Abstract
Background. Wildfires may cause serious injuries to the anatomical structure of trees that can lead to tree death or long-lasting injury recovery, limiting their growth and vitality for several years. Post-fire management involves a wide range of measures aimed at recovering and restoring burnt areas. Usually, the first step is “salvage logging”, i.e., the removal of irremediably injured trees. The burn severity depends on several parameters and is variable within the burnt area. For this reason, in some areas, the death of apparently healthy individuals has often been observed even after several years. This study aims to assess delayed/latent mortality by analyzing glucose like a tracer in wood by using a blood glucometer and HPLC. Results. The glucose in the phloem, cambium, and last xylem rings was measured using a glucometer developed for measuring glucose in the blood. The adopted approach detected glucose concentrations that were recognizable for different functional levels of the trees. Conclusions. The glucometer was suitable to detect the glucose in wood and phloem in order to define the death or health of the disturbed and undisturbed trees post-fire. Further investigations are required to find new solutions for a rapid evaluation of the abiotic and biotic factors that influence tree functionality in the forest. This approach will be used to predict the probability of the death of the individuals injured, which would improve the efficiency and the economy of recovery operations.
1. Introduction
Wildfires cause injuries and reduce the growth and functionality of trees, and depending on their extension, trees may or may not be able to recover after a wildfire. The death of a tree may occur immediately after a wildfire, or it may be anticipated by a metabolic imbalance or a progressive physical or biotic deterioration, which can last several years before the tree dies [1]. This phenomenon is reported as delayed tree mortality [2] or latent mortality [3] and is the main source of uncertainty in post-fire site management decisions. After a wildfire, several actions may be required to mitigate fire injuries, such as safeguarding and restoration, but also to minimize the loss of commercial timber, such as salvage logging practices. Although post-fire salvage logging is currently applied in many forest stands around the world, and its application in Mediterranean areas is nowadays poorly implemented and restricted to a few cases [4], the effects of this practice in these areas could have advantages on the ecosystem recovery and the conservation of biodiversity [5]. Nevertheless, it would be important to predict which plants are most likely to survive in order to preserve them, and thus reduce the impacts of salvage logging. Problems often arise during planning this operation because the vitality of the trees in a burnt area is uncertain. A common problem for technicians is to relate the degree of the injury produced by the fire and the probability that the burned trees will recover and grow in the future. The occurrence of delayed tree mortality is so crucial in forest post-fire management that it seems unreasonable to base it only on the observations of tree injury occurrence, although this is a common practice [6,7].
During the last years, empirical and process-based models have been developed to predict latent tree mortality [2,3]. In these models, large-scale tree mortality was predicted as a function of the fire behavior (i.e., fireline intensity, residence time, and the rate of spread), driving factors (e.g., topography, meteorological conditions, fuel load, suppression activities, etc.), and stem or canopy injury intensity. On the contrary, the energy and heat fluxes to which each tree and its organs are exposed can widely change during a wildfire, making it difficult to assess the fate of each tree depending on the damage degree [1].
In this context, the integrity of the cambium and related tissues (phloem and maturing xylem) would provide information on the viability of trees injured by fire. When cambium necrosis occurs around the entire stem circumference, photosynthates translocation is prevented, and the tree dies. In this frame, the collection of multiple cambial samples around the stem circumference is mandatory to define a high probability of tree mortality [8]. Thus, estimating the vitality can be considered a good proxy of the ability to recover the growth of burnt trees. However, the assessment of cambium vitality by histological observations is time-consuming and not fully applicable to large-scale forest practices.
Considering other aspects, the cambium is protected from environmental changes by the bark. Recent evidence suggests that the temperature threshold that causes irreversible injuries to the cells during the combustion phase is around 60 °C [9]. It was also found that a bark thickness of 17 mm is critical for cambium survival due to factors like heat flux and the persistence of fire along the stem and crown, which may be responsible for lethal injuries that increase latent tree mortality [10]. Therefore, the bark char code was used to determine cambium vitality at the tree level. It was successfully applied to 14 coniferous species subjected to prescribed fires in temperate forests, with the best results obtained in thin-barked species [8]. Thus, the role of the cambium and the related tissues (phloem and xylem) in the mediation of the response of trees to wildfire and the importance of understanding the physiological mechanisms that trigger tree mortality and forest dieback as a consequence of wildfire is crucial.
The intensity and duration of the disturbance determine the ability of a tree to face the injury until the stress threshold that induces the tree death is overcome [11]. Therefore, the evaluation of the tree health can be performed at a fine scale by estimating photosynthesis rates and measuring tree growth and productivity [12,13], whereas tree mortality is observed as a consequence of hydraulic failure and carbon starvation [14,15], as well as phloem dysfunction and necrosis [16]. Furthermore, oak trees’ vitality in urban environments was also investigated by using portable instruments to assess sugar concentration [17].
Indeed, the products of photosynthesis are loaded in the phloem by passing from the mesophyll cells to the sinks, where they are unloaded and metabolized. The transfer of metabolites provides energy, which is given up during subsequent metabolic reactions, and glucose is primarily utilized as an energy source [18]. In the context of post-fire damage assessment, the occurrence of phloem injury might interrupt the sap transport depending on the intensity of the damage [1]. Therefore, fire injury can lead to both immediate and delayed tree death through phloem and cambial region disruption, which impairs the translocation of processed sap rich in sugars and other substances from the crown to the rest of the organism [1]. Moreover, a decrease in non-structural carbohydrates (NSCs) has been found in fire-exposed trees, suggesting a reduction due to tree mortality or recovery [19]. This research assumes that the glucose produced (by photosynthesis in the leaves) can be used as a tracer in tracing tests to estimate the hydraulic conductivity of the phloem and, consequently, its vitality. However, glucose could also be produced by the hydrolysis of starch or by the activity of sucrose invertases to estimate the function of the enzymatic machinery of the phloem and, thus, its vitality. Thus, we hypothesized that phloem damage might be detected by glucose concentration, which could indicate widespread phloem dysfunction and cambium death. In detail, the presence of glucose along the external tissues of the stem defines the integrity of the structure, while its absence in part of the stem might indicate a hydraulic disruption. In this study, the method proposed by Martinez–Trinidad et al. [17] for measuring glucose concentration in oak trees was tested and subsequently applied to maritime pines. Therefore, the objectives of this study were (i) to assess the latent mortality at tree level by measuring glucose concentrations at different heights of the stem above the ground, where the presence/absence of glucose defines the integrity/dysfunction of the phloem; (ii) to develop a protocol for the rapid assessment of glucose using a commercially available glucometer, a portable, low cost and easy to use, device used in human medicine.
2. Materials and Methods
2.1. Protocol Setup
2.1.1. Sample Collection and Preparation
The experimental settings were carried out on samples collected from two trees of Pinus pinea grown in the garden of the University of Florence, DAGRI Department, in February 2022. Two samples per tree were collected with an increment borer (0.5 cm long and 0.3 cm wide) by retaining the two last formed rings, the cambium and phloem, and removing the outer bark (CS, core samples) [17]. Thus, the CS were composed of two wooden rings and the inner bark. The wood samples were placed in Eppendorf tubes (Merck KGaA, Darmstadt, Germany) previously filled with distilled water so that the sugars present in the samples were extracted following the procedure proposed by [20]. The Eppendorf solution was used to determine glucose and soluble sugars concentration.
2.1.2. Soluble Sugars Measurements
In order to define the reliability of measuring soluble sugars in wood samples with portable devices such as glucometers, which are generally used for medical purposes, the results obtained were compared with those quantified by High-Performance Liquid Chromatography (HPLC). The HPLC was equipped with a SHODEX SUGAR Series SC 1011 column, 8 × 300 mm (Showa Denko, Munich, Germany) set to 90 °C, combined with a Guard Pak Insert Sugar Pak II precolumn, steel injection systems (ISS101, Perkin Elmer, Waltham, MA, USA), Milli-Q water at 0.5 mL/min, and a LC-30 RI refractive index detector (Perkin Elmer, Waltham, MA, USA). Identification of soluble carbohydrates was confirmed using standard carbohydrate solutions (Sigma-Aldrich, St. Louis, MO, USA).
The solution of each Eppendorf was split into two subsamples as follows: a drop was taken by using a Pasteur pipette, placed on a glass dish, and then absorbed by the test strip of the glucometer; the remaining solution was filtered through a GVS Syringe filter PTFE, 0.22 microns, 13 mm, as used for HPLC analyses. The glucometer OneTouch Verio Flex model (LifeScan Inc., Milano, Italy) was used to quantify glucose in the extracted solution of the core samples. The glucose detection is performed by adopting the impedenzometric method by exploiting the reaction with the enzyme FAD-GDH (flavin adenine dinucleotide-dependent glucose dehydrogenase) present in a test strip. Concentrations of each soluble sugar in the solutions were measured using HPLC equipped with a SHODEX SUGAR Series SC 1011 column, 8×300 mm (Showa Denko, Munich, Germany) set to 90 °C, combined with a Guard Pak Insert Sugar Pak II precolumn (Waters), steel injection systems (ISS101, Perkin Elmer), Milli-Q water at 0.5 mL/min, and an LC-30 RI refractive index detector (Perkin Elmer). For each sample, 20 microliters of filtered solution were injected. Identification of soluble carbohydrates was performed according to [20] and was confirmed using standard carbohydrate solutions (Sigma-Aldrich, St. Louis, MO, USA). Solutions of 0.5, 0.25, 0.125, and 0.0625 mg·mL−1 for each soluble sugar were prepared and then analyzed by HPLC. Calibration curves were performed for sucrose, glucose, fructose, pinitol, stachyose, and raffinose. Quantification was performed using an internal standard.
2.1.3. Preliminary Tests
In order to assess the amount of soluble sugars with different analytical instruments and different sensitivity (from 10 ppm in HPLC to 200 ppm for glucometer), four solutions with known concentrations of glucose (12.5, 25.0, 50.0, and 100.0 mg·dL−1) were measured with both instruments. Three technical replicates were performed for each instrument and glucose concentration. Preliminary tests were performed to quantify the volume of distilled water, the extraction time, and the temperature to set the measurements of sugar concentration from a core sample. The sugar extraction was performed with the following volumes of distilled water: 1.0, 1.25, 1.50, and 1.75 mL. Three samples per volume were analyzed.
The detection limits of the glucometer were between 20 and 600 mg·dL−1. To obtain measurable sugar concentrations, adequate time and temperature for the extraction was chosen. Sixteen samples were used to define the optimal time and temperature for extraction. Each core sample was immersed in 1.0 mL of distilled water in 2 mL Eppendorf and extracted at 4 °C (eight samples) or at room temperature (20 °C) for 1, 2, 3, 4, 5, 8, 11, and 24 h.
2.2. Protocol Validation
2.2.1. Study Site
The protocol developed was applied to samples collected on the southeastern slope of the Lombardona mountain, Vicopisano (PI), Tuscany, Italy (10°34′25″ E, 43°42′23″ N). The climate at this site is humid and temperate, with summer aridity. The mean annual air temperature is 14.3 °C with an annual rainfall of 698 mm [21]. The vegetation consists mainly of Pinus pinaster and Olea europaea, secondarily of evolving forest and shrub vegetation, mixed coniferous and sclerophyll forests, and a much more restricted portion of deciduous forest. The area was affected by a wildfire on 14–15 August 2021, in which almost all the tree and shrub cover was either completely burnt or severely compromised due to the flame front intensity. However, both inside the area and along the perimeter, it was possible to identify some pine trees with significant signs of burns on the stem, but most of their foliage was still green.
2.2.2. Sample Collection
By evaluating the tree health in the area of the Lombardona mountain affected by fire, three groups of mature maritime pines were distinguished: “living” trees, L, outside the burnt area, within 100 m of the fire boundary; “dead” trees, D, inside the burnt area, with signs of carbonization on the whole stem and the crown with totally consumed and scorched foliage; and “scorched” trees (X), with signs of carbonization on part of the stem and at least 50% of the crown with consumed or scorched foliage. Seven L-trees, 3 D-trees, and 20 X-trees were sampled in February and October 2022 (6 and 10 months post-fire). The trees were sampled in an area with a surface of 1 hectare, an eastern exposition, and an average slope of 17%. The time of sampling was defined according to a previous study [19], where post-fire tree mortality and recovery patterns in Pinus ponderosa were assessed at different timesteps post-fire (4 days–16 months). A Kruskal–Wallis test performed on the “X” samples HPLC values of glucose demonstrated that both samples collected in February (n = 17) and in October (n = 18) did not show statistically significant differences (χ2 = 1.5701, df = 1, p-value = 0.2102). As trees can be affected by fires at different extents around the stem circumference [8], for each tree, the core sampling was planned by considering (i) the direction of the fire, by selecting the side of the tree that was first hit with a minimum burn height (F) and the side that was subsequently enveloped by the flames with the maximum height of the charring sign (C) in “scorched” trees; (ii) and different sampling heights at the stem, at the base (G) and at a 1.5 m height (S) in “living”, “dead” and “scorched” trees.
2.2.3. Sample Analysis
A total of 100 samples were measured as follows: 14 in L-trees, 6 in D-trees, and 80 in X-trees. To quickly obtain indications of the state of the phloem system, Δi was developed (Equation (1)).
where:
Δi = |(Si − Gi)/((Si + Gi) + 0.0001)|
Si is the value of glucose measured at a 1.5 m height of the tree “i”.
Gi is the value of glucose measured at the ground of the tree “i”,
Δi is the normalized differential index between the two measurements. The Formula (1) established in this study is applicable to conifer species, and it should be validated for broadleaves species.
Adding 0.0001 to the denominator, as suggested by [22], ensures that the denominator will never be zero, thereby preventing the equation from reaching infinity and producing unusable values. This index makes it possible to quickly assess the status of the tissues analyzed: a value of 0.00 corresponds to dead tissue, values from 0.01 to 0.50 correspond to functionality, and values from 0.51 to 1.00 correspond to impaired functionality.
For the conditions of fire direction (F) and opposite side (C), Δi was calculated.
2.2.4. Starch Analysis
We collected two samples per tree (L, D, X, n = 5, 5, and 11, respectively) at a ~50 cm height using 37 mm diameter metal punchers to determine the possible release of glucose by stored starch in the ray parenchyma of dead or X-trees. The samples, comprising phloem, cambium, and xylem, were kept on ice during the fieldwork, stored at −22 °C once in the laboratory, and freeze-dried following the protocol reported in [23]. Subsequently, the samples were prepared for biochemical analysis according to the protocol described in [24] and modified following [25]. Accordingly, samples were split along the tangential plane, and the bark side was discarded (the inner bark was dried and detached from the xylem in D-trees). Wood samples, containing from 3 to 5 rings, were cut off from the log using a chisel, and 2–3 g of mature xylem were reduced to a fine powder with an Ultra Centrifugal mill ZM 200 (Retsch, Haan, Germany) equipped with a 12-tooth rotor and ring sieves with 0.75 and 0.25 mm trapezoid holes, respectively. After homogenizing, an equal amount of wood powder per tree, a 40 mg subsample, was used for starch extraction following the procedure proposed by [22]. The starch content was measured after an extraction procedure; the residual pellet was suspended in 1.5 mL of acetate buffer (pH 5), heated at 100 °C for 1 h in a sand bath, and then cooled at room temperature. After incubation at 55 °C for 16 h with 150 μL of amyloglucosidase from Aspergillus niger (Fluka), samples were diluted with distilled water to 5 mL, and three 0.25-mL aliquots of each sample were assayed colorimetrically using glucose oxidase (Sigma-Aldrich Saint Louis, MO, USA).
2.3. Data Analysis
Statistical analyses were performed to investigate differences between Δi among the groups of trees (L, D, and X). Data were tested for normal distribution by the Lilliefors test, and the comparability of variances was checked through the Bartlett test due to uneven sample size between groups. As a consequence of the non-normal distribution and the homoscedasticity of groups, a nonparametric approach was applied. Differences between groups were tested by the Kruskal–Wallis test of variance. Multiple comparisons to highlight the relative contrast effect between the three groups were performed with the computation of simultaneous confidence intervals (SCI) as a post-hoc test using the nparcomp package [26,27]. The nparcomp package (Version, 3.0) utilizes nonparametric rank-based tests using a multivariate t–distribution with a Satterthwaite approximation as an asymptotic approximation, and unlike traditional multiple comparison procedures that might inflate the overall Type I error rate, nparcomp computes SCIs that control this error rate. An ANOVA test (p < 0.05) and a post-hoc comparison were performed to assess the effects of the plants’ conditions. Correlation matrixes were realized using the R studio “corrplot” package.
3. Results
3.1. Protocol Set Up
Preliminary tests based on the measurement of the four solutions with known concentrations of glucose (12.5, 25.0, 50.0, and 100.0 mg dL−1) performed by HPLC and glucometer recorded a RMSE of 7.10 and 6.78, respectively, demonstrating the accuracy of the glucometer.
Different volumes of distilled water were tested to quantify the optimal volume of distilled water for sugar extraction: 1.0, 1.25, 1.50, and 1.75 mL. Three samples per volume were analyzed. A linear decrease in the glucose concentration was detected with the increasing volume of distilled water (79.10 and 27.00 mg·dL−1 at 1.00 and 1.75 mL of distilled water, respectively) (Figure 1). Thus, a volume of 1 mL was chosen for the proposed protocol as it was the best solution for detecting glucose concentration using both HPLC and glucometers.
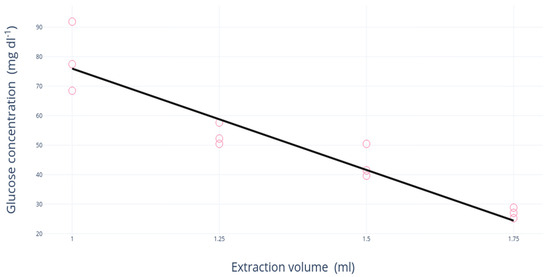
Figure 1.
Regression analysis of the extraction volume in water (mL) and glucose concentration (mg·dL−1) measured by glucometer.
Regarding the extraction time, the glucometer measured a glucose concentration of 26 mg·dL−1 after 2 h of extraction and 47 mg·dL−1 after 5 h. Over 5 h, no significant changes in glucose concentrations were observed (Figure 2). The extraction time of 5 h was therefore chosen for the proposed protocol.
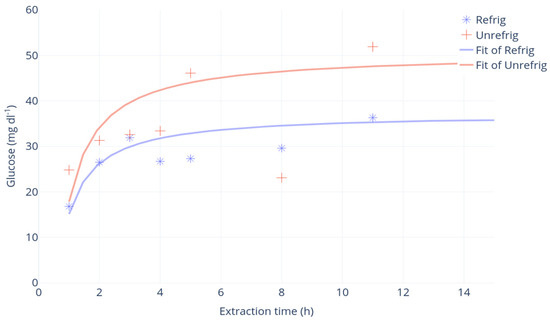
Figure 2.
Regression analysis of the extraction time (h) and glucose concentration (mg·dL−1). Samples at room temperature (“unrefrig”, red) or in refrigerated conditions, 4 °C (“refrig”, blue).
At room temperature, the glucose concentrations extracted from core samples were higher than for refrigerated samples, but the former showed the highest variability (Figure 2). Therefore, refrigerated storage was chosen for the proposed protocol.
3.2. Protocol Validation
The glucose concentrations measured with the glucometer were significantly correlated with those measured with HPLC (corr = 0.84; R2 = 0.70; p < 0.001) (Figure 3). Glucose concentration was significantly different between L-, X- and D-trees, whereas the concentrations of X-trees did not differ from those of L-trees (Figure 4), as demonstrated by Nonparametric Multiple Comparisons for relative contrast effects test results reported in Table 1.
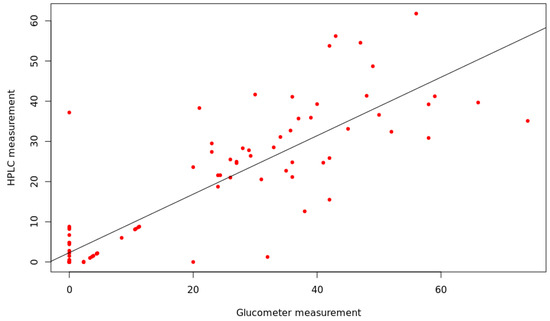
Figure 3.
Regression analysis of glucometer (mg·dL−1) and HPLC measurements (mg·dL−1). Each point represents the glucose concentration of the same woody sample measured by glucometer and HPLC after the same extraction procedure.
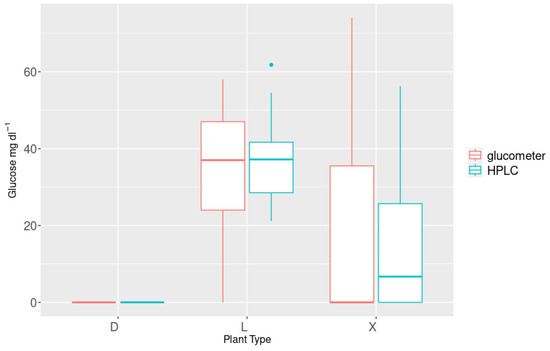
Figure 4.
Mean glucose concentrations of core samples in “dead” (D), “living” (L), and “scorched” trees (X, both sides measured). Values are shown for HPLC (cyan) and human blood glucometer (red).

Table 1.
Nonparametric Multiple Comparisons between tree groups (“dead” trees, D; “living” trees, L; “scorched” trees, X) (estimator, lower and upper probabilities, and p-value are shown).
The average glucose concentration changed between groups, with the highest values detected in L-trees (37.4 mg·dL−1), while it was not detected in D-trees (Table 2). The analysis of glucose in X-trees showed higher concentrations in the F position than in C in relation to the direction of fire (Figure 5).

Table 2.
Average and range of glucose concentration (mg·dL−1) in living, dead, and scorched trees by considering the direction of fire, at the side first hit with a minimum burn height (F) and the side enveloped by the flames with the maximum height of the charring sign (C), and at the different sampling height of the stem, the base (G) and at a 1.5 m height (S).
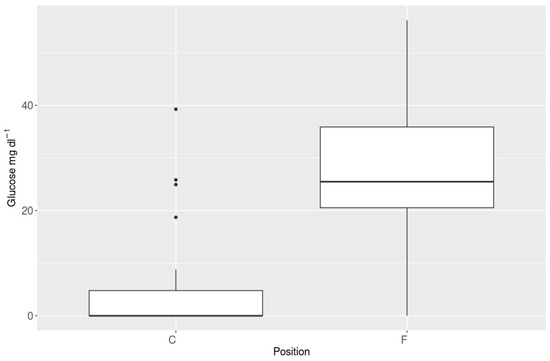
Figure 5.
Glucose values recorded with HPLC-differences between samples collected in fire front direction “F” and in the opposite direction “C”. In box plots, dots are outliers.
The Δi showed that the highest number of trees with dead tissues was detected in condition C (14 trees) and no or moderate injury in condition F (13 trees) (Figure 6).
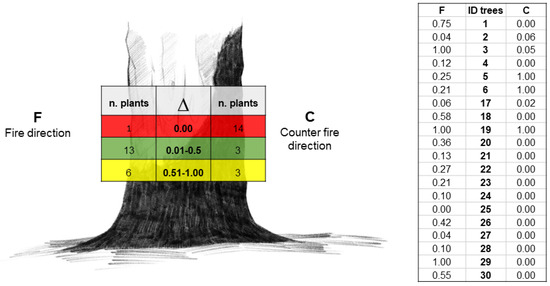
Figure 6.
Δ range and number of plants divided by injury groups (living, dead, and unknown, in green, red, and yellow, respectively) and condition F (at left) and condition C (at right) (Image: Maria Giulia Raeli); raw data are reported in the table.
Starch concentration was very low in the xylem of D-trees, while variable concentrations were measured in L- and X-trees (Table 3).

Table 3.
Average and range of starch concentration (mg/dry weight) in living, dead and scorched trees. ANOVA was shown (p < 0.001) by mean comparison (Fisher test), different letters indicate statistical difference between treatments at p < 0.05.
4. Discussion
This is the first user-friendly protocol for the rapid assessment of latent tree mortality, also known as delayed tree mortality in burnt areas. Although implementation and replication on other woody species, such as broadleaves, are welcome, the sample size in this study case is part of the early stages of conducting and assessing the potentiality of the proposed protocol. However, based on our results, glucose differential (∆) in the phloem sap seems to be a valid indicator of the phloem injury, and when it was not detected, the likelihood of latent tree mortality increased. The use of commercial tools for glucose detection can represent a good compromise between cost and goods for a practical application, even if other insights are needed. We set and validated this innovative protocol for the detection of glucose in wood through portable cheap devices (glucometers), requiring no specific skills. The method foresees the collection of core samples from scorched stems through incremental borer, which are extracted in 1 mL of distilled water for 5 h at 4 °C in a heat-insulating container refrigerated with a eutectic plate.
The comparison of the data measured through a glucometer and HPLC showed a high correlation (corr = 0.84, p < 0.001), which was higher than the results obtained by [17], although they adopted different laboratory instruments, sampling, and processing protocols. The application of the method confirmed the status of L- and D-trees, recording mean glucose concentrations of 37.4 mg·dL−1 detected in L-trees, while it was not detectable in D-trees as well as very low starch concentrations (<4 mg·g−1·DW−1). However, this distinction was not found by [17], who reported mean values equal to 7.55 mg·dL−1, 7.44 mg·dL−1, and 7.99 mg·dL−1 in dying, decaying, and healthy trees, respectively. This discrepancy may depend on the tree species (hardwood vs. softwood) and/or technical approach. The sensitivity of the glucometer allowed it to detect differences in the glucose concentrations in samples collected in the stem section at the maximum (C) and minimum (F) burn height, as well as at different heights of the stem, near the tree collar (G) and at a 1.5 m height (S).
Samples collected according to the advancement of the fire edge showed differences in the soluble sugar concentrations, which were higher in the F than in the C position, as confirmed by Δi. This suggested low injuries on the side of the tree that was first exposed to the flames compared to the tree side that was later enveloped with the advancement of the fire edge (according to [28,29]). The glucose concentrations along a height gradient of the stem highlighted the potential fire-caused damages to soluble carbon transport within the phloem, as glucose concentrations in samples collected at 1.5 m were lower than those at the collar. A higher glucose concentration at the collar might suggest the mobilization of root reserves, as supposed by [30]. However, in February 2024, trees characterized in 2022 were assessed by showing a satisfactory prediction of death in 61% of cases. The central role of glucose concentration in the phloem in defining latent death events after fire is therefore emphasized. However, the starch concentrations in wood relieved a limited availability of carbon stored in the ray parenchyma in dead plants compared to living trees, as expected. On the contrary, the high levels of starch in scorched trees (double those found in living trees) might suggest that the reduced quantity of glucose is attributable to passive carbon storage or a reduced α-amylase activity [31] and not to a lack of substrate for enzymatic activity.
The practices of safeguarding, restoration, and “salvage logging” are becoming more and more common in Tuscany as well as in other areas around the world. These practices allow to mitigate further post-fire forest and soil degradation and to minimize commercial wildfire injury. For this purpose, it is important to recognize the latent mortality of an individual tree at an early stage so that post-fire restoration efforts can be planned after a fire without affecting vital trees.
Our study is addressed to consider the potential to assess the vitality in the early stages after a fire. This study is supported by a recent study [20] that highlights the importance of NSCs for tree survival and post-fire recovery. The study [19] observed an NSC depletion, corresponding to a carbohydrate availability limitation for maintaining tree function post-fire in Pinus ponderosa. NSC depletion may be the result of fire-caused injuries, which is useful in defining post-fire tree mortality and then, in planning forest recovery.
In conclusion, the proposed protocol was developed to quickly assess the latent mortality on a single tree through the soluble sugars and glucose concentrations in tree stems at different heights by applying portable devices, such as commercial glucometers normally used in medicine. Although this approach seems to be valid and friendly, we highlight the need to deep the investigations at different levels to verify (i) the application to a wide range of species (hardwood vs. softwood, shrubs vs. herbaceous); (ii) the involvement of specific molecules translocated in the phloem sap (soluble sugars, ethanol, amino acids) produced at high temperatures and inducing cell death; (iii) the development of appropriate and specific biosensors for rapid detection in the field; (iv) and the involvement of physiological processes related to the signal transport from the burned portion of the stem to the crown to improve the definition of the Δi ranges.
Author Contributions
Conceptualization, N.F., C.C. and E.M.; methodology, N.F., A.G. and E.M; data curation, N.F., C.C., C.F., M.L.T. and A.G.; writing—original draft preparation, N.F., C.C., A.G. and E.M.; writing—review and editing, N.F., C.C., E.M., C.F., E.T., F.N., M.L.T. and A.G.; funding acquisition, C.F. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
The work was partially funded by MUR-FOE 2019-Project Cambiamento Climatico, “National Biodiversity Future Center-NBFC”, project code CN_00000033, Concession Decree No. 1034 of 17 June 2022, adopted by the Italian Ministry of University and Research, CUP B83C22002930006, and PRIN_PNRR_2022, project code P2022Z5742, Decreto Direttoriale n. 1409 del 14 settembre 2022 adopted by the Italian Ministry of University and Research, CUP B53D23023780001, funded by the European Union-Next Generation EU: PNRR Missione 4, Componente 2, Investimento 1.1. The publication was made with the contribution of the researcher Cristiano Foderi with a research contract co-funded by the European Union-PON Research and Innovation 2014–2020 in accordance with Article 24, paragraph 3a), of Law No. 240 of 30 December 2010, as amended, and Ministerial Decree No. 1062 of 10 August 2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Giulia Ferriani, Giammarco Dadà, and Diana Rodrigues for their contribution to preliminary activities in the field and lab.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bär, A.; Michaletz, S.T.; Mayr, S. Fire effects on tree physiology. New Phytol. 2019, 223, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.L.; Dickinson, M.B.; Bova, A.S. A way forward for fire-caused tree mortality prediction Modeling a physiological consequence of fire. Fire Ecol. 2010, 6, 80–94. [Google Scholar] [CrossRef]
- Youngblood, A.; Grace, J.B.; McIver, J.D. Delayed conifer mortality after fuel reduction treatments: Interactive effects of fuel, fire intensity, and bark beetles. Ecol. Appl. 2009, 19, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Pons, P.; Rost, J.; Tobella, C.; Puig Gironès, R.; Bas, J.M.; Franch, M.; Mauri, E. Towards better practices of salvage logging for reducing the ecosystem impacts in Mediterranean burned forests. iForest 2020, 13, 360–368. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Burton, P.J.; Franklin, J.F. Salvage Logging and its Ecological Conse Quences; Island Press: Washington, DC, USA, 2008; p. 227. [Google Scholar]
- Cansler, C.A.; Hood, S.M.; Varner, J.M.; van Mantgem, P.J.; Agne, M.C.; Andrus, R.A.; Ayres, M.P.; Ayres, B.D.; Bakker, J.D.; Battaglia, M.A.; et al. The Fire and Tree Mortality Database, for empirical modeling of individual tree mortality after fire. Sci. Data 2020, 7, 194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reilly, M.J.; Zuspan, A.; Yang, Z. Characterizing post-fire delayed tree mortality with remote sensing Sizing up the elephant in the room. Fire Ecol. 2023, 19, 64. [Google Scholar] [CrossRef]
- Hood, S.M.; Cluck, D.R.; Smith, S.L.; Ryan, K.C. Using bark char codes to predict post-fire cambium mortality. Fire Ecol. 2008, 4, 57–73. [Google Scholar] [CrossRef]
- Dickinson, M.B.; Johnson, E.A. Temperature-dependent rate models of vascular cambium cell mortality. Can. J. Forest Res. 2004, 34, 546–559. [Google Scholar] [CrossRef]
- Espinosa, J.; Rodríguez, D.R.O.; Madrigal, J.; Guijarro, M.; Hernando, C. Predicting potential cambium damage and fire resistance in Pinus nigra Arn. Ssp. Salzmannii. For. Ecol. Manag. 2020, 474, 118372. [Google Scholar] [CrossRef]
- Cocozza, C.; Traversi, M.L.; Giovannelli, A. Tree growth conditions are demanded when optimal, are unwanted when limited, but when are they suboptimal? Plants 2021, 10, 1943. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant physiology, development and metabolism. Plant Physiol. Develop. Metab. 2018, 25, 1–9. [Google Scholar] [CrossRef]
- Michaletz, S.T. Xylem dysfunction in fires Towards a hydraulic theory of plant responses to multiple disturbance stressors. New Phytol. 2018, 217, 1391–1393. [Google Scholar] [CrossRef]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree vitality and forest health Can tree-ring stable isotopes be used as indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar] [CrossRef]
- Partelli-Feltrin, R.; Smith, A.M.S.; Adams, H.D.; Thompson, R.A.; Kolden, C.A.; Yedinak, K.M.; Johnson, D.M. Death from hunger or thirst? phloem death, rather than xylem hydraulic failure, as a driver of fire-induced conifer mortality. New Phytol. 2022, 237, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Trinidad, T.; Watson, W.T.; Arnold, M.A.; Lombardini, L.; Appel, D.N. Comparing various techniques to measure tree vitality of live oaks. Urban Forestry Urban Green 2010, 9, 199–203. [Google Scholar] [CrossRef]
- Lambers, H.; Stuart Chapin, F.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer Nature: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Reed, C.C.; Sharon, M.H. Nonstructural carbohydrates explain post-fire tree mortality and recovery patterns. Tree Physiol. 2024, 44, tpad155. [Google Scholar] [CrossRef]
- Emiliani, G.; Traversari, S.; De Carlo, A.; Traversi, M.L.; Cantini, C.; Giovannelli, A. B-type cyclin modulation in response to carbon balance in callus of Populus alba. Plant Cell Tissue Organ Cult. 2016, 124, 283–293. [Google Scholar] [CrossRef]
- Rapetti, F. Considerazioni sui caratteri climatici del Monte Pisano. In I Monti Pisani: Il Ruolo delle ANPIL per la Conservazione e la Valorizzazione del Territorio; Felici Editore Pisa: Pisa, Italy, 2000; pp. 6–13. [Google Scholar]
- Parks, S.A.; Dillon, G.K.; Miller, C. A new metric for quantifying burn severity The relativized burn ratio. Remote Sens. 2014, 6, 1827–1844. [Google Scholar] [CrossRef]
- Simard, S.; Giovannelli, A.; Treydte, K.; Traversi, M.L.; King, G.M.; Frank, D.; Fonti, P. Intra-annual dynamics of non-structural carbohydrates in the cambium of mature conifer trees reflects radial growth demands. Tree Physiol. 2013, 33, 913–923. [Google Scholar] [CrossRef]
- Giovannelli, A.; Emiliani, G.; Traversi, M.L.; Deslauriers, A.; Rossi, S. Sampling cambial region and mature xylem for non structural carbohydrates and starch analyses. Dendrochronologia 2011, 29, 177–182. [Google Scholar] [CrossRef]
- Bellasio, C.; Fini, A.; Ferrini, F. Evaluation of a High Throughput Starch Analysis Optimised for Wood. PLoS ONE 2014, 9, e86645. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R a Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 30 May 2024).
- Konietschke, F.; Placzek, M.; Schaarschmidt, F.; Hothorn, L.A. Nparcomp An R software package for nonparametric multiple comparisons and simultaneous confidence intervals. J. Stat. Softw. 2015, 64, 1–17. [Google Scholar] [CrossRef]
- Smith, K.T.; Sutherland, E.K. Resistance of eastern hardwood stems to fire injury and damage. USDA For. Serv. 1999, 210–217. [Google Scholar]
- Chatziefstratiou, E.K.; Bohrer, G.; Bova, A.S.; Subramanian, R.; Frasson, R.P.M.; Scherzer, A.; Butler, B.W.; Dickinson, M.B. FireStem2D—A two-dimensional heat transfer model for simulating tree stem injury in fires. PLoS ONE 2013, 8, e70110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ryan, K.C.; Frandsen, W.H. Basal injury from smoldering fires in mature Pinus ponderosa laws. Int. J. Wildl. Fire 1991, 1, 107–118. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).