Pyrolysis Characteristics and Reaction Mechanism of Cement Fiberboard with Thermogravimetry/Fourier Transform Infrared Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Thermogravimetry
2.3. Kinetic Method Analysis
2.3.1. Flynn–Wall–Ozawa Method
2.3.2. Kissinger–Akahira–Sunose Method
2.3.3. Coats–Redfern Method
2.3.4. Bi-Gaussian Deconvolution Method
2.4. TG-FTIR
3. Results and Discussion
3.1. Thermogravimetric Analysis
3.2. Kinetic Parameter Calculation and Mechanism Analysis
3.2.1. Calculation of Kinetic Parameters by Model-Free Method
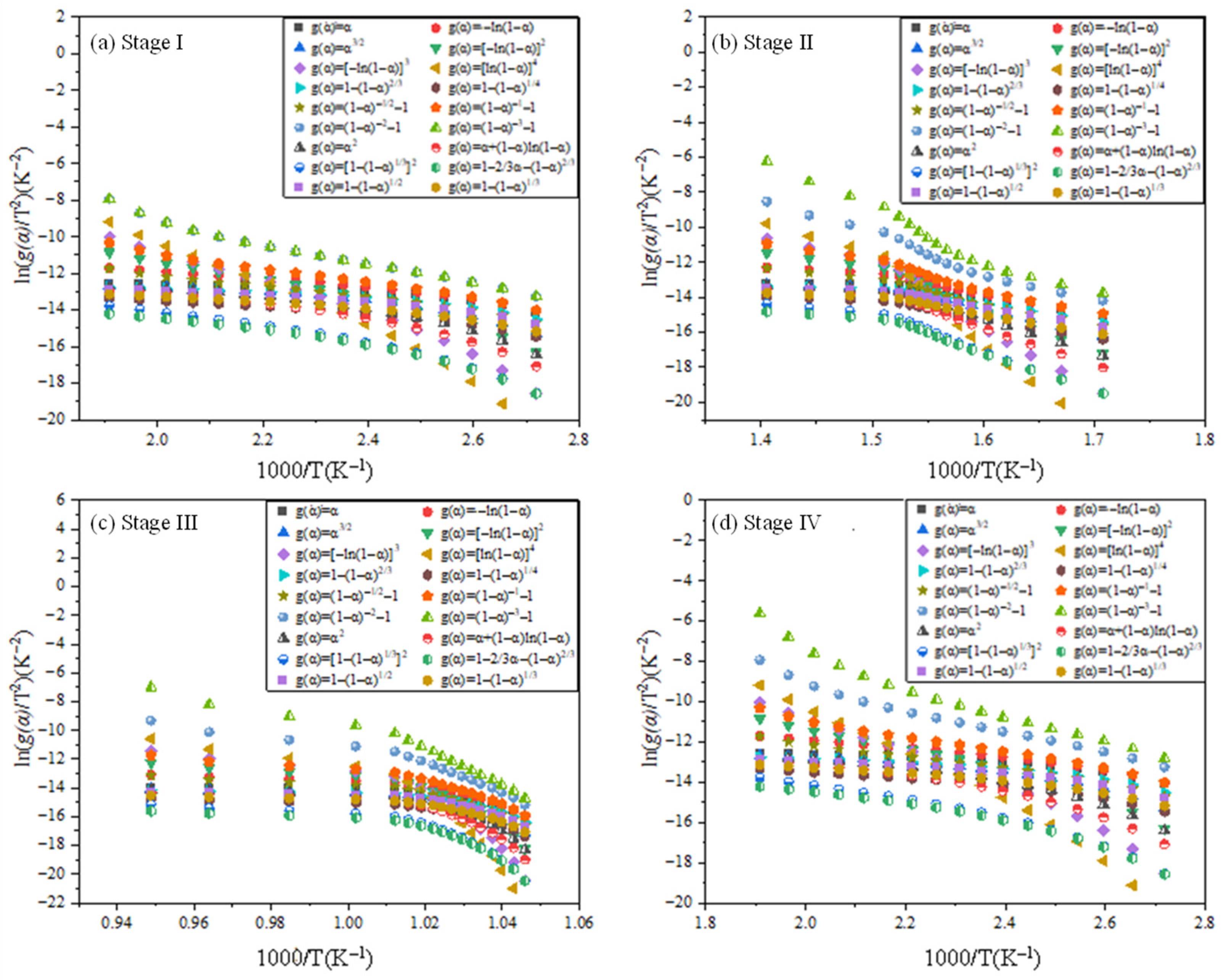
3.2.2. Estimation of Reaction Mechanism with the CR Method
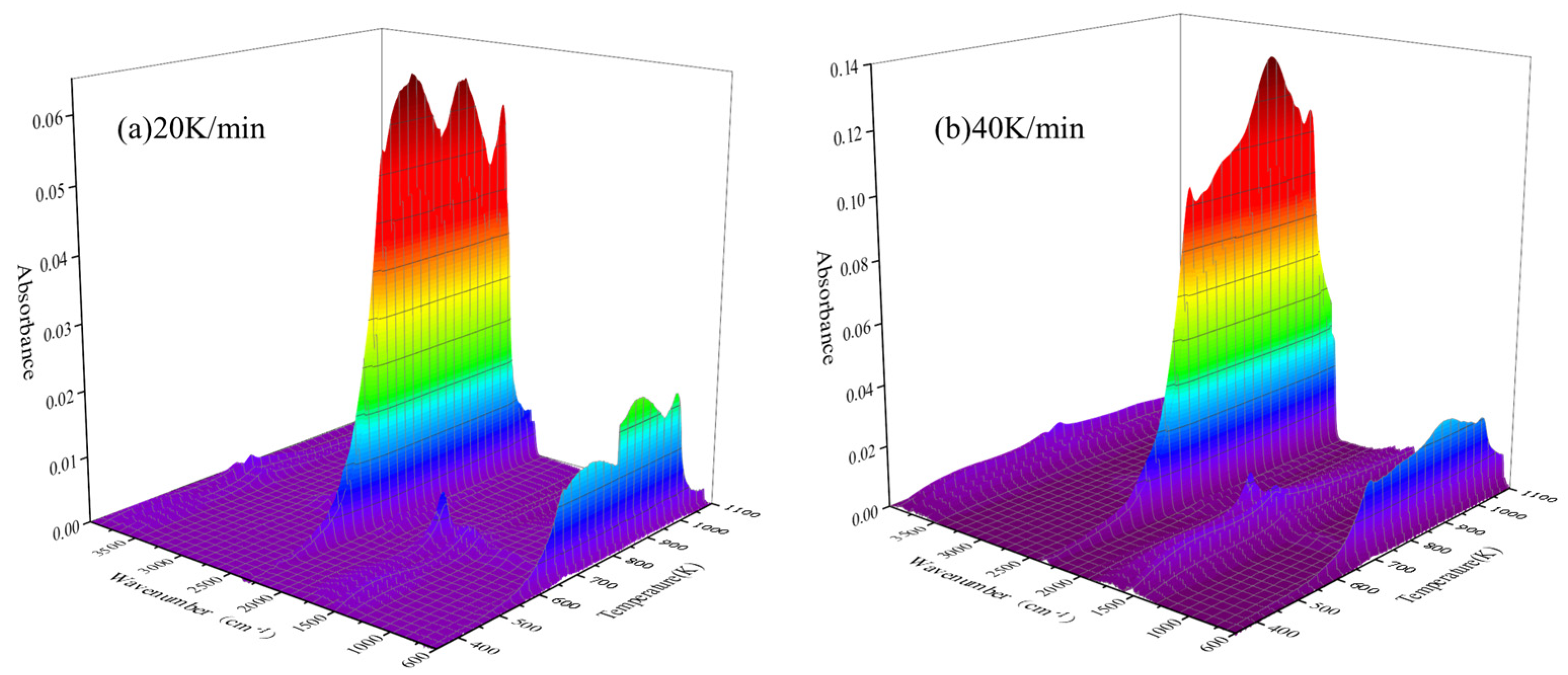
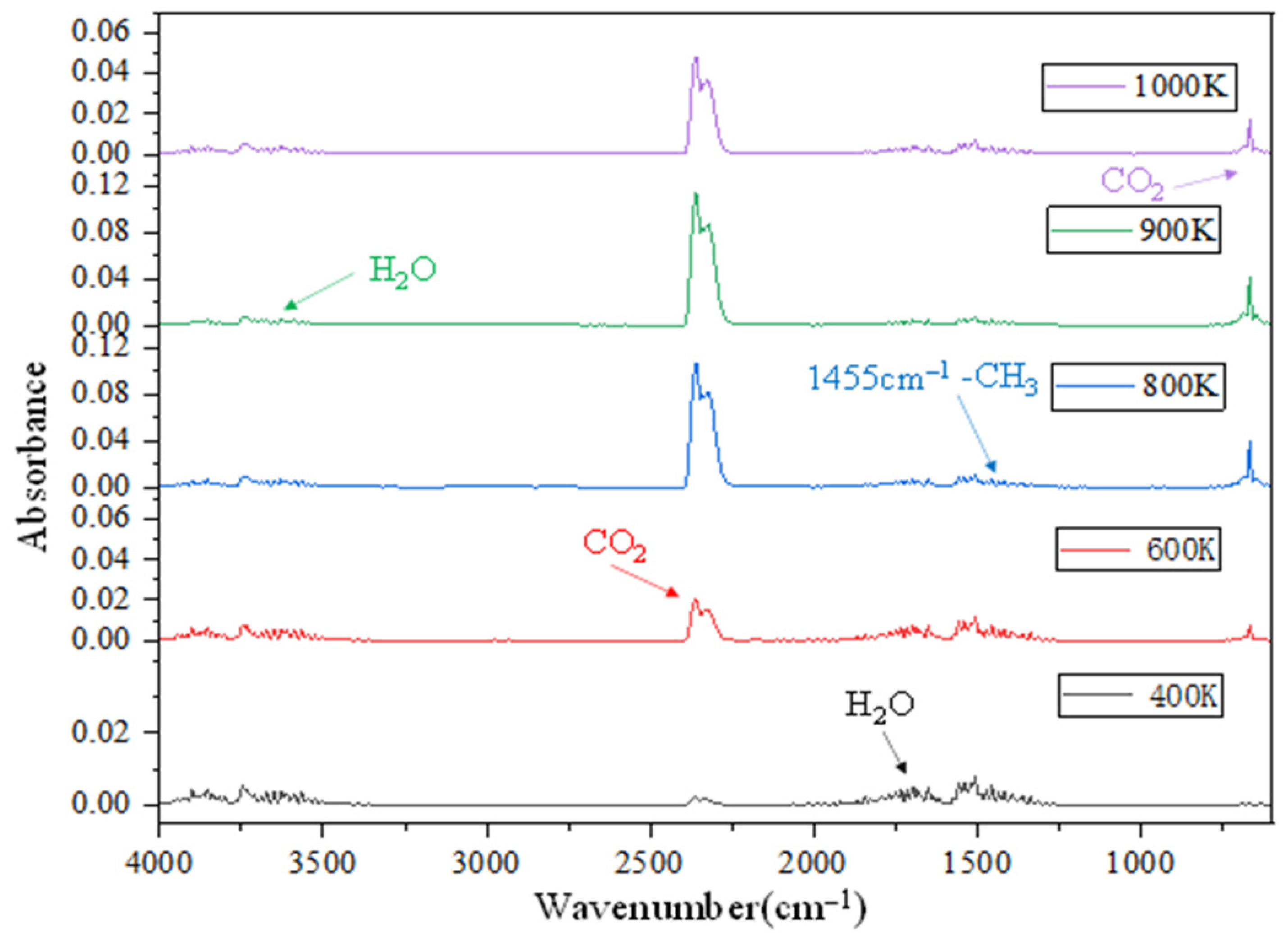
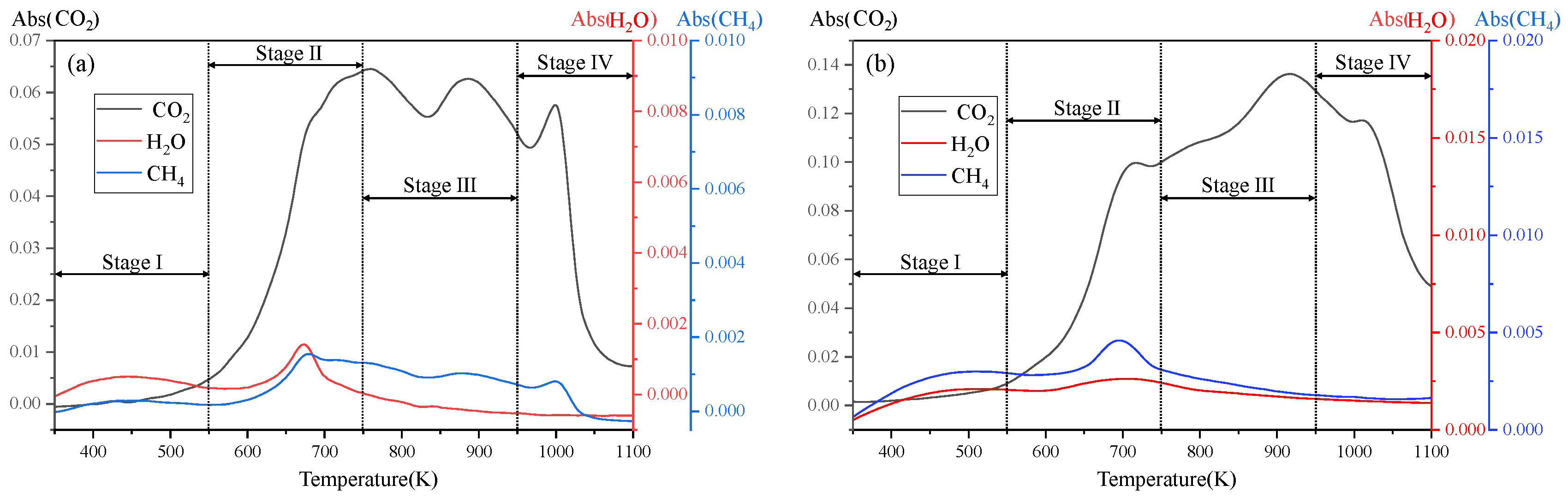
3.3. FTIR Analysis
3.4. Speculation on Reaction Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, Y.; Qiu, J.; Shah, S.P. Microstructure of extruded cement-bonded fiberboard. Cem. Concr. Res. 2001, 31, 1153–1161. [Google Scholar] [CrossRef]
- Guntekin, E.; Sahin, H.T. Accelerated weathering performance of cement bonded fiberboard. Sci. Res. Essays 2009, 4, 484–492. [Google Scholar]
- He, Z.; Jia, Y.; Wang, S.; Mahoutian, M.; Shao, Y. Maximizing CO2 sequestration in cement-bonded fiberboards through carbonation curing. Constr. Build. Mater. 2019, 213, 51–60. [Google Scholar] [CrossRef]
- Soroushian, P.; Marikunte, S.; Won, J.-P. Statistical Evaluation of Mechanical and Physical Properties of Cellulose Fiber Reinforced Cement Comr posites. Mater. J. 1995, 92, 172–180. [Google Scholar]
- ASTM E136-19; Standard Test Method for Assessing Combustibility of Materials Using a Vertical Tube Furnace at 750 °C. ASTM International: West Conshohocken, PA, USA, 2019.
- EN 13501-1:2018; Fire Classification of Construction Products and Building Elements—Part 1: Classification Using Data from Reaction to Fire Tests. European Committee for Standardization (CEN): Brussels, Belgium, 2018.
- Małaszkiewicz, D.; Sztukowska, M. Utilization of wastes from medium density fiberboards production as an aggregate for lightweight cement composite. Matec Web Conf. 2018, 174, 02005. [Google Scholar] [CrossRef]
- Shao, Y.; Shah, S. Mechanical properties of PVA fiber reinforced cement composites fabricated by extrusion processing. Mater. J. 1997, 94, 555–564. [Google Scholar]
- GB 50016-2014; Code for Fire Protection Design of Buildings. China Planning Press: Beijing, China, 2014.
- International Organization for Standardization (ISO). Fibre-ceent flat shets—Product Specification and Test Methods; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- ASTM International. Standard Specification for Mineral Fiber Block and Board Thermal Insulation; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- International Organization for Standardization (ISO). Cellular Plastics—Thermoplastic Materials—Specification for Rigid Foam for thermal Insulation of Buildings; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- International Organization for Standardization (ISO). Thermal Insulation Products for Building Equipment and Industrial Installations—Determination of Organic Content; ISO: Geneva, Switzerland, 2023. [Google Scholar]
- International Organization for Standardization (ISO). Refractory Products—Methods of Test for Ceramic Fibre Products; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- Maynet, W.; Samsudin, E.; Soh, N.N. Physical and mechanical properties of cement board made from oil palm empty fruit bunch fibre: A review. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Batu Pahat, Malaysia, 1–2 December 2020; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Ferreira, S.D.; Altafini, C.R.; Perondi, D.; Godinho, M. Pyrolysis of Medium Density Fiberboard (MDF) wastes in a screw reactor. Energy Convers. Manag. 2015, 92, 223–233. [Google Scholar] [CrossRef]
- Ranachowski, Z.; Ranachowski, P.; Dębowski, T.; Gorzelańczyk, T.; Schabowicz, K. Investigation of structural degradation of fiber cement boards due to thermal impact. Materials 2019, 12, 944. [Google Scholar] [CrossRef]
- Yu, Y.; Hou, J.; Dong, Z.; Wang, C.; Lu, F.; Song, P. Evaluating the flammability performance of Portland cement-bonded particleboards with different cement–wood ratios using a cone calorimeter. J. Fire Sci. 2016, 34, 199–211. [Google Scholar] [CrossRef]
- Fu, Z.; Yao, Y.J.C. Pyrolysis mechanism of natural fiber in cement-based composites at high temperatures. Constr. Build. Mater. 2022, 351, 128986. [Google Scholar] [CrossRef]
- Hou, J.; Jin, Y.; Che, W.; Yu, Y. Value-added utilization of wood processing residues into cement-bonded particleboards with admirable integrated performance. Constr. Build. Mater. 2022, 344, 128144. [Google Scholar] [CrossRef]
- Azni, M.E.; Norhan, A.S.; Lofflad, H.; Roslan, S.N. Feasibility study on empty fruit bunch (EFB) cement board. In Proceedings of the ISER 9th International Conference, Berlin, Germany, 30 October 2015. [Google Scholar]
- Chen, C.H.; Jang, L.-S.; Lei, M.-Y.; Chou, S. A comparative study of combustibility and surface flammability of building materials. Fire Mater. 1997, 21, 271–276. [Google Scholar] [CrossRef]
- Davies, I.; Davies, O. Agro-waste-cement particleboards: A review. MAYFEB J. Environ. Sci. 2017, 2, 10–26. [Google Scholar]
- Mayer, A.K.; Kuqo, A.; Koddenberg, T.; Mai, C. Seagrass-and wood-based cement boards: A comparative study in terms of physico-mechanical and structural properties. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106864. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, X.; Zhang, J.; He, C.; Tao, S.; Wu, Y.; Ding, Y. Pyrolysis characteristics and reaction mechanism of intumescent fire-retardant coating with thermogravimetry/Fourier transform infrared analysis. J. Anal. Appl. Pyrolysis 2024, 177, 106306. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C. Thermal degradation of beech wood with thermogravimetry/Fourier transform infrared analysis. Energy Convers. Manag. 2016, 120, 370–377. [Google Scholar] [CrossRef]
- Chen, R.; Lu, S.; Zhang, Y.; Lo, S. Pyrolysis study of waste cable hose with thermogravimetry/Fourier transform infrared/mass spectrometry analysis. Energy Convers. Manag. 2017, 153, 83–92. [Google Scholar] [CrossRef]
- Thirugnanam, S.; Srinivasan, R.; Anand, K.; Bhardwaj, A.; Puthilibai, G.; Madhu, P.; Karthick, A. Utilisation possibilities of waste medium-density fiberboard: A material recycling process. Mater. Today Proc. 2022, 59, 1362–1366. [Google Scholar] [CrossRef]
- Xu, X.; Chen, R.; Pan, R.; Zhang, D. Pyrolysis kinetics, thermodynamics, and volatiles of representative pine wood with thermogravimetry–Fourier transform infrared analysis. Energy Fuels 2020, 34, 1859–1869. [Google Scholar] [CrossRef]
- Correia, D.M.; Ribeiro, C.; Ferreira, J.C.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Sencadas, V. Influence of electrospinning parameters on poly (hydroxybutyrate) electrospun membranes fiber size and distribution. Polym. Eng. Sci. 2014, 54, 1608–1617. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, R.; Chen, R.; Xu, X. Pyrolysis characteristics and reaction mechanisms of pine needles. Appl. Biochem. Biotechnol. 2019, 189, 1056–1083. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Ding, Y.; Fukumoto, K.; Ezekoye, O.A.; Lu, S.; Wang, C.; Li, C. Experimental and numerical simulation of multi-component combustion of typical charring material. Combust. Flame 2020, 211, 417–429. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, G.; Fukumoto, K.; Zhao, M.; Zhang, X.; Wang, C.; Li, C. Experimental and numerical simulation of multi-component combustion of typical no-charring material. Energy 2023, 262, 125555. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Evrendilek, F.; Xie, W.; Kuo, J.; Zhang, X.; Buyukada, M. Kinetics, thermodynamics, gas evolution and empirical optimization of cattle manure combustion in air and oxy-fuel atmospheres. Appl. Therm. Eng. 2019, 149, 119–131. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, O.; Diwan, P.J.F. Non-isothermal kinetics of pseudo-components of waste biomass. Fuel 2019, 253, 1149–1161. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, D.; Li, M.; He, J.-J.; Gao, Z.-H.; Zhou, Y.; Sun, J.-H. Pyrolytic behavior of waste extruded polystyrene and rigid polyurethane by multi kinetics methods and Py-GC/MS. Fuel 2018, 222, 11–20. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, Y.; Chen, W.; Li, C.; Jiao, Y. Pyrolysis kinetics, thermodynamics, and interaction analysis of co-pyrolysis of bituminous coal and electrolytic aluminum solid waste. Fuel 2023, 333, 126375. [Google Scholar] [CrossRef]
- Dungani, R.; Jawaid, M.; Khalil, H.P.S.A.; Jasni, J.; Aprilia, S.; Hakeem, K.R.; Hartati, S.; Islam, M.N. A Review on Quality Enhancement of Oil Palm Trunk Waste by Resin Impregnation: Future Materials. BioResources 2013, 8, 3136–3156. [Google Scholar] [CrossRef]
- Sidhu, N.; Mastalski, I.; Zolghadr, A.; Patel, B.; Uppili, S.; Go, T.; Maduskar, S.; Wang, Z.; Neurock, M.; Dauenhauer, P.J. On the intrinsic reaction kinetics of polypropylene pyrolysis. Matter 2023, 6, 3413–3433. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K. Pyrolysis characteristics of biomass and biomass components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L.; Xu, Q. Experimental and theoretical study on thermal kinetics and reactive mechanism of nitrocellulose pyrolysis by traditional multi kinetics and modeling reconstruction. J. Hazard. Mater. 2020, 386, 121645. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, X.; Lu, S.; Zhang, Y.; Lo, S. Pyrolysis study of waste phenolic fibre-reinforced plastic by thermogravimetry/Fourier transform infrared/mass spectrometry analysis. Energy Convers. Manag. 2018, 165, 555–566. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef] [PubMed]
- Derakhshesh, Z.; Khorasani, M.; Akhlaghi, S.; Keyvani, B.; Alvani, A.A.S. Design and optimization of an intumescent flame retardant coating using thermal degradation kinetics and Taguchi’s experimental design. Polym. Int. 2012, 61, 926–933. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Xu, X.; Zhang, D. Pyrolysis kinetics and reaction mechanism of representative non-charring polymer waste with micron particle size. Energy Convers. Manag. 2019, 198, 111923. [Google Scholar] [CrossRef]
- Chen, T.; Li, L.; Zhao, R.; Wu, J. Pyrolysis kinetic analysis of the three pseudocomponents of biomass–cellulose, hemicellulose and lignin: Sinusoidally modulated temperature method. J. Therm. Anal. Calorim. 2017, 128, 1825–1832. [Google Scholar] [CrossRef]
- Zeng, F.-G.; Jia, J.-B. Reaction types and kinetics of methane generation from Huolinhe lignite pyrolysis by TG/MS experiment and quantum chemical calculations. Acta Phys.-Chim. Sin. 2009, 25, 1117–1124. [Google Scholar]
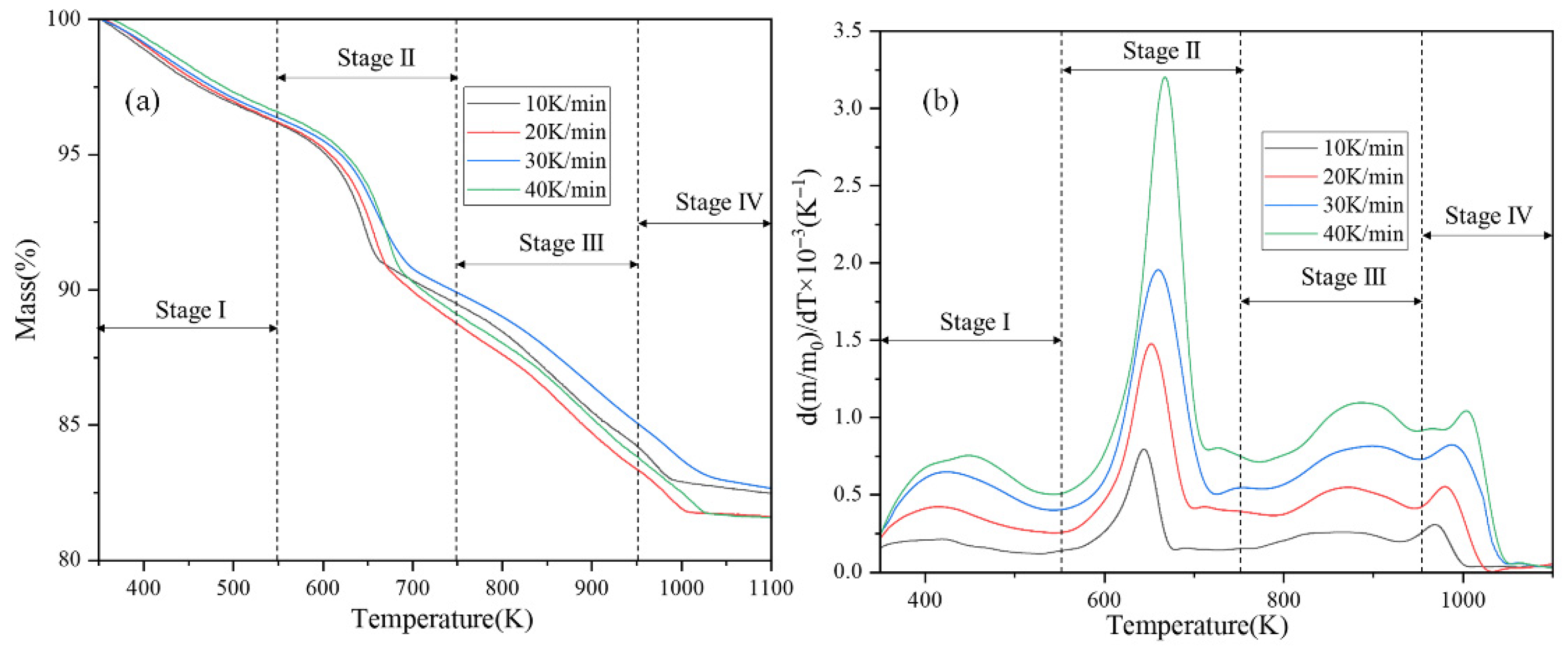
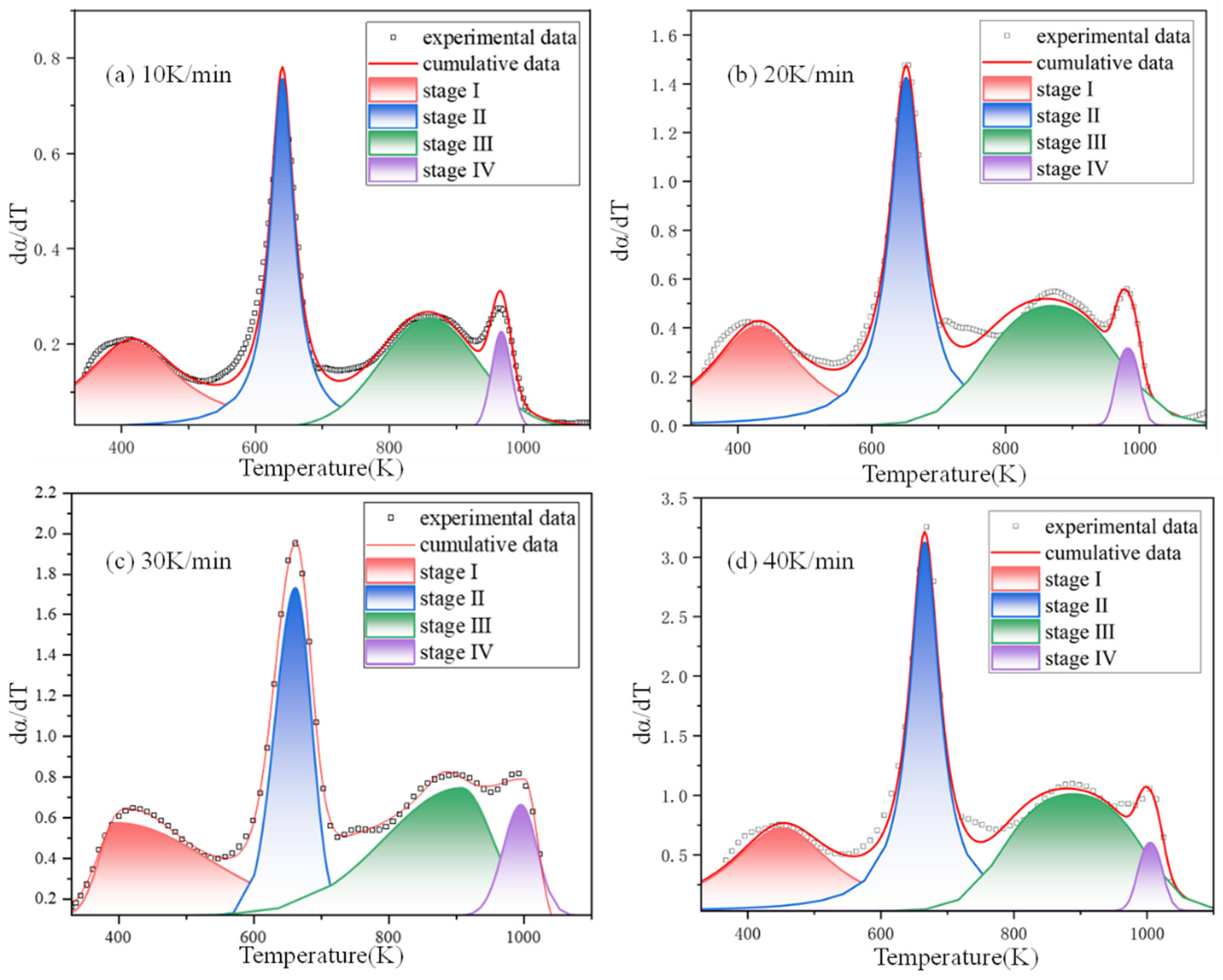
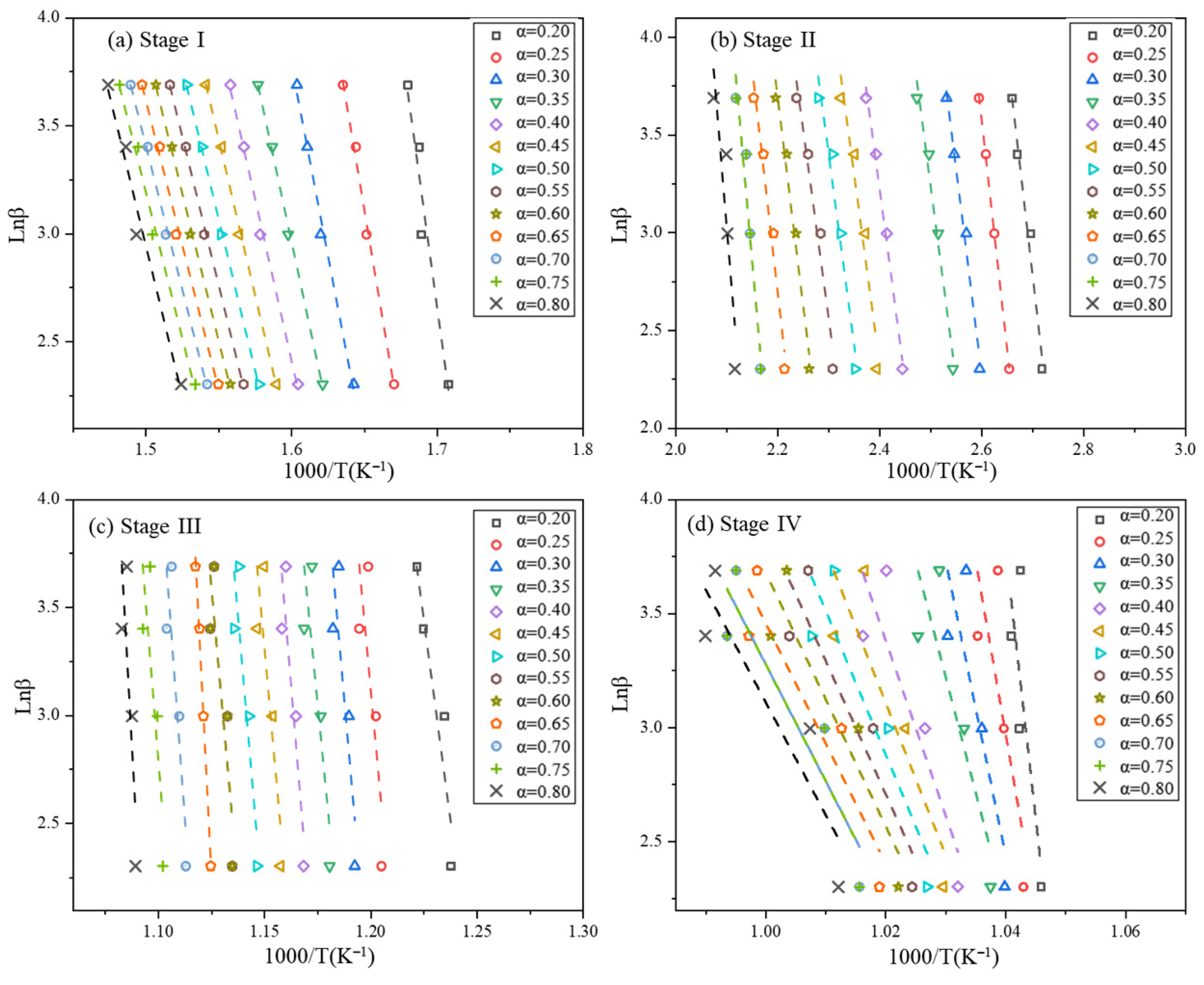
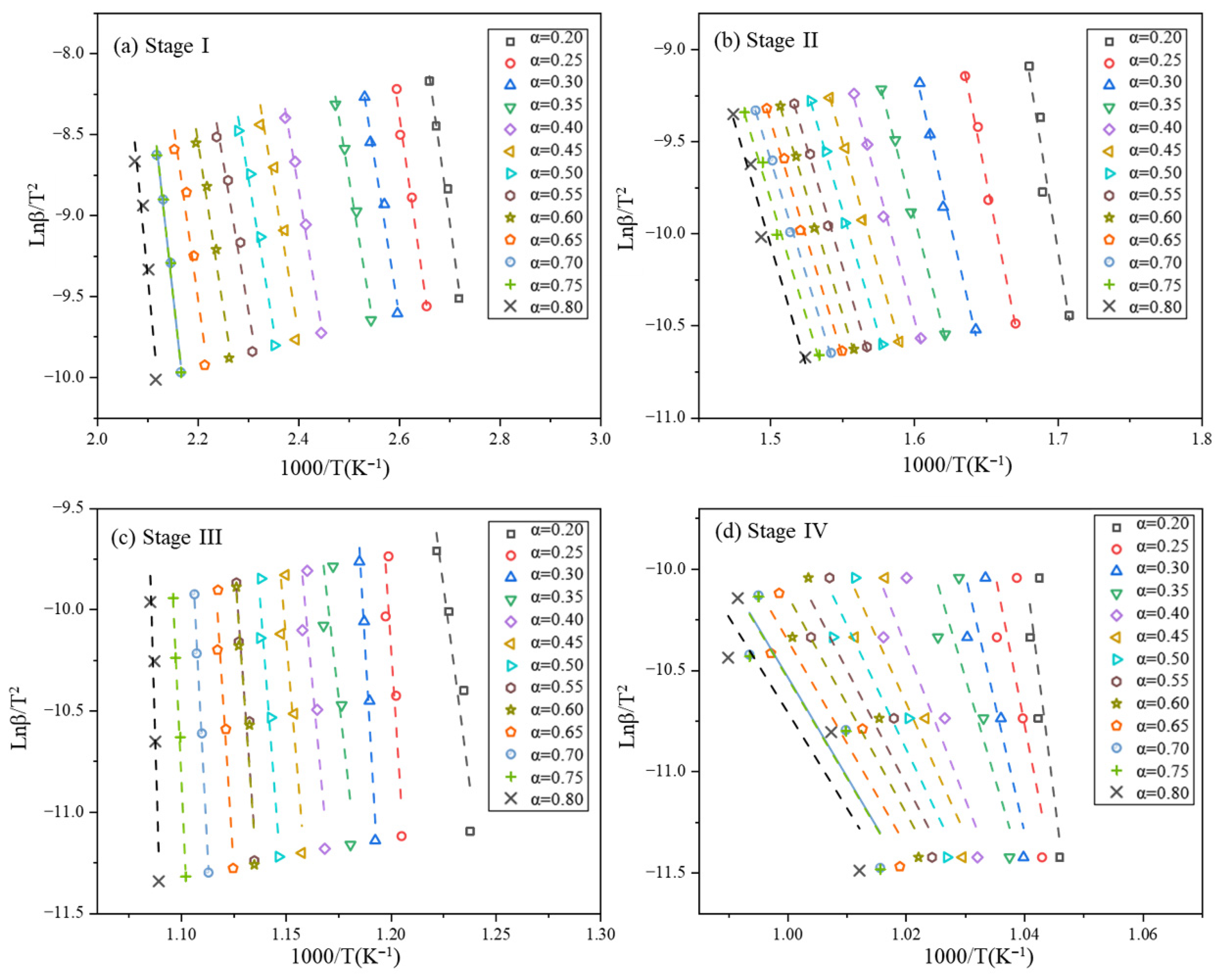
| Material Type | Fire Rating (GB 8624 Standard) | Freeze–Thaw Cycle Resistance (Cycles) | Key Freeze–Thaw Indicators (Post-Freeze Performance Retention Rate) | Applicable Building Types & Requirements | Implementation Standard/Reference |
|---|---|---|---|---|---|
| Cement fiber board | Class A (non-combustible) | ≥50 cycles (−20~20 °C) | Flexural strength retention ≥ 85%, compressive strength ≥ 90% | High-rise buildings, public places, underground projects (must meet Class A external insulation requirements of GB50016 [9]) | ISO 8336:2017 [10] |
| Rock wool board | Class A (non-combustible) | Not applicable (inorganic material, good freeze resistance) | — | Severe cold region external insulation, fire isolation belt (Class A mandatory for public buildings and residential) | ASTM C612 [11] |
| Extruded polystyrene board (XPS) | Class B1 (hardly combustible) | ≤25 cycles (prone to cracking) | Mass loss rate ≤ 5% | Low-rise residential, non-crowded public spaces (building height < 24 m, B2 grade allowed) | ISO 4898:2018 [12] |
| EPS foam board | Class B2 (combustible) | ≤15 cycles (high water absorption) | Relative dynamic modulus loss ≤ 30% | Temporary buildings, low-risk buildings (strictly prohibited in high-rise and crowded public buildings) | ISO 4898:2018 [12] |
| Perlite board | Class A (non-combustible) | Not applicable (indoor dry environment) | — | Roof insulation, top-level insulation (must meet Class A fireproof insulation, e.g., stair exits, escape routes) | ISO 6334:2023 [13] |
| Ceramic fiber board | Class A (non-combustible) | ≥100 cycles (high temperature, freeze–thaw resistant) | Mass loss rate ≤ 3% | High-temperature industrial equipment, fireproof core (dual resistance to heat and freeze–thaw) | ISO 10635:1999 [14] |
| No. | Reaction Model | ||
|---|---|---|---|
| 1 | 1 | Zero-order | |
| 2 | First-order | ||
| 3 | Nucleation | ||
| 4 | Assumed random nucleation and its subsequent growth | ||
| 5 | Assumed random nucleation and its subsequent growth | ||
| 6 | Assumed random nucleation and its subsequent growth | ||
| 7 | Chemical reaction | ||
| 8 | Chemical reaction | ||
| 9 | Chemical reaction | ||
| 10 | Chemical reaction | ||
| 11 | Chemical reaction | ||
| 12 | Chemical reaction | ||
| 13 | One-dimensional diffusion | ||
| 14 | ) | Two-dimensional diffusion | |
| 15 | Three-dimensional diffusion, spherical symmetry | ||
| 16 | Three-dimensional diffusion, cylindrical symmetry | ||
| 17 | Contracting cylinder | ||
| 18 | Contracting sphere |
| Stage | Parameter | 10 K/min | 20 K/min | 30 K/min | 40 K/min |
|---|---|---|---|---|---|
| Stage I | 0 | 0 | 0 | 0 | |
| 364.99 | 384.82 | 405.43 | 449.06 | ||
| H | 0.16 | 0.33 | 0.45 | 0.64 | |
| 18.83 | 26.79 | 32.79 | 76.53 | ||
| 160.59 | 91.36 | 77.11 | 215.41 | ||
| Stage II | 0 | 0 | 0 | 0 | |
| 645.35 | 656.45 | 662.87 | 667.81 | ||
| H | 0.55 | 1.14 | 1.51 | 2.53 | |
| 29.46 | 27.17 | 30.36 | 23.33 | ||
| 16.75 | 16.18 | 21.85 | 17.75 | ||
| Stage III | 0 | 0 | 0 | 0 | |
| 887.79 | 914.37 | 906.63 | 885.01 | ||
| H | 0.22 | 0.49 | 0.57 | 0.70 | |
| 126.55 | 270.01 | 202.78 | 140.70 | ||
| 36.54 | 33.91 | 43.44 | 32.77 | ||
| Stage IV | 0 | 0 | 0 | 0 | |
| 968.79 | 984.91 | 1000.97 | 1004.98 | ||
| H | 0.22 | 0.49 | 0.75 | 0.95 | |
| 23.55 | 20.85 | 20.18 | 77.80 | ||
| 17.86 | 17.43 | 19.36 | 18.74 |
| (a) Stage I and II | |||||||||
| Stage I α | * FWO | R2 | * KAS | R2 | Stage II α | * FWO | R2 | * KAS | R2 |
| 0.30 | 137.003 | 0.98 | 138.615 | 0.98 | 0.30 | 240.465 | 0.99 | 260.791 | 0.99 |
| 0.35 | 131.347 | 0.97 | 132.544 | 0.97 | 0.35 | 227.344 | 1.00 | 247.241 | 1.00 |
| 0.40 | 131.179 | 0.99 | 132.495 | 0.99 | 0.40 | 219.026 | 1.00 | 238.6855 | 1.00 |
| 0.45 | 130.241 | 0.96 | 131.097 | 0.96 | 0.45 | 212.088 | 1.00 | 231.544 | 1.00 |
| 0.50 | 130.423 | 0.98 | 131.355 | 0.97 | 0.50 | 208.779 | 1.00 | 228.173 | 0.99 |
| 0.55 | 129.325 | 0.96 | 129.974 | 0.96 | 0.55 | 206.456 | 1.00 | 225.820 | 1.00 |
| 0.60 | 142.879 | 0.98 | 143.498 | 0.99 | 0.60 | 201.986 | 1.00 | 221.225 | 1.00 |
| 0.70 | 137.385 | 0.97 | 138.575 | 0.97 | 0.65 | 200.899 | 0.99 | 220.149 | 1.00 |
| * Ave | 133.200 | 134.226 | 0.70 | 204.361 | 1.00 | 223.811 | 1.00 | ||
| 0.75 | 209.658 | 0.99 | 231.64 | 0.99 | |||||
| 0.80 | 206.480 | 0.98 | 228.384 | 0.98 | |||||
| * Ave | 211.635 | 233.455 | |||||||
| (b) Stage III and IV | |||||||||
| Stage III α | * FWO | R2 | * KAS | R2 | Stage IV α | * FWO | R2 | * KAS | R2 |
| 0.30 | 270.881 | 0.90 | 272.661 | 0.93 | 0.30 | 457.531 | 0.92 | 454.545 | 0.93 |
| 0.40 | 286.094 | 0.93 | 284.765 | 0.92 | 0.40 | 403.806 | 0.92 | 403.105 | 0.92 |
| 0.50 | 210.583 | 0.94 | 209.794 | 0.93 | 0.50 | 427.963 | 0.90 | 425.515 | 0.91 |
| 0.60 | 248.098 | 0.95 | 247.135 | 0.96 | 0.70 | 457.531 | 0.92 | 454.545 | 0.92 |
| 0.70 | 225.608 | 0.96 | 223.965 | 0.97 | 0.80 | 403.806 | 0.93 | 403.105 | 0.91 |
| 0.80 | 241.147 | 0.97 | 239.154 | 0.96 | * Ave | 427.963 | 425.51 | ||
| * Ave | 247.069 | 246.246 | |||||||
| (a) Stage I | |||||||||
| NO. | 10 K/min | R2 | 20 K/min | R2 | 30 K/min | R2 | 40 K/min | R2 | |
| 1 | 13.60 | 0.82 | 14.22 | 0.85 | 14.64 | 0.84 | 15.33 | 0.89 | |
| 2 | 22.25 | 0.96 | 23.08 | 0.97 | 23.74 | 0.97 | 24.48 | 0.98 | |
| 3 | 24.02 | 0.87 | 24.97 | 0.89 | 25.59 | 0.88 | 26.67 | 0.92 | |
| 4 | 51.75 | 0.97 | 53.44 | 0.98 | 54.74 | 0.98 | 56.31 | 0.99 | |
| 5 | 81.25 | 0.98 | 83.81 | 0.98 | 85.74 | 0.98 | 88.15 | 0.99 | |
| 6 | * 110.75 | * 0.98 | * 114.18 | * 0.98 | * 116.75 | * 0.98 | * 119.98 | * 0.99 | |
| 7 | 16.12 | 0.88 | 16.80 | 0.90 | 17.28 | 0.9 | 18.00 | 0.93 | |
| 8 | 19.77 | 0.94 | 20.54 | 0.95 | 21.13 | 0.95 | 21.87 | 0.97 | |
| 9 | 27.87 | 0.99 | 28.82 | 0.99 | 29.65 | 0.99 | 30.39 | 0.99 | |
| 10 | 34.32 | 0.99 | 35.40 | 0.99 | 36.44 | 0.99 | 37.17 | 0.99 | |
| 11 | 49.29 | 0.98 | 50.69 | 0.97 | 52.19 | 0.98 | 52.88 | 0.96 | |
| 12 | 49.29 | 0.98 | 67.97 | 0.95 | 70.00 | 0.96 | 70.63 | 0.93 | |
| 13 | 34.44 | 0.89 | 35.73 | 0.90 | 36.54 | 0.9 | 38.01 | 0.93 | |
| 14 | 39.19 | 0.92 | 40.60 | 0.93 | 41.53 | 0.93 | 43.05 | 0.96 | |
| 15 | 45.24 | 0.95 | 46.78 | 0.96 | 47.88 | 0.96 | 49.44 | 0.98 | |
| 16 | 41.18 | 0.93 | 42.63 | 0.94 | 43.62 | 0.94 | 45.15 | 0.96 | |
| 17 | 17.51 | 0.91 | 18.22 | 0.92 | 18.75 | 0.92 | 19.47 | 0.95 | |
| 18 | 18.99 | 0.93 | 19.74 | 0.94 | 20.31 | 0.94 | 21.04 | 0.97 | |
| (b) Stage II | |||||||||
| NO. | g( ) | 10 K/min | R2 | 20 K/min | R2 | 30 K/min | R2 | 40 K/min | R2 |
| 1 | 54.60 | 0.86 | 54.48 | 0.90 | 47.86 | 0.92 | 59.03 | 0.96 | |
| 2 | 81.95 | 0.94 | 81.22 | 0.97 | 71.78 | 0.98 | 86.23 | 0.98 | |
| 3 | 87.25 | 0.87 | 87.14 | 0.91 | 77.19 | 0.93 | 93.98 | 0.96 | |
| 4 | 174.60 | 0.95 | 173.26 | 0.97 | 154.36 | 0.98 | 183.31 | 0.99 | |
| 5 | 267.25 | 0.95 | 265.31 | 0.98 | 236.93 | 0.99 | 280.39 | 0.99 | |
| 6 | 359.90 | 0.95 | 357.35 | 0.98 | 319.51 | 0.99 | 377.47 | 0.99 | |
| 7 | 62.51 | 0.89 | 62.24 | 0.93 | 54.81 | 0.94 | 66.96 | 0.97 | |
| 8 | 74.07 | 0.93 | 73.53 | 0.96 | 64.92 | 0.97 | 78.45 | 0.98 | |
| 9 | 99.88 | 0.97 | 98.65 | 0.98 | 87.34 | 0.99 | 103.83 | 0.98 | |
| 10 | 120.56 | 0.98 | 118.71 | 0.99 | 105.23 | 0.99 | 124.02 | 0.97 | |
| 11 | 168.64 | 0.98 | 165.30 | 0.97 | 146.75 | 0.98 | 170.80 | 0.93 | |
| 12 | * 222.93 | * 0.96 | * 217.92 | * 0.95 | * 203.66 | * 0.96 | * 223.60 | * 0.90 | |
| 13 | 119.90 | 0.88 | 119.79 | 0.92 | 106.52 | 0.93 | 128.92 | 0.96 | |
| 14 | 134.79 | 0.90 | 134.41 | 0.94 | 119.63 | 0.95 | 143.89 | 0.97 | |
| 15 | 153.90 | 0.93 | 153.08 | 0.96 | 136.32 | 0.97 | 162.86 | 0.98 | |
| 16 | 141.07 | 0.91 | 140.55 | 0.95 | 125.12 | 0.96 | 150.13 | 0.98 | |
| 17 | 66.90 | 0.91 | 66.54 | 0.94 | 58.66 | 0.96 | 71.34 | 0.98 | |
| 18 | 71.60 | 0.92 | 71.13 | 0.95 | 62.77 | 0.97 | 76.00 | 0.98 | |
| (c) Stage III | |||||||||
| NO. | g( ) | 10 K/min | R2 | 20 K/min | R2 | 30 K/min | R2 | 40 K/min | R2 |
| 1 | 135.98 | 0.82 | 142.19 | 0.85 | 166.94 | 0.88 | 153.30 | 0.89 | |
| 2 | 222.54 | 0.96 | 230.76 | 0.97 | 253.15 | 0.96 | 244.82 | 0.98 | |
| 3 | * 240.19 | * 0.87 | * 249.74 | * 0.89 | * 269.86 | * 0.89 | * 266.70 | * 0.92 | |
| 4 | 517.53 | 0.97 | 534.45 | 0.98 | 545.19 | 0.97 | 563.14 | 0.99 | |
| 5 | 812.53 | 0.97 | 838.14 | 0.98 | 837.26 | 0.97 | 881.46 | 0.99 | |
| 6 | 1141.83 | 0.98 | 1387.39 | 0.97 | 1129.32 | 0.97 | 1533.35 | 0.94 | |
| 7 | 161.16 | 0.88 | 167.98 | 0.90 | 192.02 | 0.91 | 180.01 | 0.93 | |
| 8 | 197.73 | 0.93 | 205.39 | 0.95 | 228.42 | 0.95 | 218.66 | 0.97 | |
| 9 | 278.70 | 0.98 | 288.15 | 0.99 | 309.06 | 0.98 | 303.94 | 0.99 | |
| 10 | 343.20 | 0.99 | 354.03 | 0.99 | 373.32 | 0.99 | 371.71 | 0.99 | |
| 11 | 492.92 | 0.97 | 506.88 | 0.97 | 522.45 | 0.98 | 528.79 | 0.96 | |
| 12 | 662.17 | 0.95 | 679.66 | 0.95 | 691.11 | 0.96 | 706.33 | 0.93 | |
| 13 | 367.11 | 0.90 | 357.29 | 0.90 | 372.79 | 0.9 | 380.10 | 0.93 | |
| 14 | 391.94 | 0.92 | 405.98 | 0.93 | 420.11 | 0.93 | 430.54 | 0.96 | |
| 15 | 452.35 | 0.95 | 467.79 | 0.96 | 480.28 | 0.95 | 494.37 | 0.98 | |
| 16 | 411.81 | 0.93 | 426.31 | 0.94 | 439.91 | 0.94 | 451.55 | 0.96 | |
| 17 | 175.09 | 0.93 | 182.23 | 0.92 | 205.88 | 0.93 | 194.74 | 0.95 | |
| 18 | 189.94 | 0.94 | 197.42 | 0.94 | 220.67 | 0.94 | 210.44 | 0.97 | |
| (d) Stage IV | |||||||||
| NO. | g( ) | 10 K/min | R2 | 20 K/min | R2 | 30 K/min | R2 | 40 K/min | R2 |
| 1 | 136.76 | 0.94 | 325.76 | 0.91 | 209.49 | 0.89 | 232.06 | 0.93 | |
| 2 | 214.44 | 0.90 | 468.74 | 0.98 | 323.63 | 0.94 | 333.68 | 0.98 | |
| 3 | 213.46 | 0.96 | 496.81 | 0.91 | 330.16 | 0.92 | 356.33 | 0.93 | |
| 4 | 445.54 | 0.91 | 953.82 | 0.98 | 679.09 | 0.95 | 683.83 | 0.98 | |
| 5 | 676.63 | 0.92 | 1438.90 | 0.98 | 1034.59 | 0.95 | 1033.99 | 0.98 | |
| 6 | 907.72 | 0.92 | 1923.97 | 0.98 | 1390.08 | 0.95 | 1384.14 | 0.98 | |
| 7 | 158.86 | 0.89 | 367.33 | 0.94 | 242.31 | 0.95 | 261.70 | 0.95 | |
| 8 | 191.73 | 0.96 | 427.73 | 0.96 | 290.57 | 0.90 | 304.62 | 0.97 | |
| 9 | 266.72 | 0.86 | 561.69 | 0.99 | 399.11 | 0.89 | 399.36 | 0.99 | |
| 10 | * 327.65 | * 0.92 | * 468.51 | * 1.00 | * 486.49 | * 0.92 | * 474.65 | * 0.99 | |
| 11 | 470.37 | 0.89 | 916.53 | 0.99 | 690.44 | 0.96 | 649.25 | 0.96 | |
| 12 | 631.69 | 0.92 | 1196.85 | 0.97 | 921.19 | 0.98 | 846.64 | 0.94 | |
| 13 | 290.17 | 0.97 | 667.85 | 0.91 | 450.85 | 0.93 | 480.60 | 0.93 | |
| 14 | 331.48 | 0.91 | 746.24 | 0.94 | 512.46 | 0.97 | 536.57 | 0.95 | |
| 15 | 385.98 | 0.97 | 846.06 | 0.96 | 592.34 | 0.91 | 607.46 | 0.97 | |
| 16 | 349.34 | 0.93 | 779.06 | 0.95 | 538.68 | 0.88 | 559.90 | 0.96 | |
| 17 | 171.28 | 0.92 | 390.32 | 0.95 | 260.62 | 0.97 | 278.06 | 0.96 | |
| 18 | 184.67 | 0.95 | 414.86 | 0.96 | 280.25 | 0.89 | 295.49 | 0.97 | |
| Gas Products/Functional Groups | Wavenumber Band (cm−1) |
|---|---|
| CO2 | 2400–2260, 680–660 |
| H2O | 4000–3500, 1800–1300 |
| -CH3 | 3100–2750, 1380–1350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Tang, L.; Hu, Y.; Yang, C.; Deng, W.; Ding, Y. Pyrolysis Characteristics and Reaction Mechanism of Cement Fiberboard with Thermogravimetry/Fourier Transform Infrared Analysis. Fire 2025, 8, 426. https://doi.org/10.3390/fire8110426
Zhu Y, Tang L, Hu Y, Yang C, Deng W, Ding Y. Pyrolysis Characteristics and Reaction Mechanism of Cement Fiberboard with Thermogravimetry/Fourier Transform Infrared Analysis. Fire. 2025; 8(11):426. https://doi.org/10.3390/fire8110426
Chicago/Turabian StyleZhu, Yuxiang, Longjiang Tang, Ying Hu, Chunlin Yang, Weijian Deng, and Yanming Ding. 2025. "Pyrolysis Characteristics and Reaction Mechanism of Cement Fiberboard with Thermogravimetry/Fourier Transform Infrared Analysis" Fire 8, no. 11: 426. https://doi.org/10.3390/fire8110426
APA StyleZhu, Y., Tang, L., Hu, Y., Yang, C., Deng, W., & Ding, Y. (2025). Pyrolysis Characteristics and Reaction Mechanism of Cement Fiberboard with Thermogravimetry/Fourier Transform Infrared Analysis. Fire, 8(11), 426. https://doi.org/10.3390/fire8110426








