Comprehensive Evaluation of a High-Resistance Fire Retardant via Simultaneous Thermal Analysis, Gas Chromatography–Mass Spectrometry, and Mass Loss Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Simultaneous Thermal Analysis (STA)
2.3. Gas Chromatography–Mass Spectrometry
2.4. Mass Loss Analysis
2.5. Data Analysis and Reproducibility
3. Results
3.1. Simultaneous Thermal Analysis (STA) Results
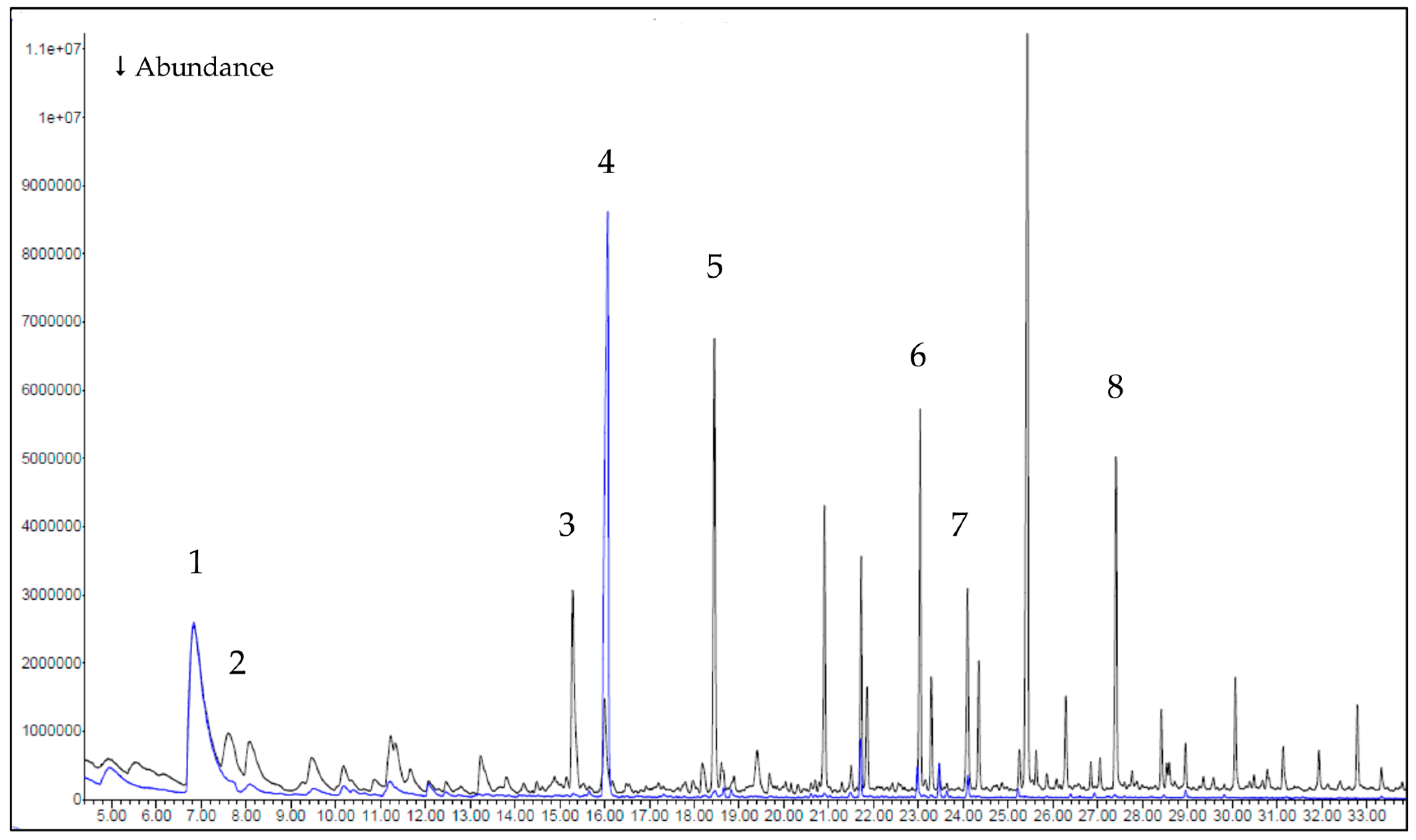
3.2. Gas Chromatography–Mass Spectrometry (GC-MS) Results
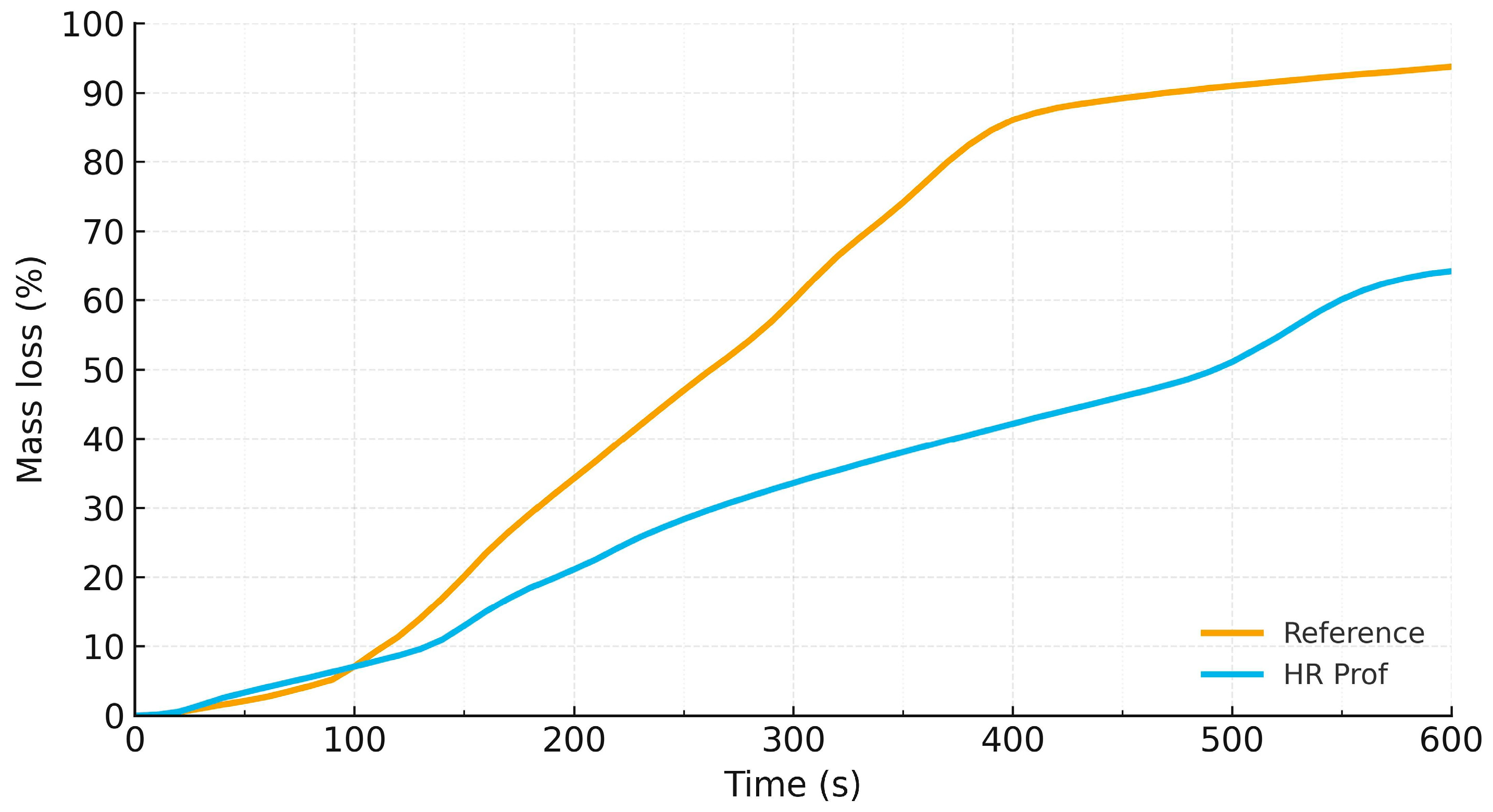
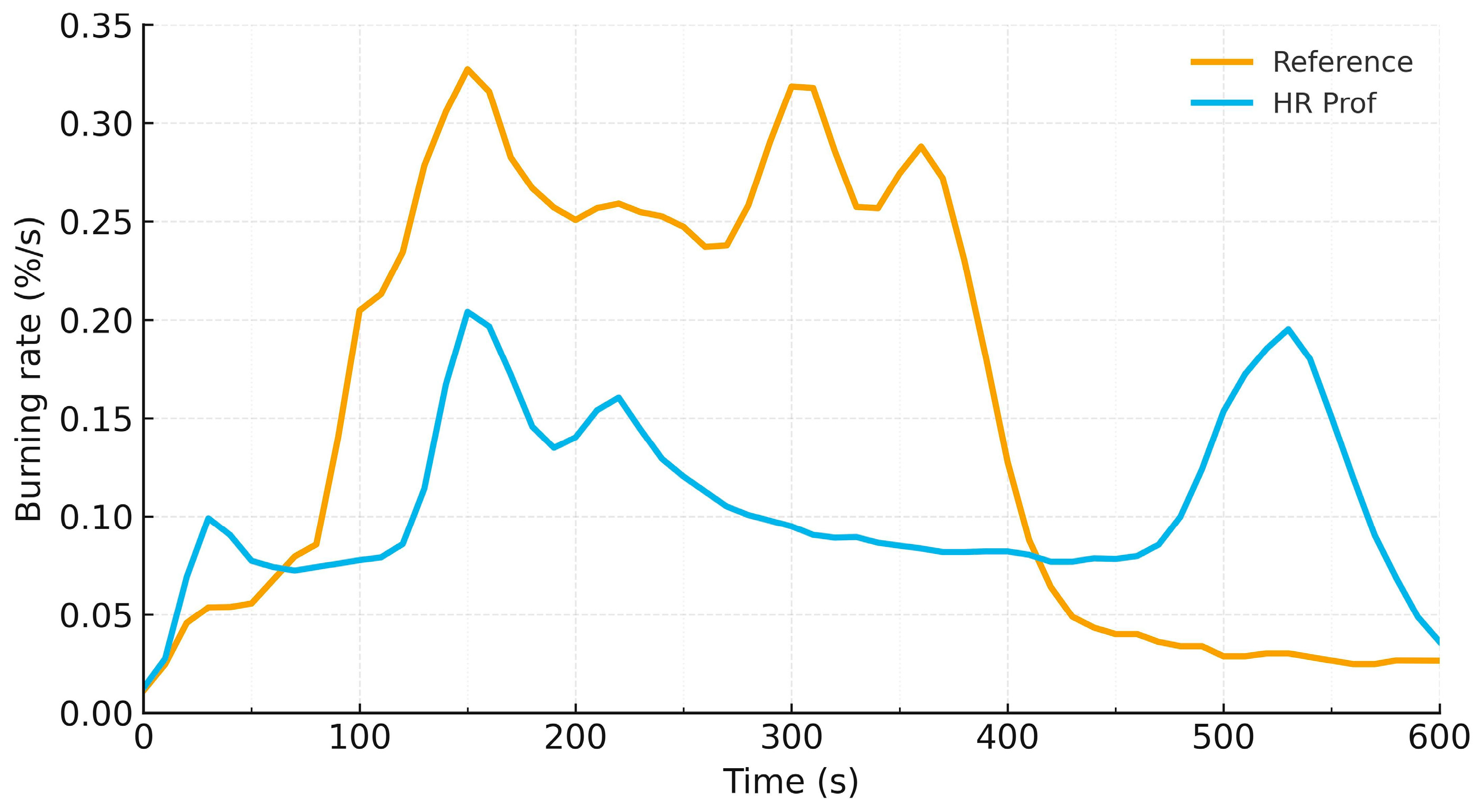
3.3. Mass Loss Analysis Results
3.4. Integrated Evaluation of Fire Retardant Performance
4. Discussion
5. Conclusions
- Thermal behavior (STA): in air, HR Prof redirects decomposition toward earlier, dehydration-dominated pathways: the main TG step is broadened and shifted to lower temperature, and the DSC peak occurs at lower temperature with reduced magnitude. A DTG feature near ~600 °C indicates oxidation of transient char at high temperatures.
- Evolved gases (GC-MS): between 150 and 250 °C, HR Prof releases fewer and less energetic volatiles than the reference (identified components 5/9/9 vs. 20/24/51 at 150/200/250 °C), with a marked reduction at 250 °C (51 → 9) and suppression of phenolic/furanic markers. The simpler, more oxygen-rich volatile pool supports the dehydration-biased pathway.
- Bench-scale kinetics (mass loss): under 600 s of radiant heating, HR Prof lowers both the average burning rate (0.107 vs. 0.156%/s) and the cumulative mass loss (64.2 ± 9.5% vs. 93.7 ± 2.1%). ANOVA confirms a significant reduction in the percentage endpoint at α = 0.05; the grams-lost trend is lower but is not significant at the present n.
- Mechanistic coherence and safety relevance: the datasets are consistent: earlier condensed-phase dehydration/char (STA) → fewer/less energetic volatiles (GC-MS) → lower and flatter burning-rate profiles with smaller material consumption (mass loss). The reduction and simplification of evolved volatiles also indicate a less hazardous combustible/explosive mixture, aligning with the observed damping of combustion intensity.
- Overall mechanism: HR Prof operates via a dual-mode mechanism: condensed-phase stabilization (phosphate-catalyzed dehydration/crosslinking yielding transient protective char) complemented by gas-phase inhibition (phosphorus-containing species), with the char ultimately oxidized in air at higher temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance. |
| CI | Confidence Interval. |
| DSC | Differential Scanning Calorimetry. |
| DTG | Derivative Thermogravimetry (mass-loss rate). |
| EGA | Evolved Gas Analysis. |
| FR/FRs | Fire Retardant/Fire Retardants. |
| GS-MS | Gas Chromatography–Mass Spectrometry. |
| GLM | Generalized Linear Model. |
| HR Prof | Commercial phosphorus-based fire retardant used in this study. |
| DOAJ | Heat Release Rate. |
| HRR | Heat Release Rate. |
| LOI | Limiting Oxygen Index. |
| MCC | Microscale Combustion Calorimetry. |
| NIST | National Institute of Standards and Technology. |
| OLTI-EGA | On-Line Thermally Induced Evolved Gas Analysis. |
| P–N | Phosphorus–Nitrogen (synergy). |
| RH | Relative Humidity. |
| RR | Rate Ratio. |
| RT | Retention Time. |
| SD | Standard Deviation. |
| STA | Simultaneous Thermal Analysis. |
| TGA/TG | Thermogravimetric Analysis/Thermogravimetry. |
| THR | Total Heat Released. |
| UL-94 | Underwriters Laboratories 94 flammability test. |
| VOC(s) | Volatile Organic Compound(s). |
References
- Li, F.-F. Comprehensive Review of Recent Research Advances on Flame-Retardant Coatings for Building Materials: Chemical Ingredients, Micromorphology, and Processing Techniques. Molecules 2023, 28, 1842. [Google Scholar] [CrossRef]
- Patel, R.; Chaudhary, M.L.; Patel, Y.N.; Chaudhari, K.; Gupta, R.K. Fire-Resistant Coatings: Advances in Flame-Retardant Technologies, Sustainable Approaches, and Industrial Implementation. Polymers 2025, 17, 1814. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, S.; He, L.; Xu, S. Advances in the Study of Flame-Retardant Cellulose and Its Application in Polymers: A Review. Polymers 2025, 17, 1249. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, O.B.; Amelkovich, Y.A.; Bannov, A.G.; Berdyugina, I.S.; Maniyan, V.P. Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate. Polymers 2021, 13, 638. [Google Scholar] [CrossRef]
- Mitrenga, P.; Vandlíčková, M.; Konárik, M. Experimental Investigation of Fire—Technical Characteristics of Selected Flame Retardants for the Protection of Wooden Structures. Coatings 2025, 15, 193. [Google Scholar] [CrossRef]
- Atay, G.Y.; Wilk-Jakubowski, J.L.; Loboichenko, V. Novel Flame-Retardant Wood-Polymer Composites by Using Inorganic Mineral Huntite and Hydromagnesite: An Aspect of Application in Electrical Engineering. Materials 2025, 18, 2652. [Google Scholar] [CrossRef]
- Feng, B.; Yu, S.; Xiang, H.; Li, L.; Zhu, M. Current Status and Future Trends for Modification Technology of Flame Retardant Nylon 66. Polymers 2025, 17, 1074. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.H.; Zope, I.S.; Dasari, A.; Tan, K.H. Correlating the Performance of a Fire-Retardant Coating across Different Scales of Testing. Polymers 2020, 12, 2271. [Google Scholar] [CrossRef] [PubMed]
- Holeček, T.; Šedivka, P.; Sahula, L.; Berčák, R.; Zeidler, A.; Hájková, K. Investigation of Pressure Vacuum Impregnation Using Inorganic, Organic, and Natural Fire Retardants on Beech Wood (Fagus sylvatica) and Its Impact on Fire Resistance. Fire 2025, 8, 318. [Google Scholar] [CrossRef]
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers 2022, 14, 362. [Google Scholar] [CrossRef]
- Trojanová, K.; Veľková, V.; Kačík, F. Volatile Organic Compounds Arising from Wood Polymers on Thermal Loading of Spruce Wood. Polymers 2025, 17, 875. [Google Scholar] [CrossRef]
- Grześkowiak, W.Ł.; Ratajczak, I.; Zborowska, M.; Przybylska, M.; Patora, M. Phosphorus–Nitrogen Interaction in Fire Retardants and Its Impact on the Chemistry of Treated Wood. Materials 2024, 17, 5283. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Martinasso, G. Intumescent Fire-Retardant Systems. Polym. Degrad. Stab. 1989, 23, 359–376. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Gullifa, G.; Papa, E.; Putzolu, G.; Rizzo, G.; Ruocco, M.; Albertini, C.; Risoluti, R.; Materazzi, S. MS and GC–MS Analytical Methods for On-Line Thermally Induced Evolved Gas Analysis (OLTI-EGA). Chemosensors 2025, 13, 258. [Google Scholar] [CrossRef]
- Yuen, A.C.Y.; Chen, T.B.Y.; Yeoh, G.H.; Yang, W.; Cheung, S.C.-P.; Cook, M.; Yu, B.; Chan, Q.N.; Yip, H.L. Establishing pyrolysis kinetics for the modelling of the flammability and burning characteristics of solid combustible materials. J. Fire Sci. 2018, 36, 494–517. [Google Scholar] [CrossRef]
- STN EN 13238:2011; Reaction to Fire Tests for Building Products—Conditioning Procedures and General Rules for Selection of Substrates. Slovak Office of Standards, Metrology and Testing: Bratislava, Slovakia, 2011.
- Hájková, K.; Šedivka, P.; Holeček, T.; Berčák, R.; Sahula, L. The Effect of Chemical Modification by Synthetic and Natural Fire-Retardants on Burning and Chemical Characteristics of Structural Fir (Abies alba L.) Wood. Fire 2025, 8, 116. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of Fire-Retarded Materials—Interpretation of Cone Calorimeter Data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability Behaviour of Wood and a Review of the Methods for Its Reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Green, J. A Review of Phosphorus-Containing Flame Retardants. J. Fire Sci. 1992, 10, 470–487. [Google Scholar] [CrossRef]
- Weng, M.; Fu, Y.; Xu, W. Flame-Retardant Coating on Wood Surface by Natural Biomass Polyelectrolyte via a Layer-by-Layer Self-Assembly Approach. Forests 2024, 15, 1362. [Google Scholar] [CrossRef]
- Yan, Y.; Dong, S.; Jiang, H.; Hou, B.; Wang, Z.; Jin, C. Efficient and Durable Flame-Retardant Coatings on Wood Fabricated by Chitosan, Graphene Oxide and Ammonium Polyphosphate Ternary Complexes via a Layer-by-Layer Self-Assembly Approach. ACS Omega 2022, 7, 29369–29379. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Ji, Y.; Zhu, M.; Liang, Y.; Jian, H.; Yan, Z.; Wen, M.; Park, H. Preparation of Organic-Inorganic Phosphorus-Nitrogen-Based Flame Retardants and Their Application to Plywood. Polymers 2023, 15, 3112. [Google Scholar] [CrossRef]
- Lin, C.-F.; Karlsson, O.; Kim, I.; Myronycheva, O.; Mensah, R.A.; Försth, M.; Das, O.; Mantanis, G.I.; Jones, D.; Sandberg, D. Fire Retardancy and Leaching Resistance of Furfurylated Pine Wood (Pinus sylvestris L.) Treated with Guanyl-Urea Phosphate. Polymers 2022, 14, 1829. [Google Scholar] [CrossRef]
- Gebke, S.; Thümmler, K.; Sonnier, R.; Tech, S.; Wagenführ, A.; Fischer, S. Flame Retardancy of Wood Fiber Materials Using Phosphorus-Modified Wheat Starch. Molecules 2020, 25, 335. [Google Scholar] [CrossRef]
- Markwart, J.C.; Battig, A.; Zimmermann, L.; Wagner, M.; Fischer, J.; Schartel, B.; Wurm, F.R. Systematically Controlled Decomposition Mechanism in Phosphorus Flame Retardants by Precise Molecular Architecture: P–O vs P–N. ACS Appl. Polym. Mater. 2019, 1, 1118–1128. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Liu, D. Synthesis and application of novel magnesium phosphate ester flame retardants for transparent intumescent fire-retardant coatings applied on wood substrates. Prog. Org. Coat. 2019, 129, 327–337. [Google Scholar] [CrossRef]
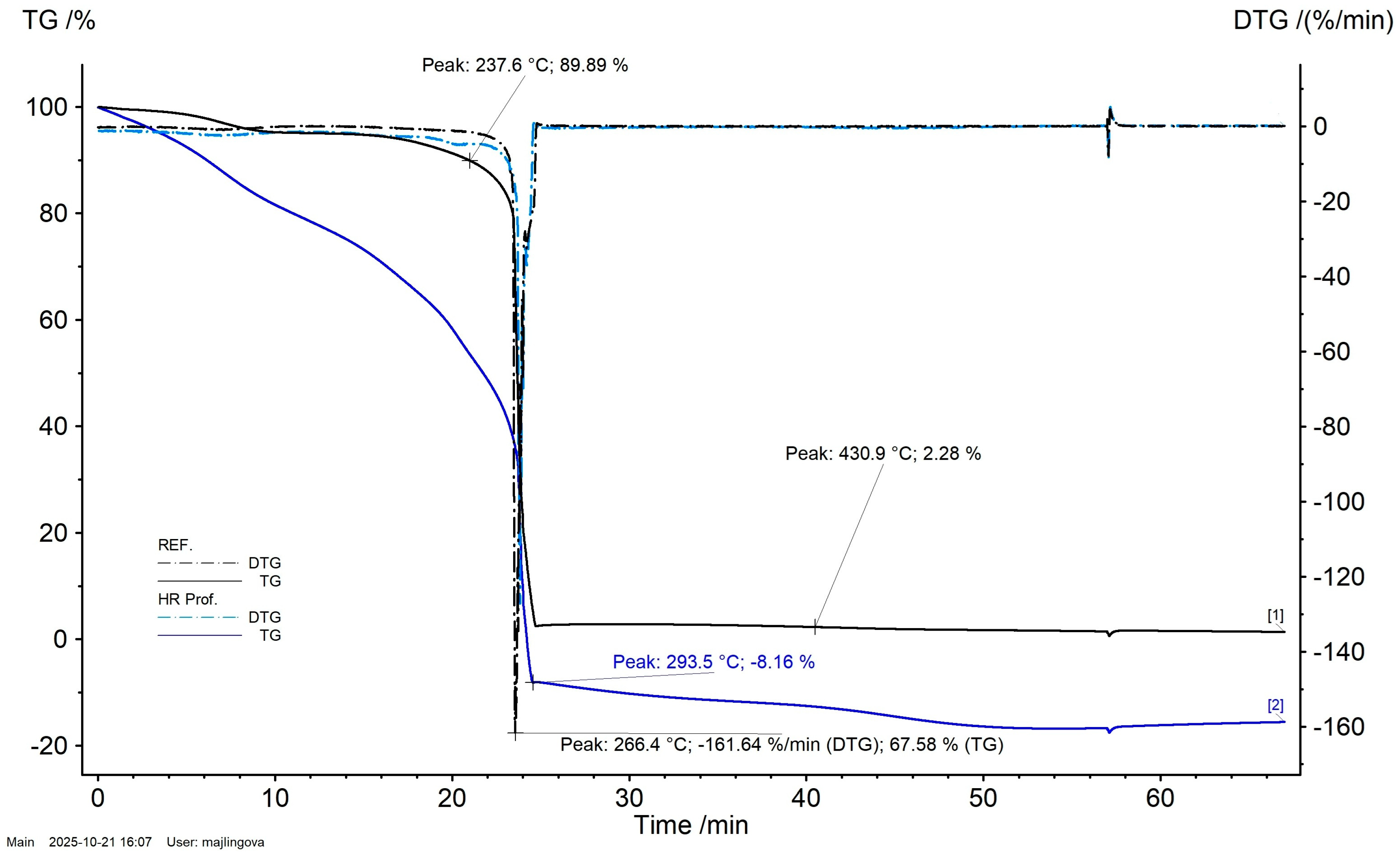
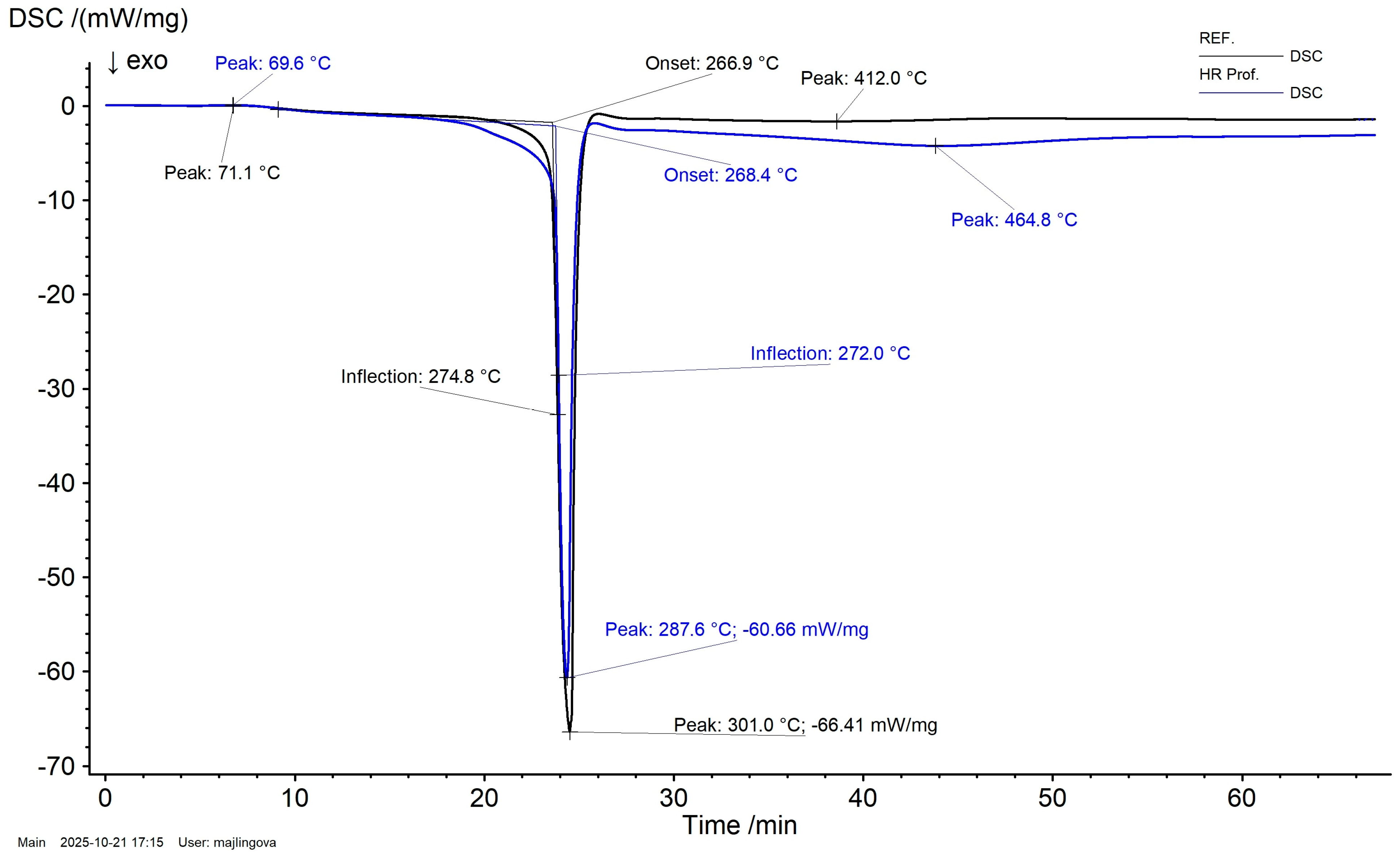
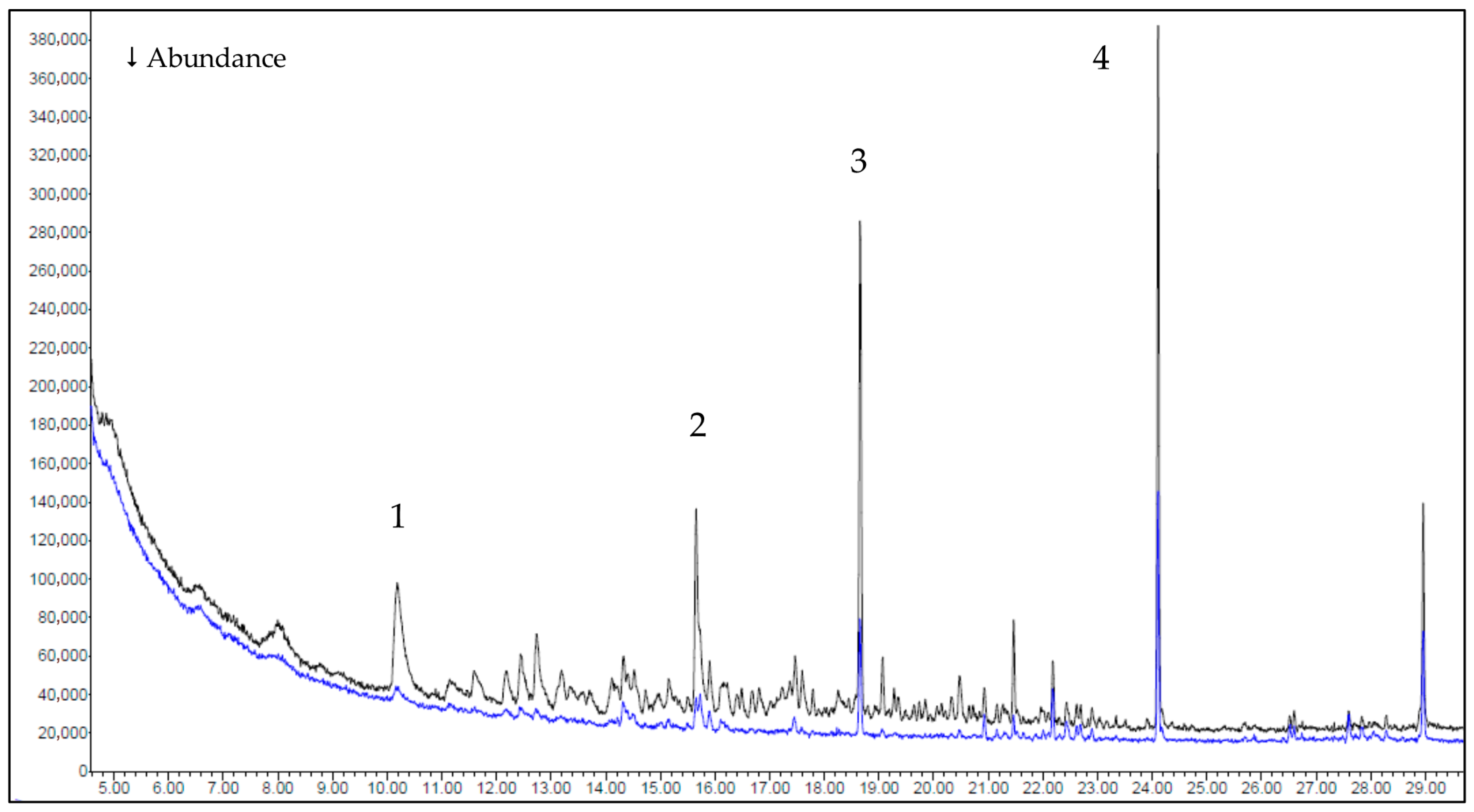
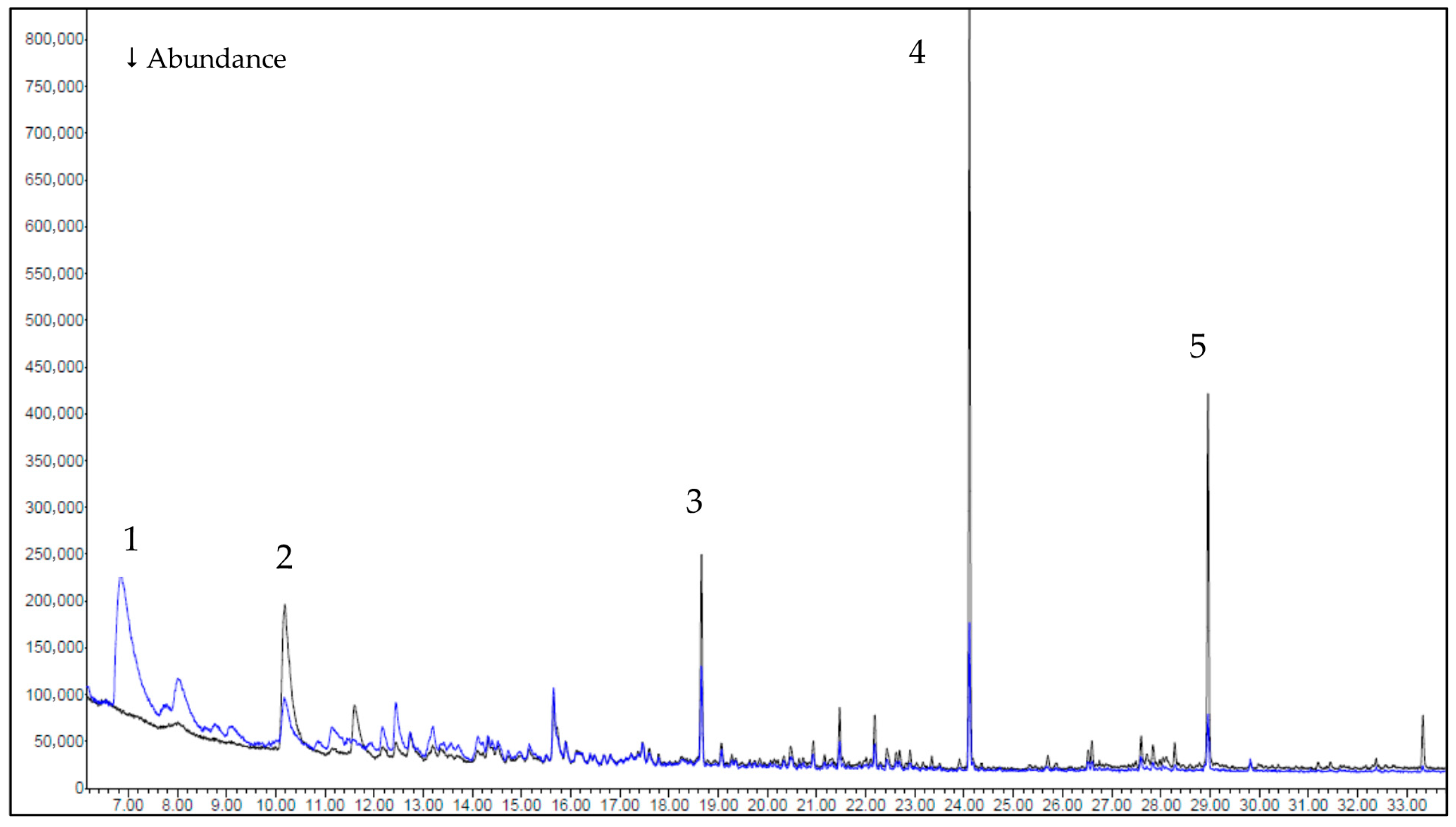
| Sample | Temperature Interval (°C) | Mass Change (%) | Residual Mass (%) |
|---|---|---|---|
| REF | 30.9–38.5 | −0.59 | 99.10 |
| 38.5–137.2 | −4.05 | 95.05 | |
| 137.2–301.6 | −92.7 | 2.58 | |
| 301.6–312.9 | 0.24 | 2.83 | |
| 312.9–545.6 | −1.18 | 1.65 | |
| 545.6–607.2 | −0.03 | 1.61 | |
| 607.3–695.7 | −0.25 | 1.36 | |
| HR Prof | 29.9–54.4 | −7.19 | 91.14 |
| 54.4–130.5 | −12.14 | 79.30 | |
| 130.5–389.6 | −91.06 | −11.76 | |
| 389.6–591.4 | −4.97 | −16.73 | |
| 591.4–695.6 | 1.15 | −15.18 |
| Sample | Temperature | Compounds Group | ||
|---|---|---|---|---|
| 150 °C | 200 °C | 250 °C | ||
| Reference | 20 | 24 | 51 | Aldehydes, ketones, phenols, alcohols, aliphatic hydrocarbons, esters, terpenes, acids, furans |
| HR Prof | 5 | 9 | 9 | Aldehydes, ketones, terpenes, aliphatic |
| Temperature (°C) | Reference (n) | HR Prof (n) | Rate ratio (HR/Ref) | 95% CI |
|---|---|---|---|---|
| 150 | 20 | 5 | 0.250 | 0.094–0.666 |
| 200 | 24 | 9 | 0.375 | 0.174–0.807 |
| 250 | 51 | 9 | 0.176 | 0.087–0.358 |
| Parameter | Reference (Mean ± SD) | HR Prof (Mean ± SD) | ANOVA F(1,4), p | Tukey, Mean Diff [95% CI], p |
|---|---|---|---|---|
| Cumulative mass loss (%) | 93.74 ± 1.6 | 64.22 ± 9.51 | 27.56, 0.0063 | 29.53 [13.91, 45.14], 0.0063 |
| Mass lost (g) | 8.16 ± 0.59 | 6.59 ± 1.77 | 2.12, 0.219 | 1.57 [−1.42, 4.56], 0.219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitterová, I.; Veľková, V.; Majlingová, A. Comprehensive Evaluation of a High-Resistance Fire Retardant via Simultaneous Thermal Analysis, Gas Chromatography–Mass Spectrometry, and Mass Loss Study. Fire 2025, 8, 432. https://doi.org/10.3390/fire8110432
Mitterová I, Veľková V, Majlingová A. Comprehensive Evaluation of a High-Resistance Fire Retardant via Simultaneous Thermal Analysis, Gas Chromatography–Mass Spectrometry, and Mass Loss Study. Fire. 2025; 8(11):432. https://doi.org/10.3390/fire8110432
Chicago/Turabian StyleMitterová, Iveta, Veronika Veľková, and Andrea Majlingová. 2025. "Comprehensive Evaluation of a High-Resistance Fire Retardant via Simultaneous Thermal Analysis, Gas Chromatography–Mass Spectrometry, and Mass Loss Study" Fire 8, no. 11: 432. https://doi.org/10.3390/fire8110432
APA StyleMitterová, I., Veľková, V., & Majlingová, A. (2025). Comprehensive Evaluation of a High-Resistance Fire Retardant via Simultaneous Thermal Analysis, Gas Chromatography–Mass Spectrometry, and Mass Loss Study. Fire, 8(11), 432. https://doi.org/10.3390/fire8110432







