Development and Evaluation of Steel Component Coatings for Substations/Converter Stations with Both Fire and Corrosion Prevention Functions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Development of Dual-Function Coating Formula
3.1.1. Substrate
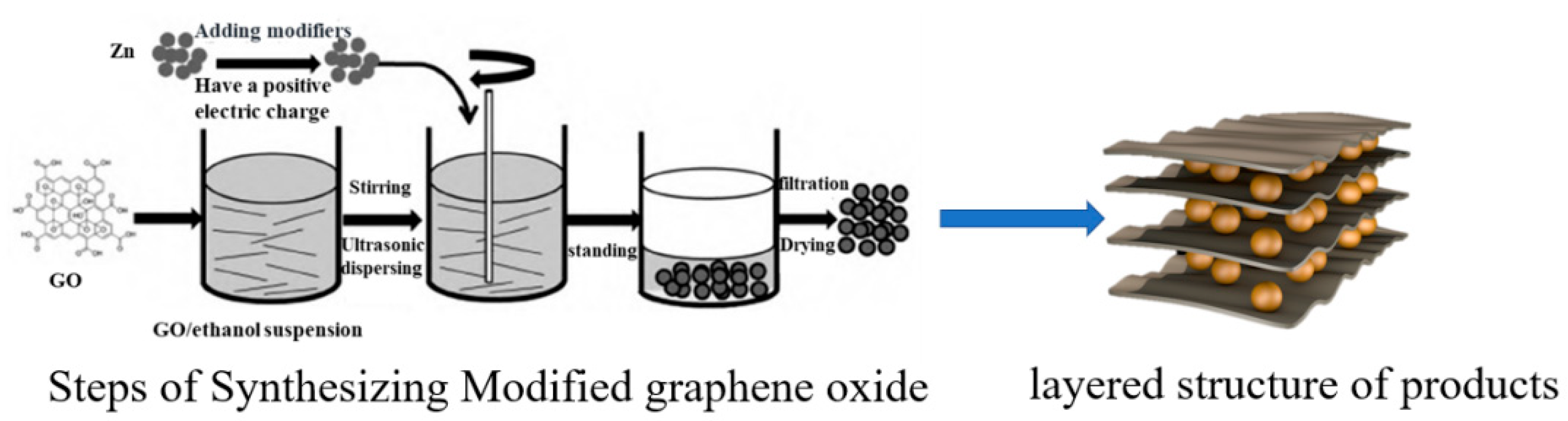
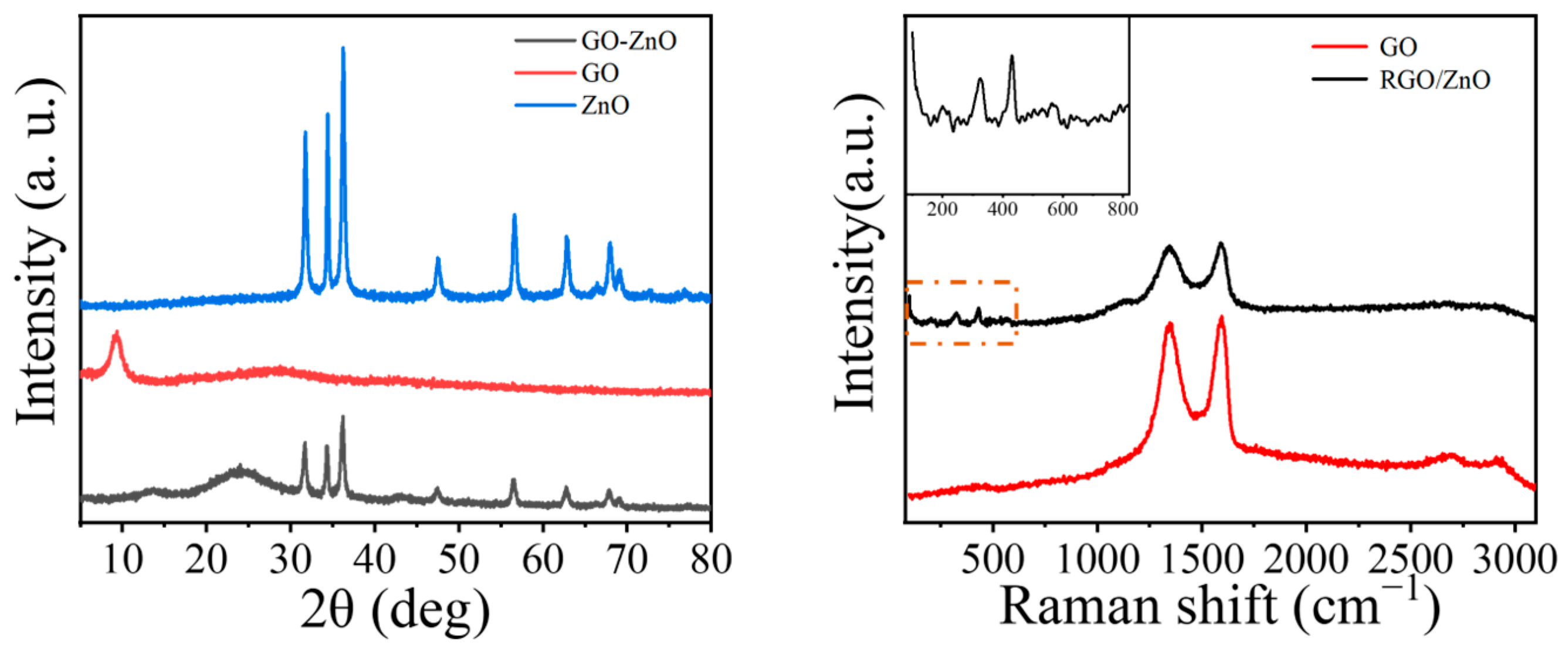
3.1.2. Synthesis of Two-Dimensional Material GO/ZnO
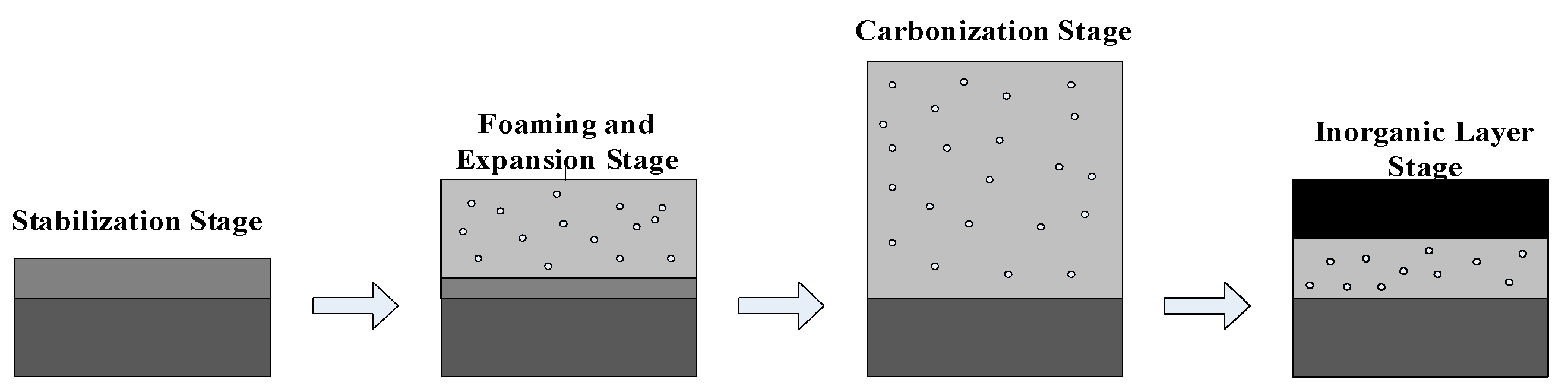
3.1.3. Synthesis of Fire-Retardant and Anti-Corrosion Coatings
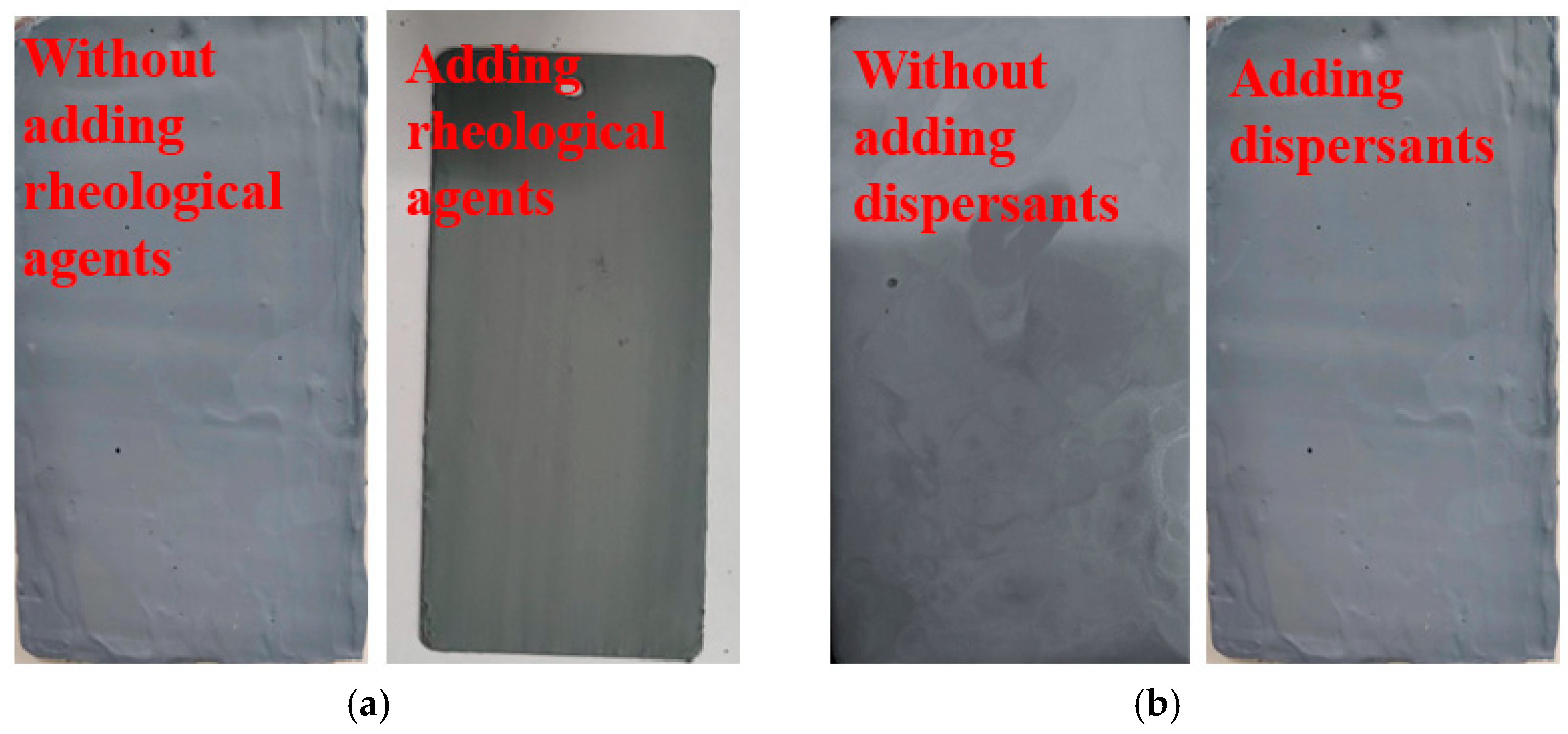
3.1.4. Additives
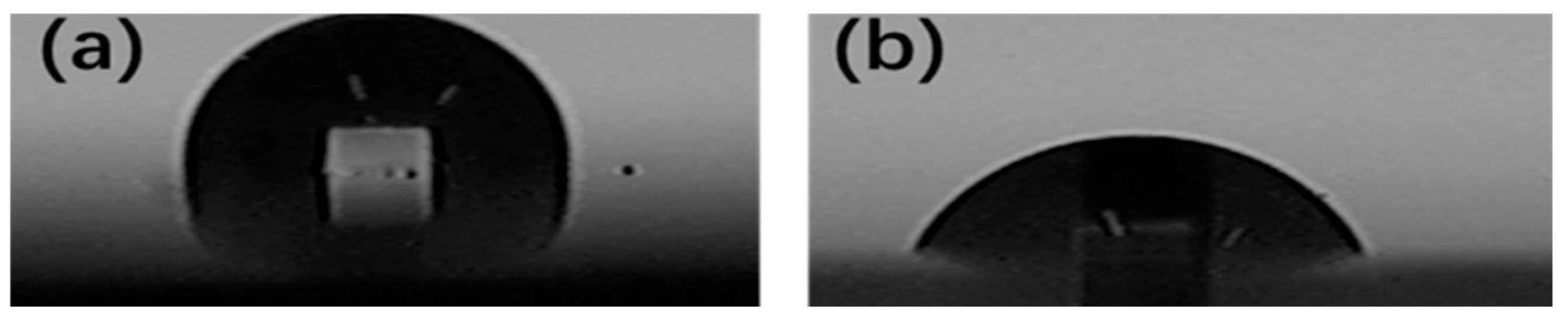
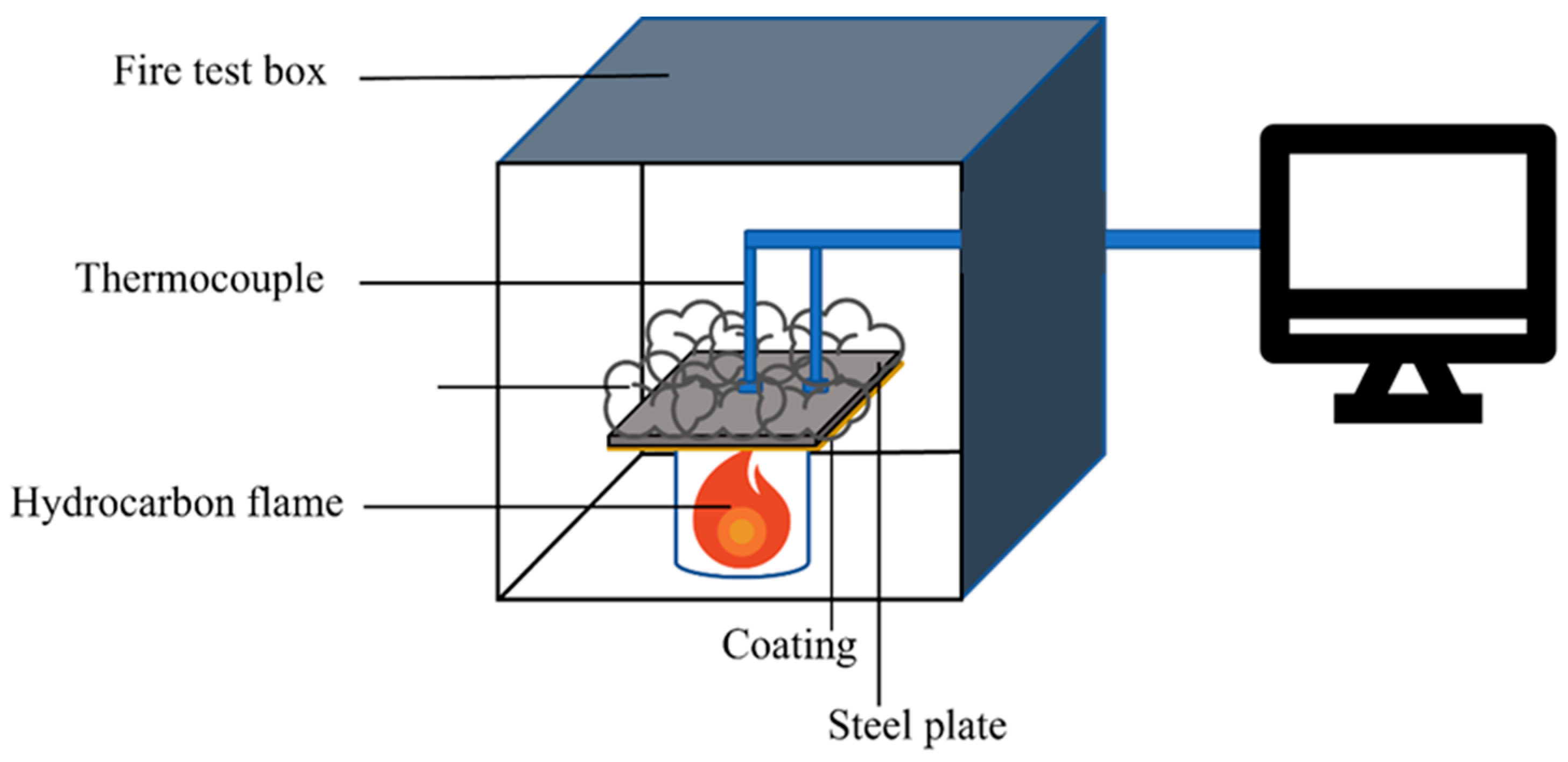
3.2. Dual-Function Coating Performance Test
- (1)
- Coatings of various thicknesses were applied to carbon steel substrates (a Q235 steel plate with a thickness of 0.5 mm, a yield strength of 235 MPa, a maximum tensile strength of 460 MPa, an elongation of 26%, and an elastic modulus of 200 GPa), and their adhesion strength (or pull strength) was repeatedly tested according to standard tensile testing methods. The testing ensured that the adhesion strength of the 3 mm thick coating met or exceeded Grade I standards (pull strength ≥ 0.5 MPa).
- (2)
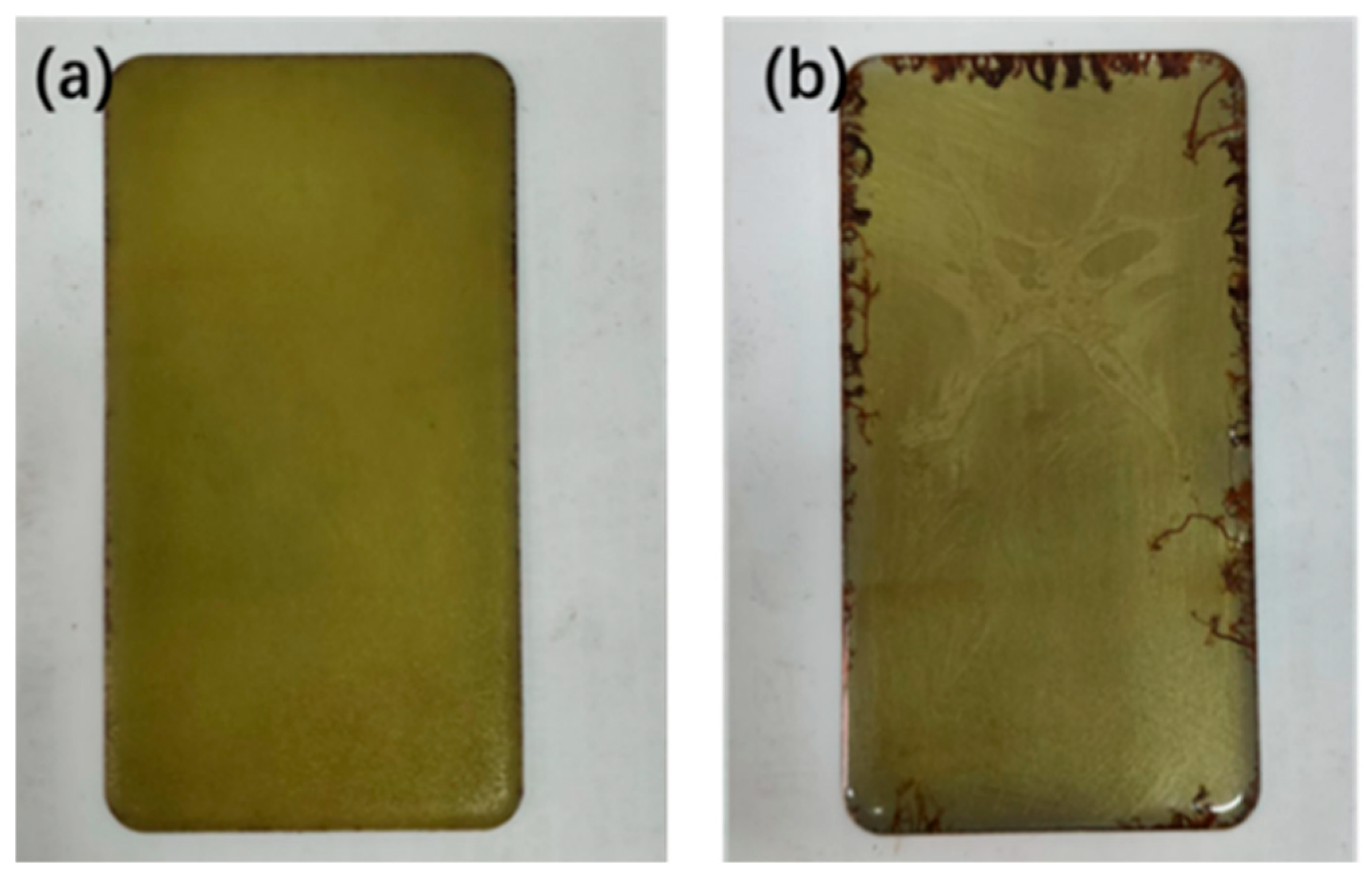
- Neutral salt spray corrosion resistance tests were conducted until the time to the first appearance of rust was no less than 1000 h.
- (3)
- Coatings were subjected to irradiation aging tests using the filtered xenon-arc lamp light, with continuous exposure for 1000 h. The comprehensive aging performance level of the protective coatings should not be lower than Grade 2.
4. Conclusions
- (1)
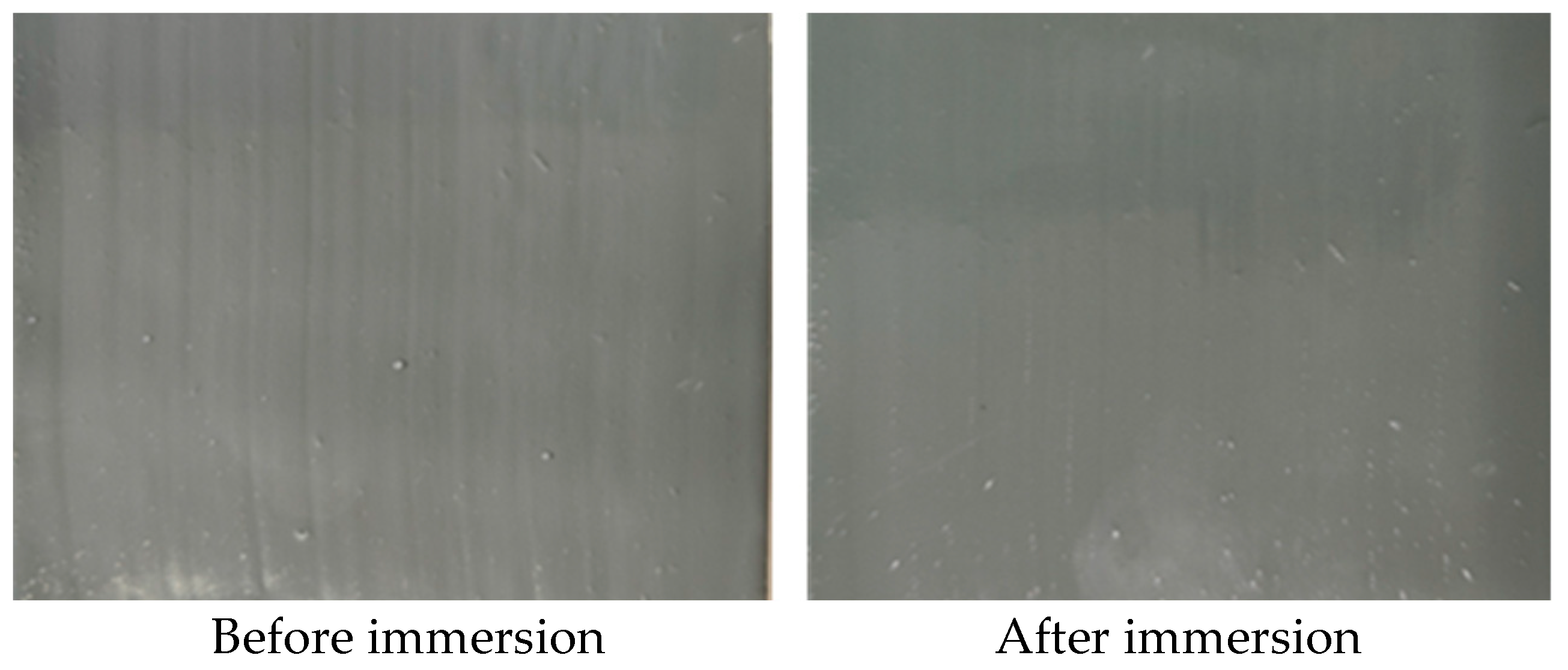
- By modifying epoxy resin with silicone, silicone segments were successfully introduced into the epoxy resin. Contact angle tests and salt-water corrosion experiments demonstrated that the modified resin exhibits good anti-corrosion properties.
- (2)
- Zinc oxide-modified graphene oxide was prepared as fireproof filler through electrostatic self-assembly. By adjusting the ratio of coating resin material to fireproof filler, an intumescent fireproof coating was obtained. In the laboratory fire resistance limit test, the coating exhibited excellent intumescent fireproof performance.
- (3)
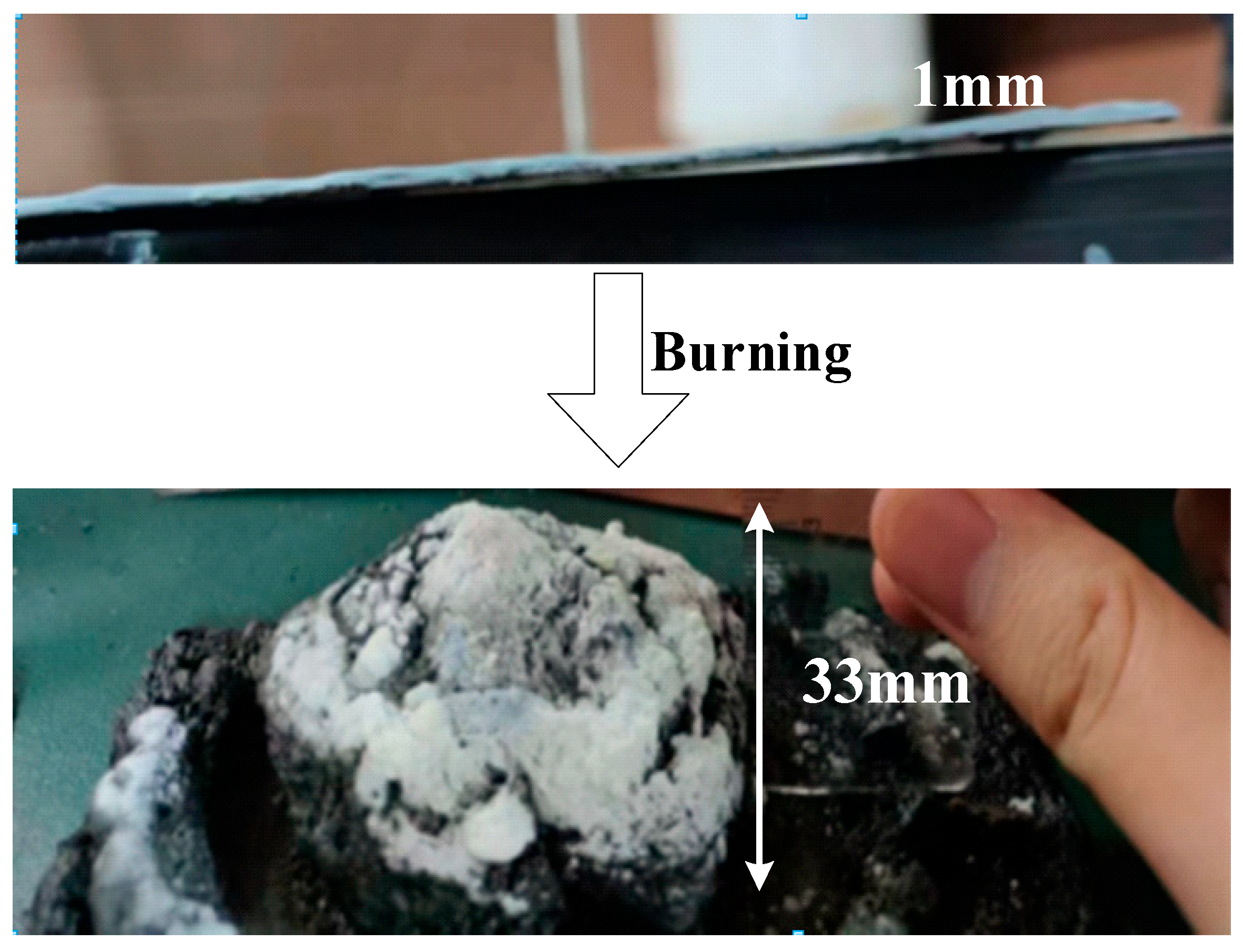
- In accordance with GB14907-2018 Fire Resistive Coating for Steel Structure, the fireproof performance of the material was evaluated. During the fire-resistance limit test, the average temperature of the specimen remained at 505 °C within 2 h, meeting China’s national standards for fireproof coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, H. Research on New Type of Steel Structure Coating with Fireproof, Heat Preservation and Antiseptic Functions. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2018. [Google Scholar]
- Huang, Y.; Jiang, S.; Liang, R.; Sun, P.; Hai, Y.; Zhang, L. Thermal-triggered insulating fireproof layers: A novel fire-extinguishing MXene composites coating. Chem. Eng. J. 2020, 391, 123621. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, F.; Yu, J.; Yu, K.; Tian, L. Development of ultra-high ductility engineered cementitious composites as a novel and resilient fireproof coating. Constr. Build. Mater. 2021, 288, 123090. [Google Scholar] [CrossRef]
- Ma, X.; Pan, J.; Cai, J.; Zhang, Z.; Han, J. A review on cement-based materials used in steel structures as fireproof coating. Constr. Build. Mater. 2022, 315, 125623. [Google Scholar] [CrossRef]
- Dagdag, O.; El Harfi, A.; Essamri, A.; El Gouri, M.; Chraibi, S.; Assouag, M.; Benzidia, B.; Hamed, O.; Lgaz, H.; Jodeh, S. Phosphorous-based epoxy resin composition as an effective anticorrosive coating for steel. Int. J. Ind. Chem. 2018, 9, 231–240. [Google Scholar] [CrossRef]
- Dagdag, O.; Hsissou, R.; El Harfi, A.; Berisha, A.; Safi, Z.; Verma, C.; Ebenso, E.; Touhami, M.E.; El Gouri, M. Fabrication of polymer based epoxy resin as effective anti-corrosive coating for steel: Computational modeling reinforced experimental studies. Surfaces Interfaces 2020, 18, 100454. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Cui, G.; Bi, Z.; Wang, S.; Liu, J.; Xing, X.; Li, Z.; Wang, B. A comprehensive review on smart anti-corrosive coatings. Prog. Org. Coat. 2020, 148, 105821. [Google Scholar] [CrossRef]
- Kumar, S.A.; Narayanan, T.S. Thermal properties of siliconized epoxy interpenetrating coatings. Prog. Org. Coat. 2002, 45, 323–330. [Google Scholar] [CrossRef]
- Díaz, I.; Chico, B.; De La Fuente, D.; Simancas, J.; Vega, J.; Morcillo, M. Corrosion resistance of new epoxy–siloxane hybrid coatings. A laboratory study. Prog. Org. Coat. 2010, 69, 278–286. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y.; Fuseini, M. Recent progress in Epoxy Nanocomposites: Corrosion, structural, flame retardancy and appli-cations—A comprehensive review. Polym. Adv. Technol. 2023, 34, 3438–3472. [Google Scholar] [CrossRef]
- Luo, F.; Wu, K.; Guo, H.; Zhao, Q.; Lu, M. Simultaneous reduction and surface functionalization of graphene oxide for enhancing flame retardancy and thermal conductivity of mesogenic epoxy composites. Polym. Int. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Li, X.; Yu, L.; Zhang, Z.; Wu, Z. Zinc ferrite nanoparticle decorated boron nitride nanosheet: Preparation, magnetic field arrangement, and flame retardancy. Chem. Eng. J. 2019, 356, 680–692. [Google Scholar] [CrossRef]
- Yokomachi, K.; Seino, M.; Grunzinger, S.J.; Hayakawa, T.; Kakimoto, M.-A. Synthesis and degree of branching of epoxy-terminated hyperbranched polysiloxysilane. Polym. J. 2008, 40, 198–204. [Google Scholar] [CrossRef]
- Li, X. POSS Modified Bisphthalonitrile Heat-Resistant Resin System Design, Preparation, and Modification Mechanism. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- Yang, X. Study on Modification of Bisphenol A Type Nitrile Resin and Its Composite Materials. Ph.D. Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2019. [Google Scholar]
- Wang, P. Study on the Synergistic Effects of Components in Intumescent Fire Retardant Coating. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2017. [Google Scholar]
- Chen, Y.; Zhou, C.; Chang, J.; Zou, H.; Liang, M. The effect of epoxy–silicone copolymer content on the thermal and mechanical properties of cured epoxy resin modified with siloxane. RSC Adv. 2014, 4, 60685–60693. [Google Scholar] [CrossRef]
- Heng, Z.; Zeng, Z.; Chen, Y.; Zou, H.; Liang, M. Silicone modified epoxy resins with good toughness, damping properties and high thermal residual weight. J. Polym. Res. 2015, 22, 203. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Gong, X.; Yao, E.; Liu, T.; Zhang, Y.; Zou, H. Thermal conductivity of Graphene-polymer composites: Implications for thermal management. Heat Mass Transf. 2020, 56, 1931–1945. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications; Springer: Berlin/Heidelberg, Germany, 2015; pp. 61–95. [Google Scholar]
- Morales-Acosta, D.; Flores-Oyervides, J.; Rodríguez-González, J.; Sánchez-Padilla, N.; Benavides, R.; Fernández-Tavizón, S.; Mercado-Silva, J. Comparative methods for reduction and sulfonation of graphene oxide for fuel cell electrode applications. Int. J. Hydrogen Energy 2019, 44, 12356–12364. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, J.J.; Fang, H.F.; Wang, C.Y.; Wang, Z.L.; Xie, S.S. Hierarchical shelled ZnO structures made of bunched nanowire arrays. Adv. Funct. Mater. 2007, 17, 1303–1310. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Yan, L.; Jia, H. Comparative study of the fire protection performance and thermal stability of intumescent fire-retardant coatings filled with three types of clay nano-fillers. Fire Mater. 2020, 44, 112–120. [Google Scholar] [CrossRef]
- Liu, M. Study on Magnesium Phosphate Cement Based Fireproof and Anticorrosive Coating for Steel Structure. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2022. [Google Scholar]
- Haeri, Z.; Ramezanzadeh, B.; Ramezanzadeh, M. Recent progress on the metal-organic frameworks decorated graphene oxide (MOFs-GO) nano-building application for epoxy coating mechanical-thermal/flame-retardant and anti-corrosion features improvement. Prog. Org. Coat. 2022, 163, 106645. [Google Scholar] [CrossRef]
- Mariappan, T. Recent developments of intumescent fire protection coatings for structural steel: A review. J. Fire Sci. 2016, 34, 120–163. [Google Scholar] [CrossRef]
- Zhou, S.; Song, L.; Wang, Z.; Hu, Y.; Xing, W. Flame retardation and char formation mechanism of intumescent flame retarded polypropylene composites containing melamine phosphate and pentaerythritol phosphate. Polym. Degrad. Stab. 2008, 93, 1799–1806. [Google Scholar] [CrossRef]
- Sun, L.; Qu, Y.; Li, S. Co-microencapsulate of ammonium polyphosphate and pentaerythritol in intumescent flame-retardant coatings. J. Therm. Anal. Calorim. 2013, 111, 1099–1106. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Wang, Q. Flame-retarded poly (propylene) with melamine phosphate and pentaerythritol/polyurethane composite charring agent. Macromol. Mater. Eng. 2007, 292, 206–213. [Google Scholar] [CrossRef]
- Li, Y. Study on Intumescent Flame Retardant Polypropylene and Preparation of the Flame-Retardant Color Masterbatch. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2015. [Google Scholar]
- Li, H.; Hu, Z.; Zhang, S.; Gu, X.; Wang, H.; Jiang, P.; Zhao, Q. Effects of titanium dioxide on the flammability and char formation of water-based coatings containing intumescent flame retardants. Prog. Org. Coat. 2015, 78, 318–324. [Google Scholar] [CrossRef]
- Mariappan, T.; Agarwal, A.; Ray, S. Influence of titanium dioxide on the thermal insulation of waterborne intumescent fire protective paints to structural steel. Prog. Org. Coat. 2017, 111, 67–74. [Google Scholar] [CrossRef]
- GB 14907-2018; Fire Resistive Coating for Steel Structure. 2018. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=3CBC40297F2E9BE7ED81670597BF5134 (accessed on 16 March 2022).
- Teijido, R.; Ruiz-Rubio, L.; Echaide, A.G.; Vilas-Vilela, J.L.; Lanceros-Mendez, S.; Zhang, Q. State of the art and current trends on layered inorganic-polymer nanocomposite coatings for anticorrosion and multi-functional applications. Prog. Org. Coat. 2022, 163, 106684. [Google Scholar] [CrossRef]
- Kalendova, A.; Veselý, D.; Kalenda, P. A study of the effects of pigments and fillers on the properties of anticorrosive paints. Pigment. Resin Technol. 2006, 35, 83–94. [Google Scholar] [CrossRef]
- Xing, Y. Preparation of Waterborne Epoxy Zinc-Rich Coatings. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2020. [Google Scholar]
- GB/T 10125-2012; Corrosion Tests in Artificial Atmospheres-Salt Spray Tests. 2012. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=92EA45A859F9F460D8073CBC277D06AA (accessed on 16 March 2022).
- GB/T 1865-2009; Paints and Varnishes—Artificial Weathering and Exposure to Artificial Radiation. 2009. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=EAB09FD32943FBAC1F617C5E815AA7EB (accessed on 16 March 2022).
- GB/T 1766-2008; Paints and Varnishes—Rating Schemes of Degradation of Coats. 2009. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=083B5597CE568F666F1305FB52B7D2B8 (accessed on 16 March 2022).
- Zhang, Y. Study on Preparation and Performance of Self Expanding Flame Retardant Epoxy Resin Based Fire Retardant and Anti-Corrosion Coating. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2022. [Google Scholar]
- Grigonis, M.; Mačiulaitis, R.; Lipinskas, D. Fire Resistance Tests of Various Fire Protective Coatings. 2011. Available online: https://etalpykla.vilniustech.lt/handle/123456789/130001 (accessed on 16 March 2022).
- Fan, F. Preparation and Fire Retardant Mechanism of Waterborne Ultra-thin Fire Retardant Coatings for Steel Structure. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2013. [Google Scholar]




| E44 (g) | PDMS (g) | KH-550 (g) | Xylene and Cyclohexanone (in a 1:1 Ratio) (g) | |
|---|---|---|---|---|
| Coupling agent blending method | 50 | 7 | 1.5 | 17 |
| Coupling agent grafting method | 50 | 7 | 1.5 | 17 |
| Chemical grafting method | 50 | 5 | 17 |
| Material | Modified GO | Pentaerythritol | Melamine | TiO2 | Ammonium Polyphosphate |
|---|---|---|---|---|---|
| Mass of material (g) | 0.3 g | 10.2 g | 17.2 g | 10.2 g | 19.4 g |
| Material | Modified epoxy resin | BYK-110 | fumed silica | Zinc phosphate | |
| Mass of material (g) | 20 g | 0.05 g | 0.5 g | 1 g |
| No. | Item | Reference Value | Test Results | Conclusion |
|---|---|---|---|---|
| 1 | Adhesion strength, MPa | ≥0.15 | 0.63 | Pass |
| 2 | Fire resistance | FP ≥ 2.00 h; average specimen temperature ≤ 538 °C | FP = 2.00 h; average specimen temperature: 505 °C | Pass |
| 3 | Resistance to neutral salt spray | Time to first appearance of rust not less than 1000 h | No rust after 1000 h | Pass |
| 4 | Xenon lamp irradiation aging | Continuous exposure for 1000 h, comprehensive aging performance level of coating not lower than Grade 2 | Continuous exposure for 1000 h, comprehensive aging performance level of coating not lower than Grade 2 | Pass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, B.; Wu, C.; Zhou, T.; Pan, B. Development and Evaluation of Steel Component Coatings for Substations/Converter Stations with Both Fire and Corrosion Prevention Functions. Fire 2025, 8, 1. https://doi.org/10.3390/fire8010001
Liu Y, Chen B, Wu C, Zhou T, Pan B. Development and Evaluation of Steel Component Coatings for Substations/Converter Stations with Both Fire and Corrosion Prevention Functions. Fire. 2025; 8(1):1. https://doi.org/10.3390/fire8010001
Chicago/Turabian StyleLiu, Yu, Baohui Chen, Chuanping Wu, Tiannian Zhou, and Bichen Pan. 2025. "Development and Evaluation of Steel Component Coatings for Substations/Converter Stations with Both Fire and Corrosion Prevention Functions" Fire 8, no. 1: 1. https://doi.org/10.3390/fire8010001
APA StyleLiu, Y., Chen, B., Wu, C., Zhou, T., & Pan, B. (2025). Development and Evaluation of Steel Component Coatings for Substations/Converter Stations with Both Fire and Corrosion Prevention Functions. Fire, 8(1), 1. https://doi.org/10.3390/fire8010001





