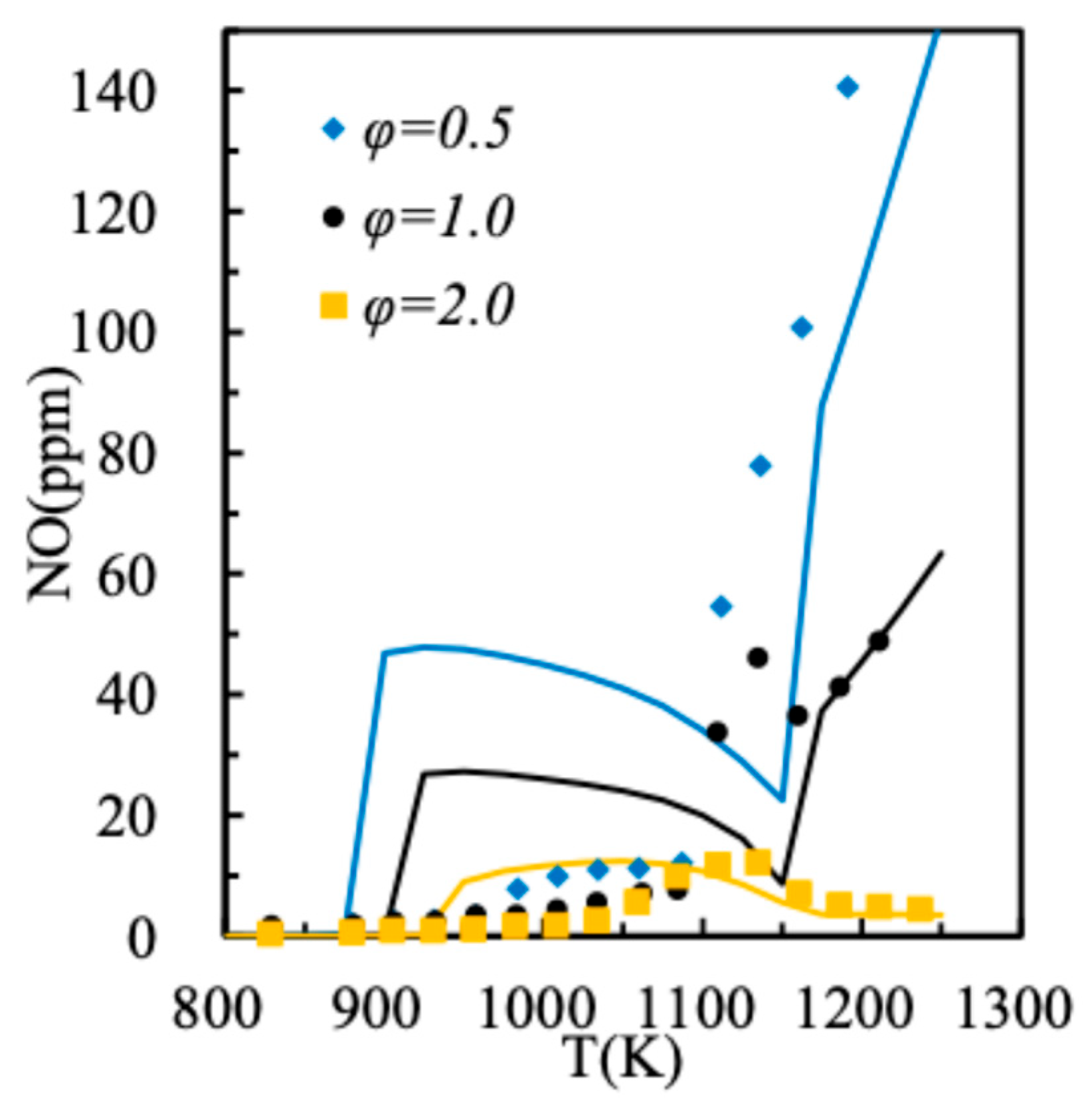
2.3. Spray Model Verification
3. Results and Discussion
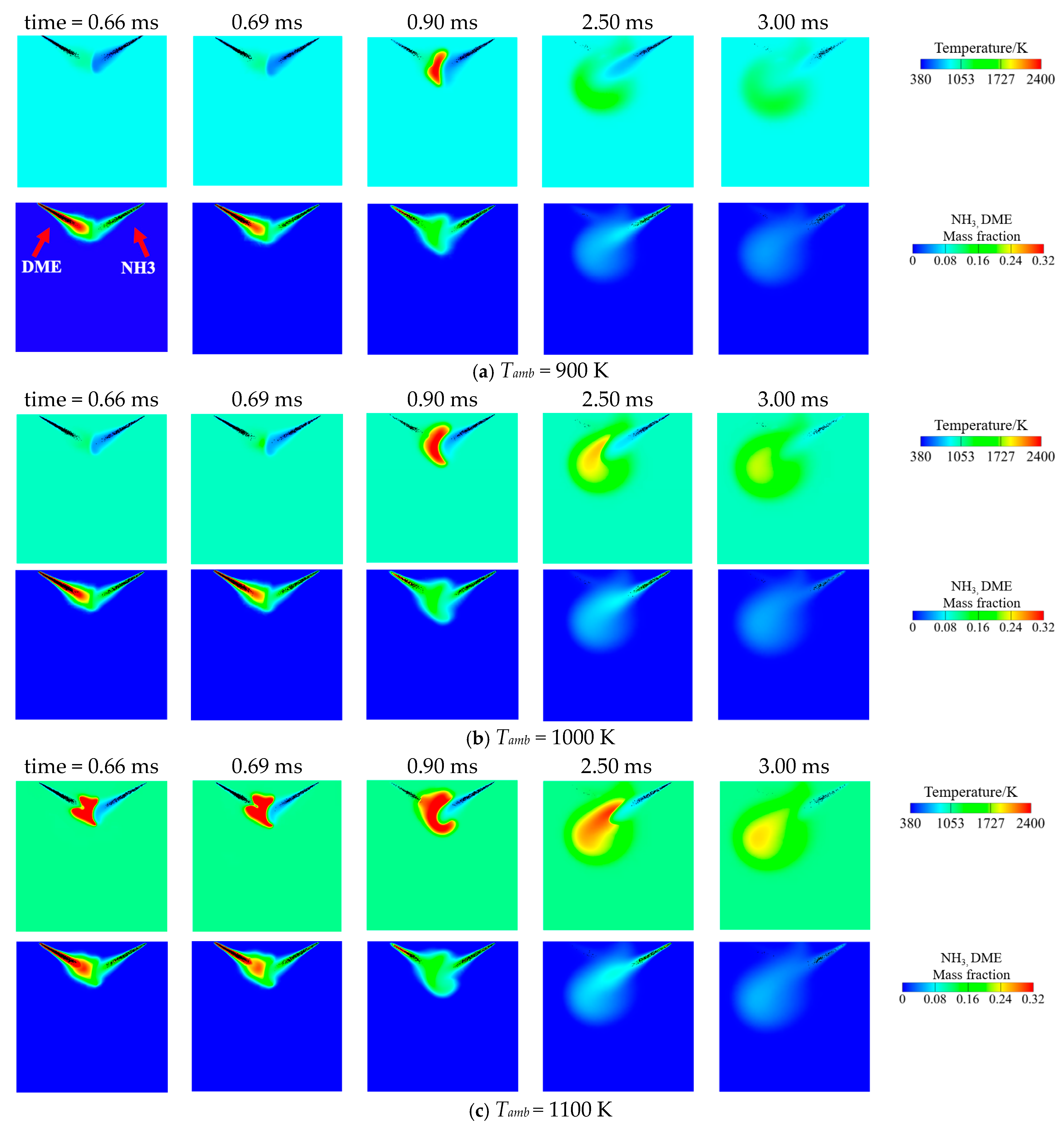
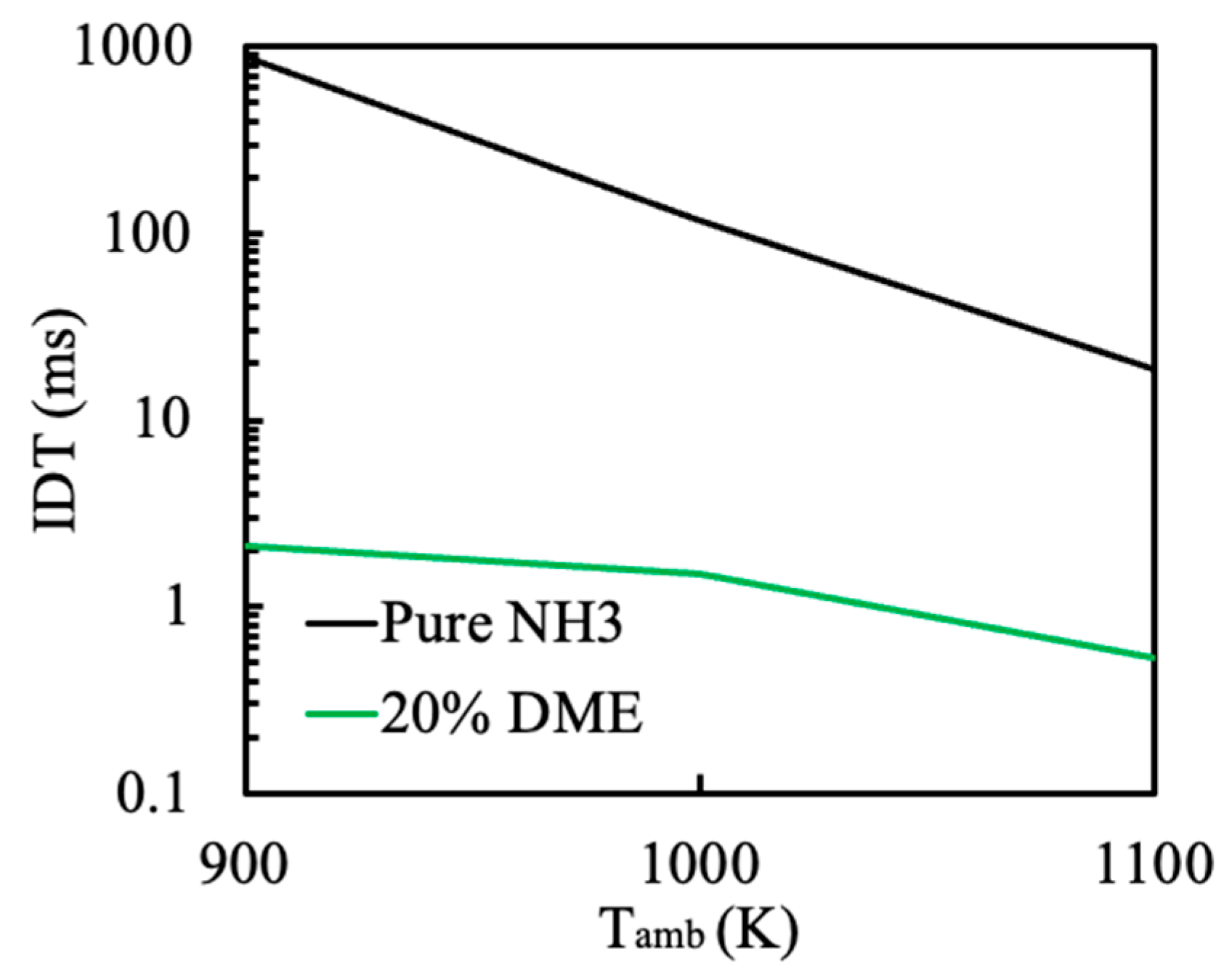
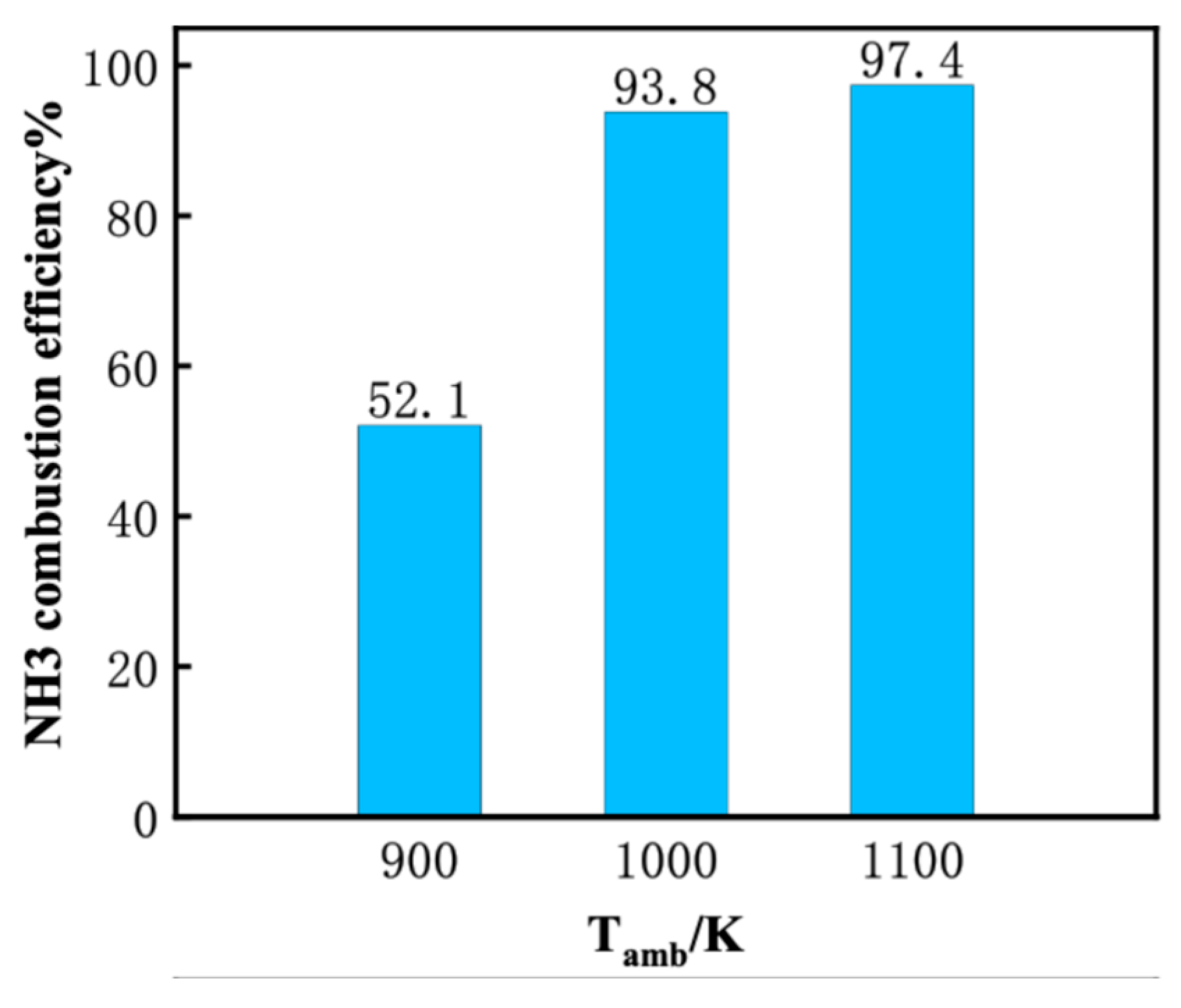
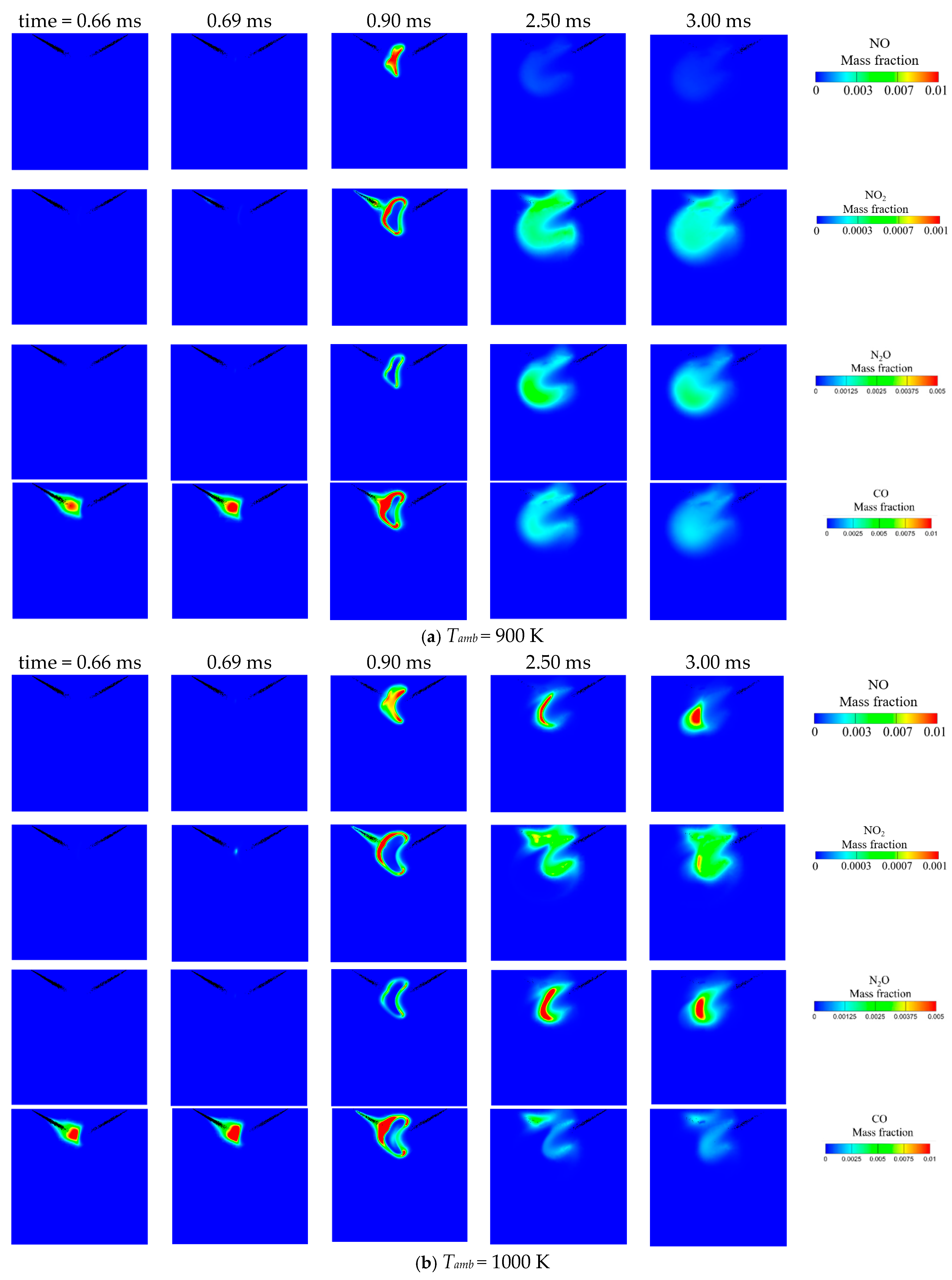
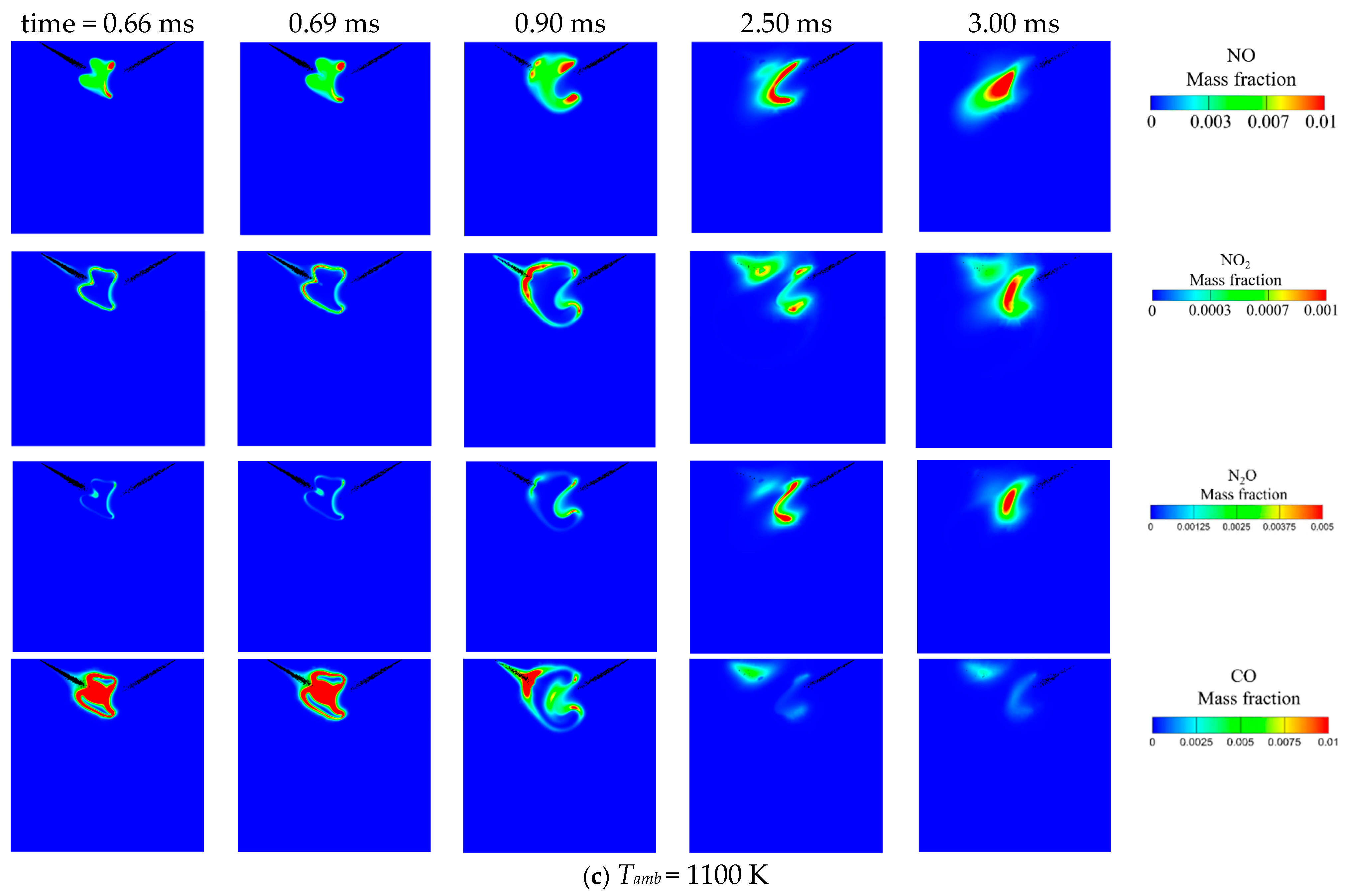
3.1. Effect of Ambient Temperature on the Ignition Process
3.2. Effect of AER at Tamb = 1100 K
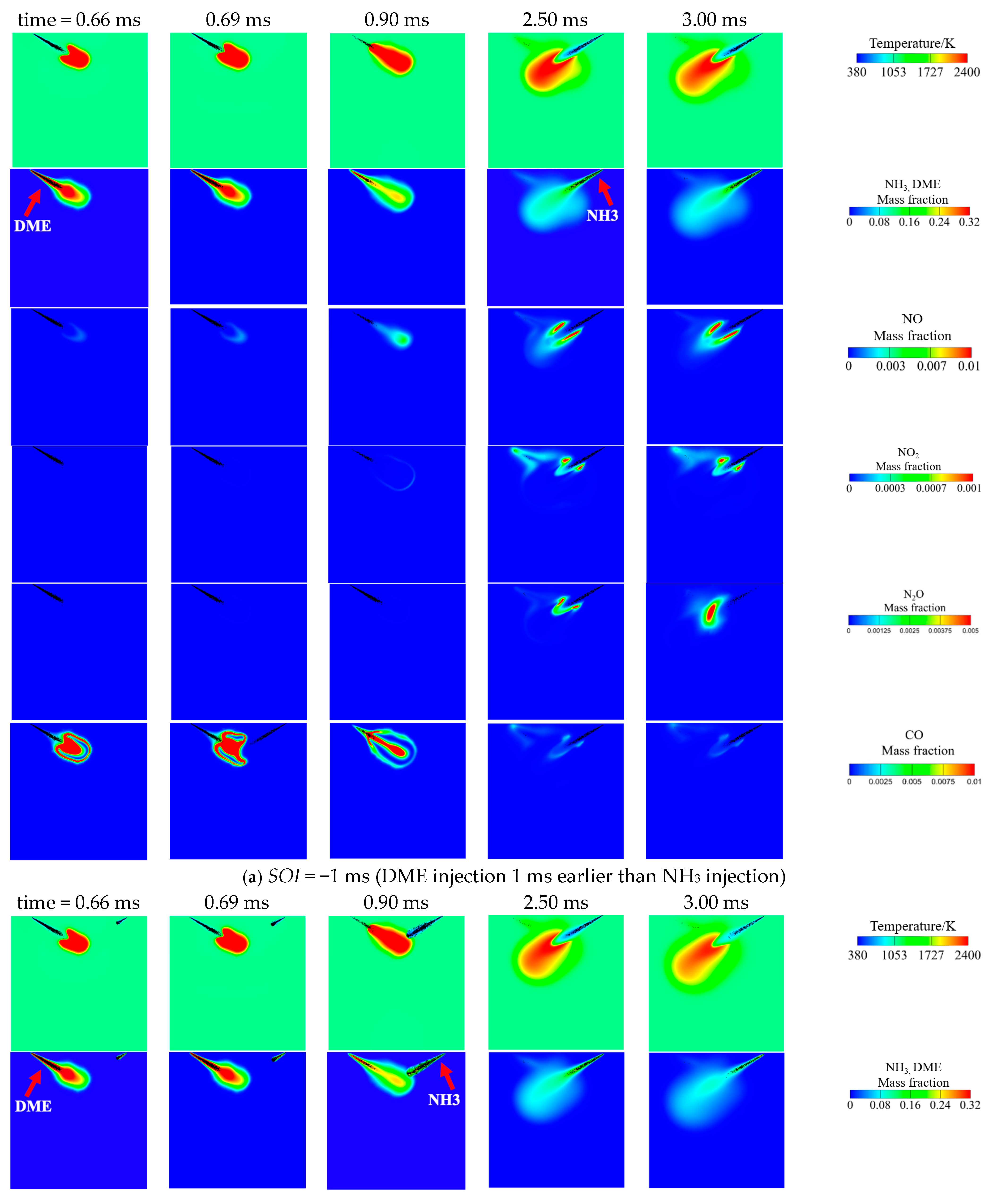
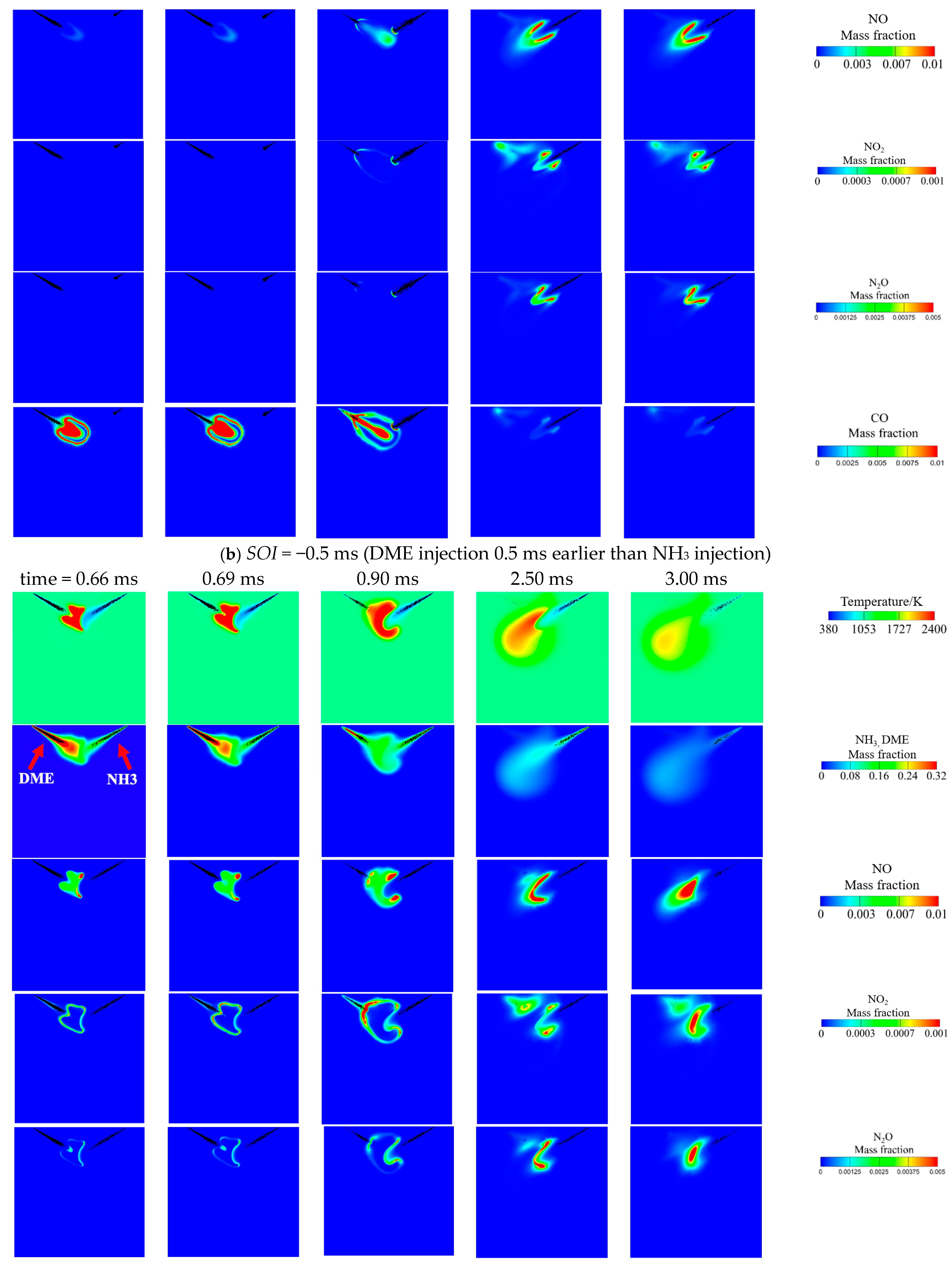
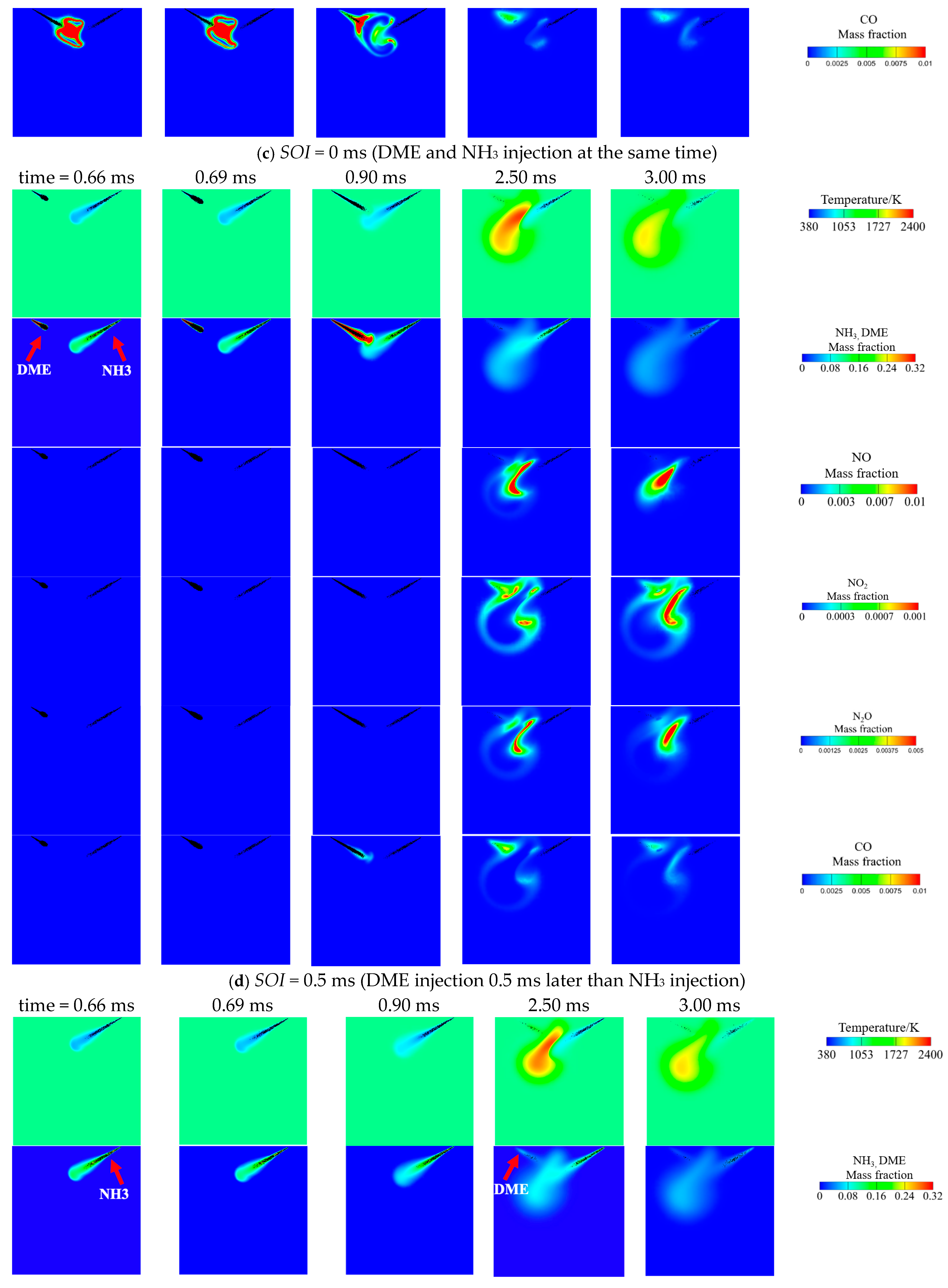
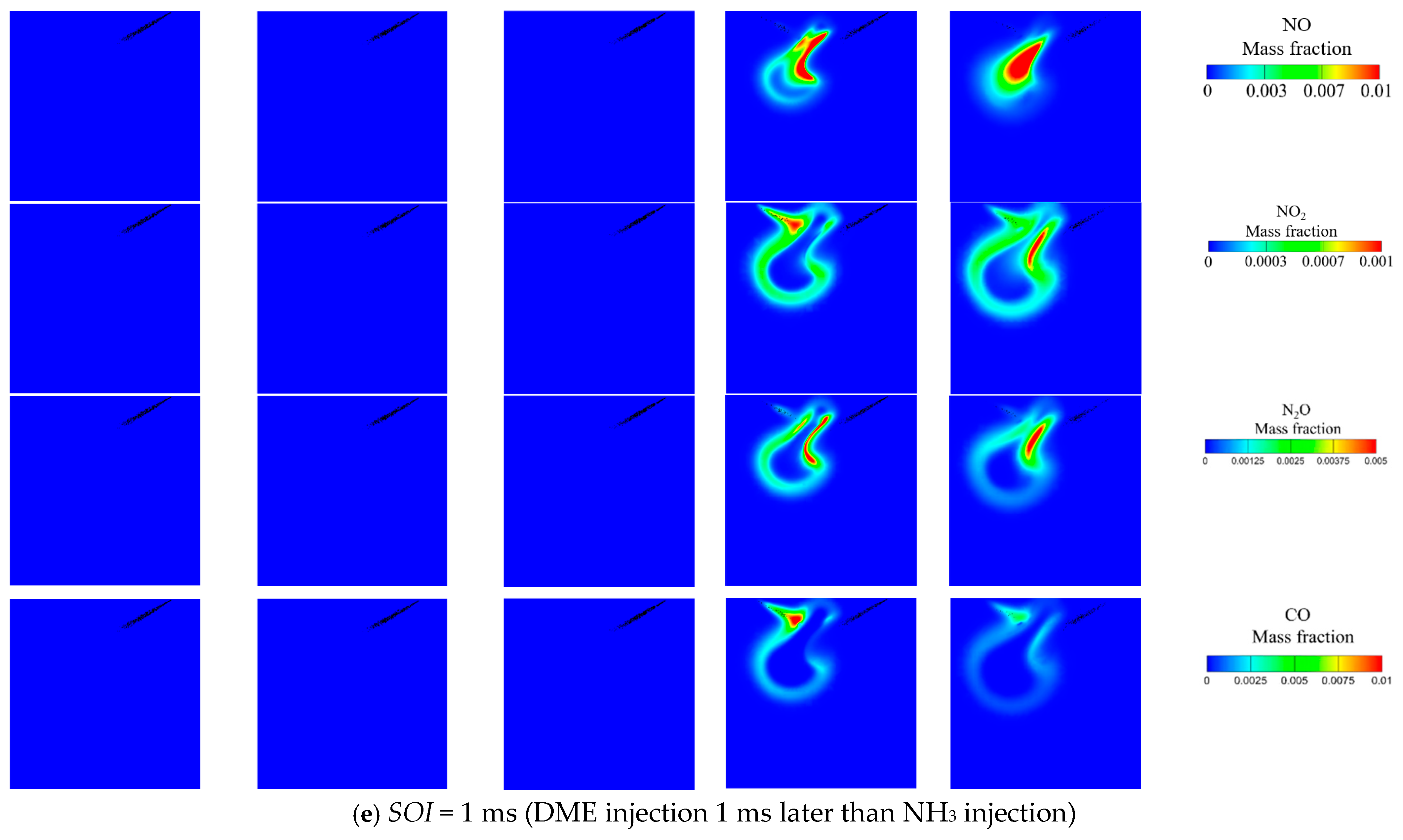
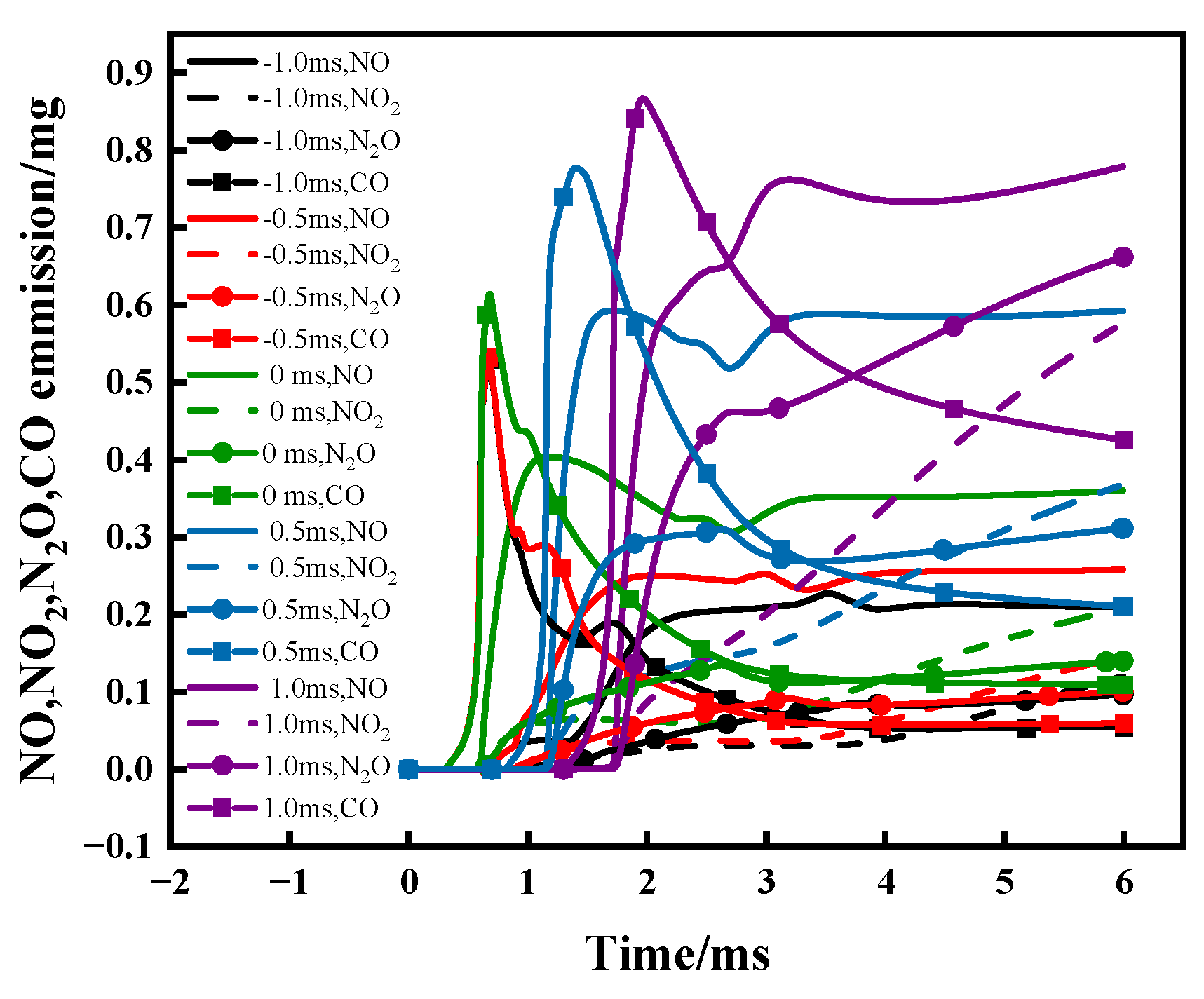
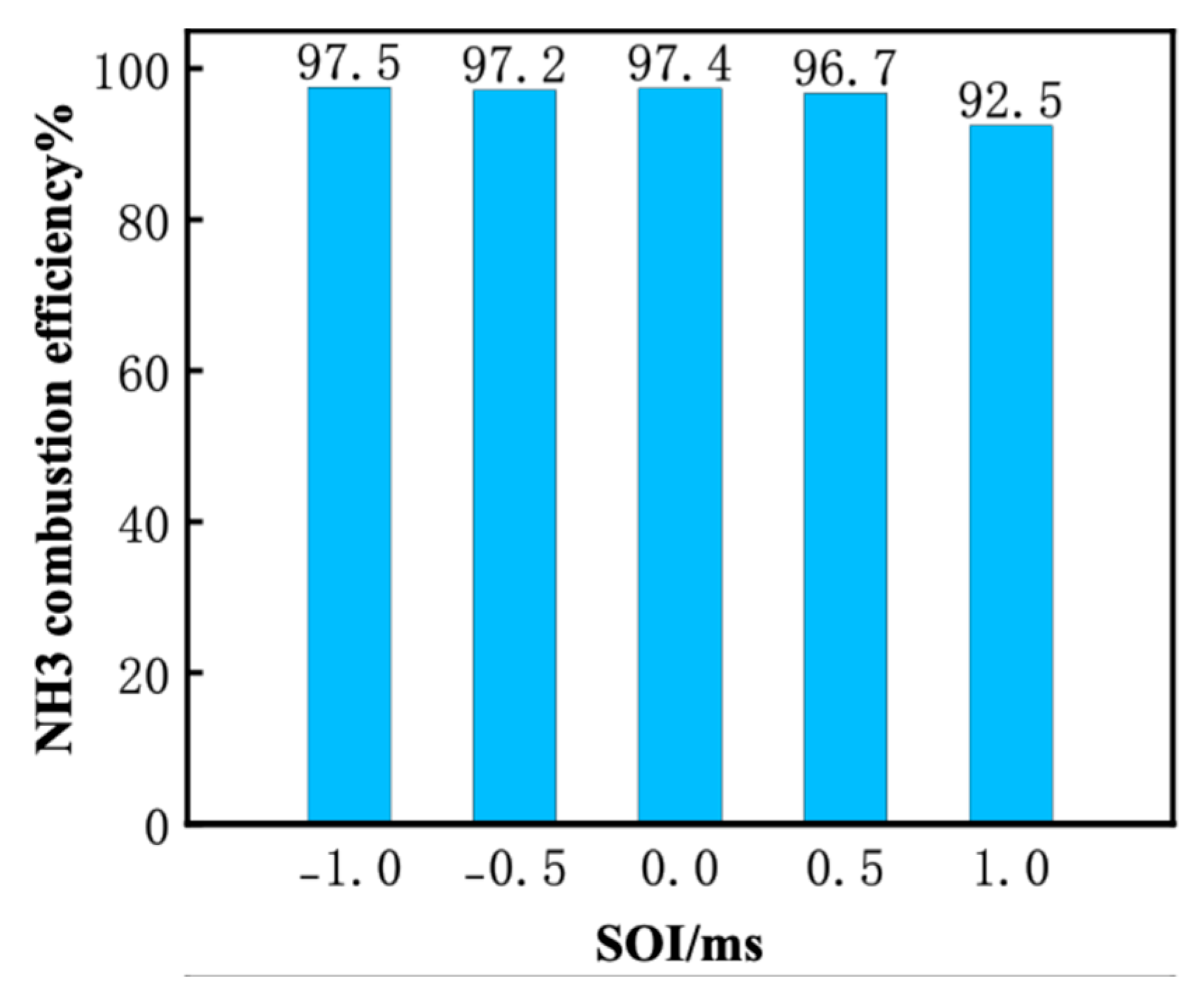
3.3. Effect of DME Injection Timing at Tamb = 1100 K and AER = 80%
4. Conclusions
- Increasing the ambient temperature from 900 K to 1100 K significantly accelerates the autoignition of both DME and NH3, reducing IDT by two orders of magnitude. Consequently, NH3 combustion efficiency increases from 52.1% to 97.4%. N2O and CO are significantly reduced at higher ambient temperature owing to higher flame temperature. However, a substantial increase in NO and NO2 emission is observed. NO formation has a strong correlation with flame temperature. NO2 is usually formed around the periphery of NO because the two primary NO2 formation channels HO2 + NO = NO2 + OH (R2) and NO + O(+M) = NO2(+M) (R3) favor intermediate flame temperature.
- Raising the AER from 60% to 90% results in a slight decline in combustion efficiency of NH3 from 98.7% to 94% due to enhanced evaporative cooling and reduced pilot fuel energy. NO emission has a non-monotonical relationship with AER, which can be attributed to the ‘trade-off’ relationship between NO formation precursor (HNO) and the radical pool at varying AERs. N2O formation is promoted due to increased NH concentration at high AER.
- Advancing DME injection not only benefits combustion efficiency but also reduces NO, NO2, N2O and CO emissions. The reduced NO can be attributed to increased equivalence ratio caused by faster DME combustion, thus facilitating de-NOx reactions (NH2 + NO = N2 + H2O). Meanwhile, reduced NO2, N2O and CO are caused by increased flame temperature with earlier DME injection.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| NH3 | ammonia |
| DME | dimethyl ether |
| H2 | hydrogen |
| AER | ammonia energy ratio |
| NOx | nitrogen oxides |
| NO | nitric oxide |
| NO2 | nitrogen dioxide |
| N2O | nitrous oxide |
| RANS | Reynolds-Averaged Navier–Stokes |
| CO | carbon monoxide |
| UHC | unburned hydrocarbons |
| LBV | laminar burning velocity |
| CO2 | carbon dioxide |
| IDT | ignition delay time |
| JSR | jet stirred reactor |
| SOI | start of injection |
| HPDI | high-pressure dual-fuel direct injection |
| CVCC | constant volume combustion chamber |
| KHRT | Kelvin–Helmholtz Rayleigh–Taylor |
References
- Nazir, M.J.; Li, G.; Nazir, M.M.; Zulfiqar, F.; Siddique, K.H.; Iqbal, B.; Du, D. Harnessing soil carbon sequestration to address climate change challenges in agriculture. Soil Tillage Res. 2024, 237, 105959. [Google Scholar] [CrossRef]
- Sun, J.; Zhai, N.; Miao, J.; Sun, H. Can Green Finance Effectively Promote the Carbon Emission Reduction in “Local-Neighborhood” Areas?—Empirical Evidence from China. Agriculture 2022, 12, 1550. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 1. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Chen, L.; Ren, M.; Fakayode, O.A.; Han, J.; Zhou, C. Efficient hydrogen evolution reaction performance using lignin-assisted chestnut shell carbon-loaded molybdenum disulfide. Ind. Crops Prod. 2023, 193, 116214. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crops Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Thomas, G.; Parks, G. Potential Roles of Ammonia in a Hydrogen Economy; U.S. Department of Energy: Washington, DC, USA, 2006.
- Kroch, E. Ammonia—A Fuel for Motor Buses. J. Inst. Pet. 1945, 31, 213. [Google Scholar]
- Seaman, R.; Huson, G. The choice of NH3 to fuel the X-15 Rocket plane, NH3 Association. In Proceedings of the 8th NH3 Fuel Association Conference, Boston, MA, USA, 19 September 2011. [Google Scholar]
- Dai, L.; Hashemi, H.; Glarborg, P.; Gersen, S.; Marshall, P.; Mokhov, A.; Levinsky, H. Ignition delay times of NH3/DME blends at high pressure and low DME fraction: RCM experiments and simulations. Combust. Flame 2021, 227, 120. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185. [Google Scholar] [CrossRef]
- Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Kondo, S.; Sekiya, A. Burning velocity measurements of nitrogen-containing compounds. J. Hazard. Mater. 2008, 155, 144. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Demonstration of compression-ignition engine combustion using ammonia in reducing greenhouse gas emissions. Energy Fuels 2008, 22, 2963. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Zhou, X.; Huang, S.; Chen, R.; Yi, P.; Lv, Y.; Wang, Y.; Rao, H.; Liu, Y.; et al. Reductions in GHG and unburned ammonia of the pilot diesel-ignited ammonia engines by diesel injection strategies. Appl. Therm. Eng. 2025, 260, 124967. [Google Scholar] [CrossRef]
- Zhang, Z.; Long, W.; Dong, P.; Tian, H.; Tian, J.; Li, B.; Wang, Y. Performance characteristics of a two-stroke low speed engine applying ammonia/diesel dual direct injection strategy. Fuel 2023, 332, 126086. [Google Scholar] [CrossRef]
- Bjørgen, K.O.P.; Emberson, D.R.; Løvås, T. Combustion of liquid ammonia and diesel in a compression ignition engine operated in high-pressure dual fuel mode. Fuel 2024, 360, 130269. [Google Scholar] [CrossRef]
- Li, T.; Zhou, X.; Wang, N.; Wang, X.; Chen, R.; Li, S.; Yi, P. A comparison between low- and high-pressure injection dual-fuel modes of diesel-pilot-ignition ammonia combustion engines. J. Energy Inst. 2022, 102, 362. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Zhong, W.; Jiang, P.; Xu, M.; Guo, B. An optical investigation of the effects of diesel injection pressure and intake air temperature in an ammonia-diesel dual-fuel engine under low-load conditions. Appl. Therm. Eng. 2025, 261, 125174. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. Process Intensif. 2014, 82, 150. [Google Scholar] [CrossRef]
- Li, Y.; Obadi, M.; Qi, Y.; Shi, J.; Liu, S.; Sun, J.; Chen, Z.; Xu, B. Extraction of Oat Lipids and Phospholipids Using Subcritical Propane and Dimethyl Ether: Experimental Data and Modeling. Eur. J. Lipid Sci. Technol. 2021, 123, 2000092. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S.-C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.-C. Performance characteristics of compression-ignition engine using high concentration of ammonia mixed with dimethyl ether. Appl. Energy 2014, 113, 488. [Google Scholar] [CrossRef]
- Leng, Y.; Dai, L.; Wang, Q.; Lu, J.; Yu, O.; Simms, N.J. Numerical Study on the Combustion and Emissions Characteristics of Liquid Ammonia Spray Ignited by Dimethyl Ether Spray. Fire 2024, 8, 14. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, L.; Wang, Y.; Qu, X.; Chen, X.; Wang, Q. Experimental and kinetic study on O2 reduced and enriched premixed ammonia/DME flames. J. Energy Inst. 2025, 123, 102276. [Google Scholar] [CrossRef]
- Yin, G.; Xiao, B.; Zhao, H.; Zhan, H.; Hu, E.; Huang, Z. Jet-stirred reactor measurements and chemical kinetic study of ammonia with dimethyl ether. Fuel 2023, 341, 127542. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86, 848. [Google Scholar] [CrossRef]
- Lu, T.; Law, C.K. A directed relation graph method for mechanism reduction. Proc. Combust. Inst. 2005, 30, 1. [Google Scholar] [CrossRef]
- Niemeyer, K.E.; Sung, C.-J. On the importance of graph search algorithms for DRGEP-based mechanism reduction methods. Combust. Flame 2011, 158, 1439. [Google Scholar] [CrossRef]
- Han, Z.; Reitz, R.D. Turbulence Modeling of Internal Combustion Engines Using RNG κ-ε Models. Combust. Sci. Technol. 1995, 106, 267. [Google Scholar] [CrossRef]
- He, C.; Zhang, P.; Law, C.K. Dynamics of binary droplet collisions. Prog. Energy Combust. Sci. 2025, 111, 101252. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Dhanasekaran, R.; Rana, D.; Saravanan, S.; Rajesh Kumar, B. A comparative assessment of ternary blends of three bio-alcohols with waste cooking oil and diesel for optimum emissions and performance in a CI engine using response surface methodology. Energy Convers. Manag. 2018, 156, 337. [Google Scholar] [CrossRef]
- O’ROurke, P.J.; Amsden, A.A. A Spray/Wall Interaction Submodel for the KIVA-3 Wall Film Model. SAE Trans. 2000, 109, 281. [Google Scholar]
- Schmidt, D.P.; Rutland, C.J. A New Droplet Collision Algorithm. J. Comput. Phys. 2000, 164, 62. [Google Scholar] [CrossRef]
- Hiroyasu, H.; Kadota, T. Models for Combustion and Formation of Nitric Oxide and Soot in Direct Injection Diesel Engines. SAE Pap. 1976, 85, 513–526. [Google Scholar] [CrossRef]
- Dai, L.; Ma, W.; Wang, Q.; Maas, U.; Yu, C. Numerical investigation of the effect of H2 and O2 addition on laminar counterflow flames of ammonia: A comparative study. Fuel 2025, 399, 135452. [Google Scholar] [CrossRef]
- Nakamura, H.; Shindo, M. Effects of radiation heat loss on laminar premixed ammonia/air flames. Proc. Combust. Inst. 2019, 37, 2. [Google Scholar] [CrossRef]
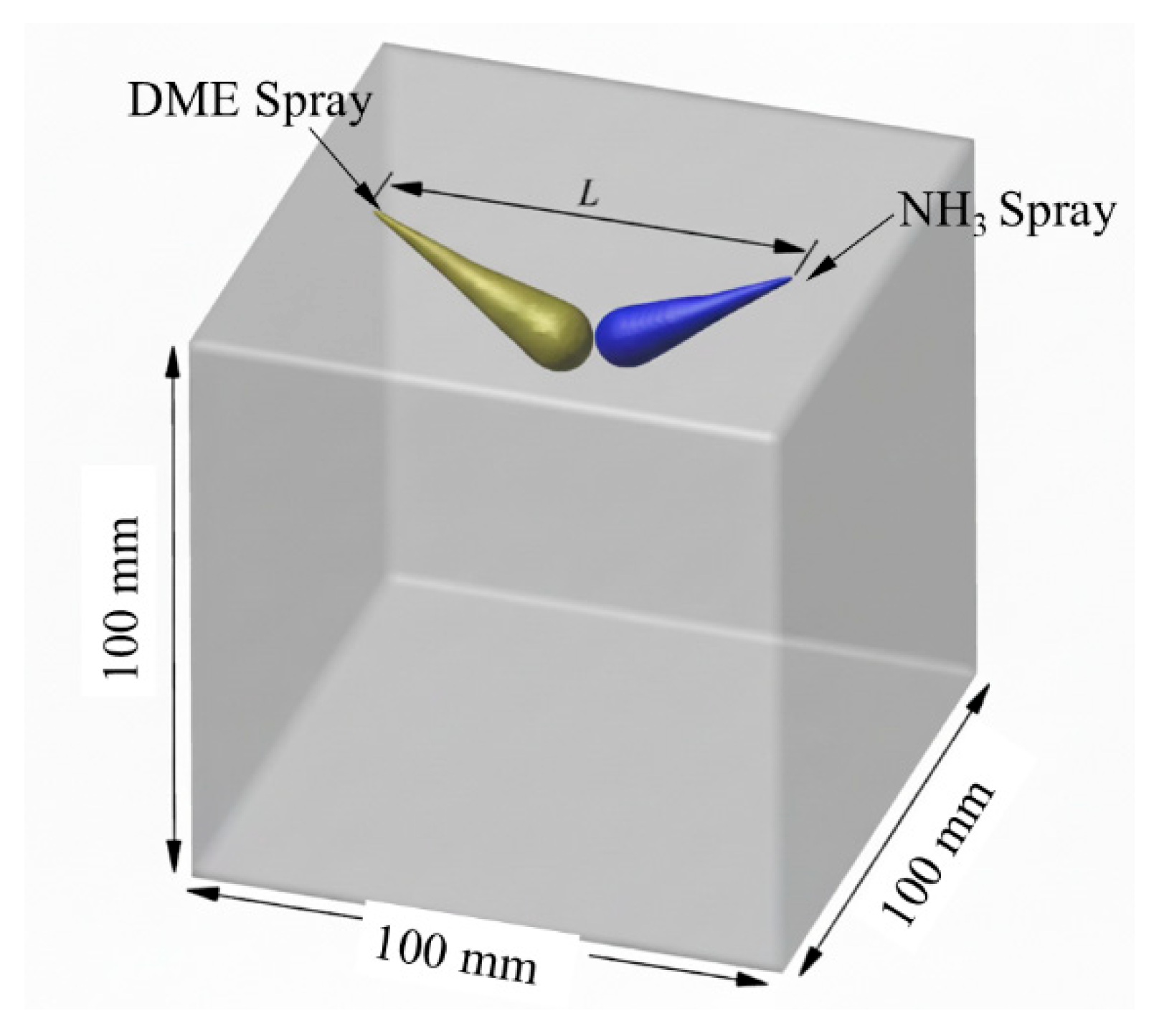






| Parameter | Ammonia | DME | Diesel |
|---|---|---|---|
| Boiling point/K | 239.8 | 358 | 450–643 K |
| Autoignition temperature/K | 924 | 623 | 226–233 |
| Octane number | 130 | - | - |
| Cetane number | - | 55–60 | 40–55 |
| Lower heating value/(MJ/kg) | 18.8 | 28.43 | 43.5 |
| Latent heat of vaporization/(KJ/kg) | 1.37 | 460 | 270 |
| Laminar burning velocity/(m/s) | 0.07 | 0.54 | - |
| Parameter | Value |
|---|---|
| Chamber size/mm | 100 × 100 × 100 |
| Ambient temperature/K | 900, 1000, 1100 |
| Ambient gas | Air |
| Injector distance/cm | 7.0 |
| DME injection pressure/MPa | 75 |
| Ammonia Nozzle diameter/mm | 0.22 |
| DME Nozzle diameter/mm | 0.18 |
| Ammonia injection mass/mg | 18.15 |
| DME injection mass/mg | 3 |
| Nozzle angle/° | 120 |
| NH3 injection pressure/MPa | 75 |
| AER/% | 60, 80, 90, 95 |
| SOI of DME/ms | −1, 0.5, 0, 0.5, 1 |
| Ambient pressure/MPa | 3.8 |
| Parameter | Value |
|---|---|
| B1 of DME | 11 |
| B1 of NH3 | 8 |
| B0 | 0.61 |
| Cτ | 1 |
| CRT | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, Q.; Dai, L. Pilot Ignition of Ammonia Spray Using Dimethyl Ether Spray at Elevated Temperature: A Numerical Study. Fire 2025, 8, 436. https://doi.org/10.3390/fire8110436
Zhang C, Wang Q, Dai L. Pilot Ignition of Ammonia Spray Using Dimethyl Ether Spray at Elevated Temperature: A Numerical Study. Fire. 2025; 8(11):436. https://doi.org/10.3390/fire8110436
Chicago/Turabian StyleZhang, Chengcheng, Qian Wang, and Liming Dai. 2025. "Pilot Ignition of Ammonia Spray Using Dimethyl Ether Spray at Elevated Temperature: A Numerical Study" Fire 8, no. 11: 436. https://doi.org/10.3390/fire8110436
APA StyleZhang, C., Wang, Q., & Dai, L. (2025). Pilot Ignition of Ammonia Spray Using Dimethyl Ether Spray at Elevated Temperature: A Numerical Study. Fire, 8(11), 436. https://doi.org/10.3390/fire8110436






