Experimental Study on Explosion Characteristics of LPG/Air Mixtures Suppressed by CO2 Synergistic Inert Powder
Abstract
1. Introduction
2. Experiment
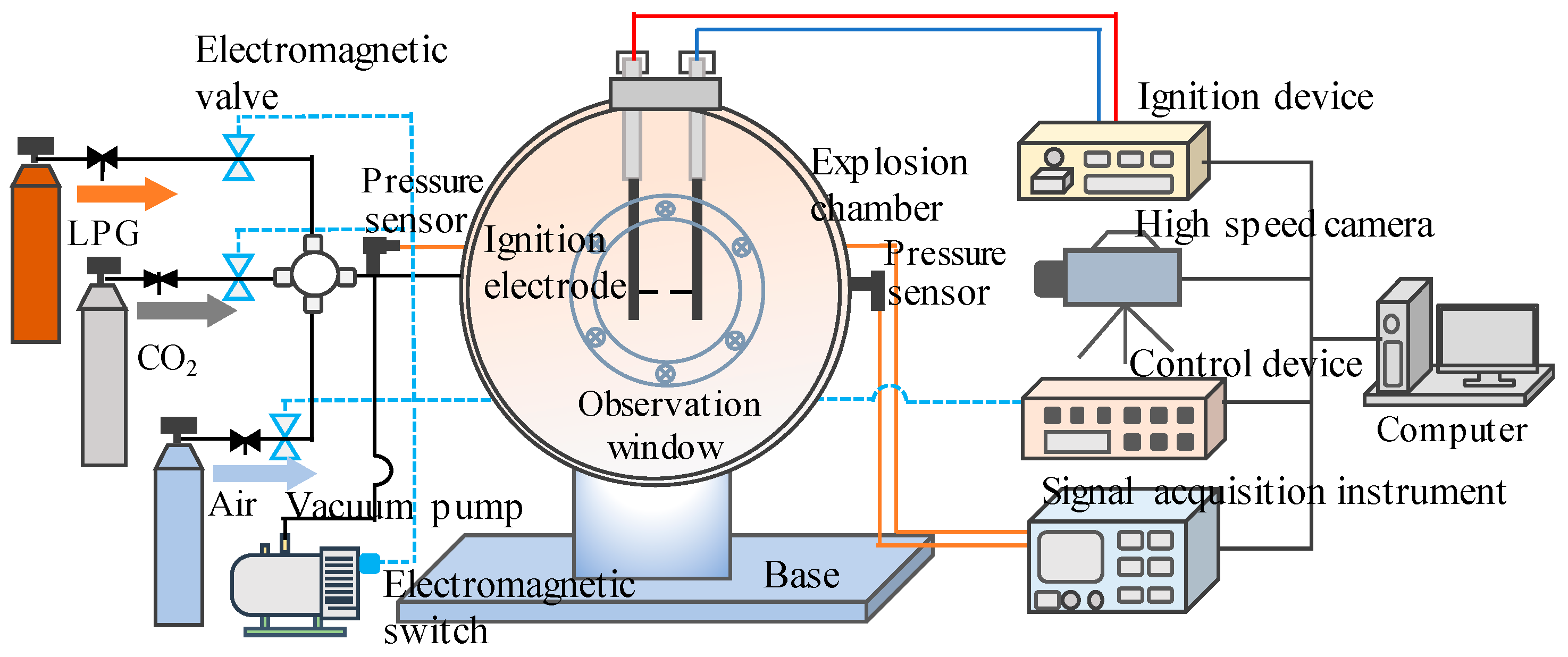
2.1. Explosion Testing System
2.2. Experiment Material
2.3. Experimental Procedure
3. Results and Discussion
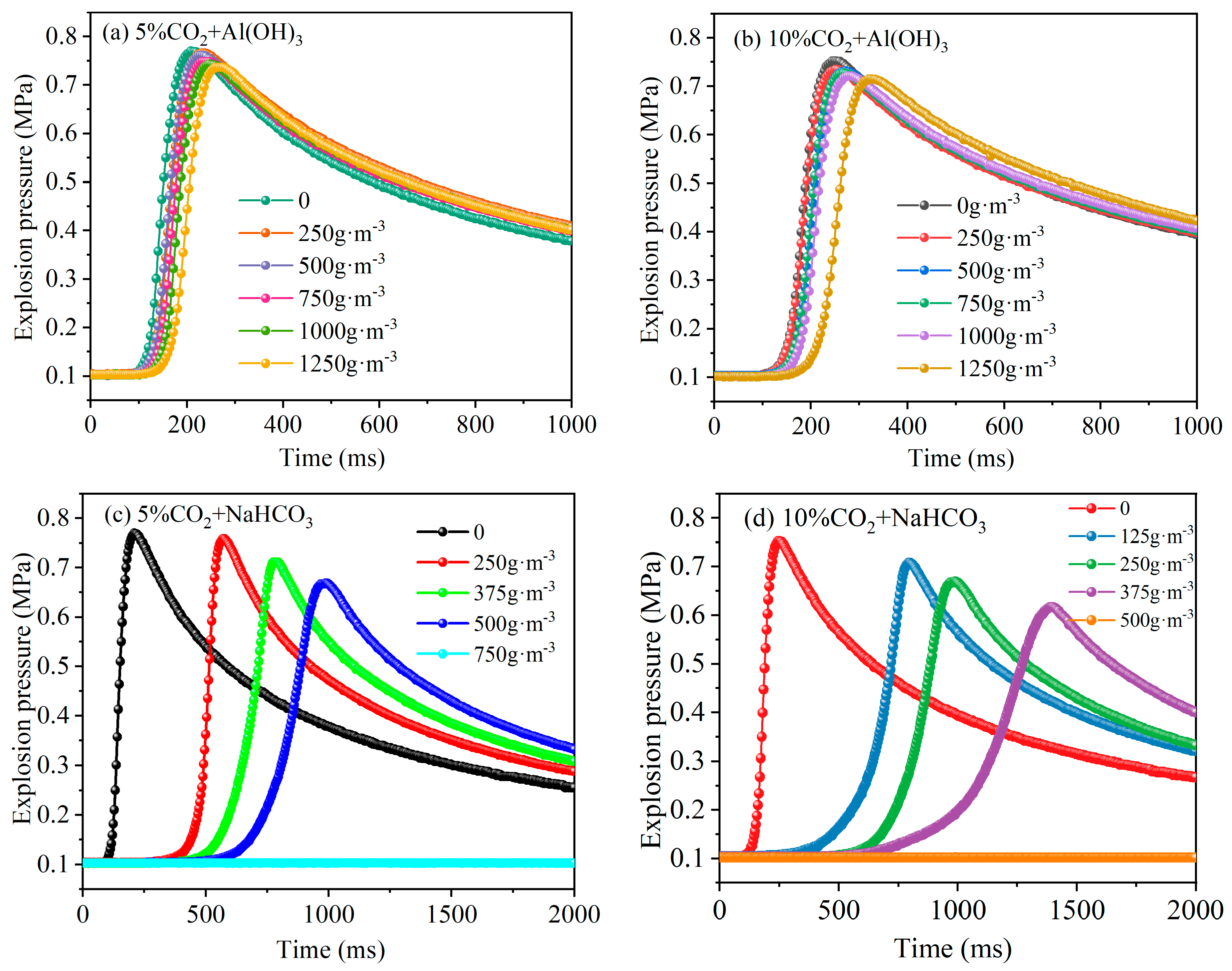
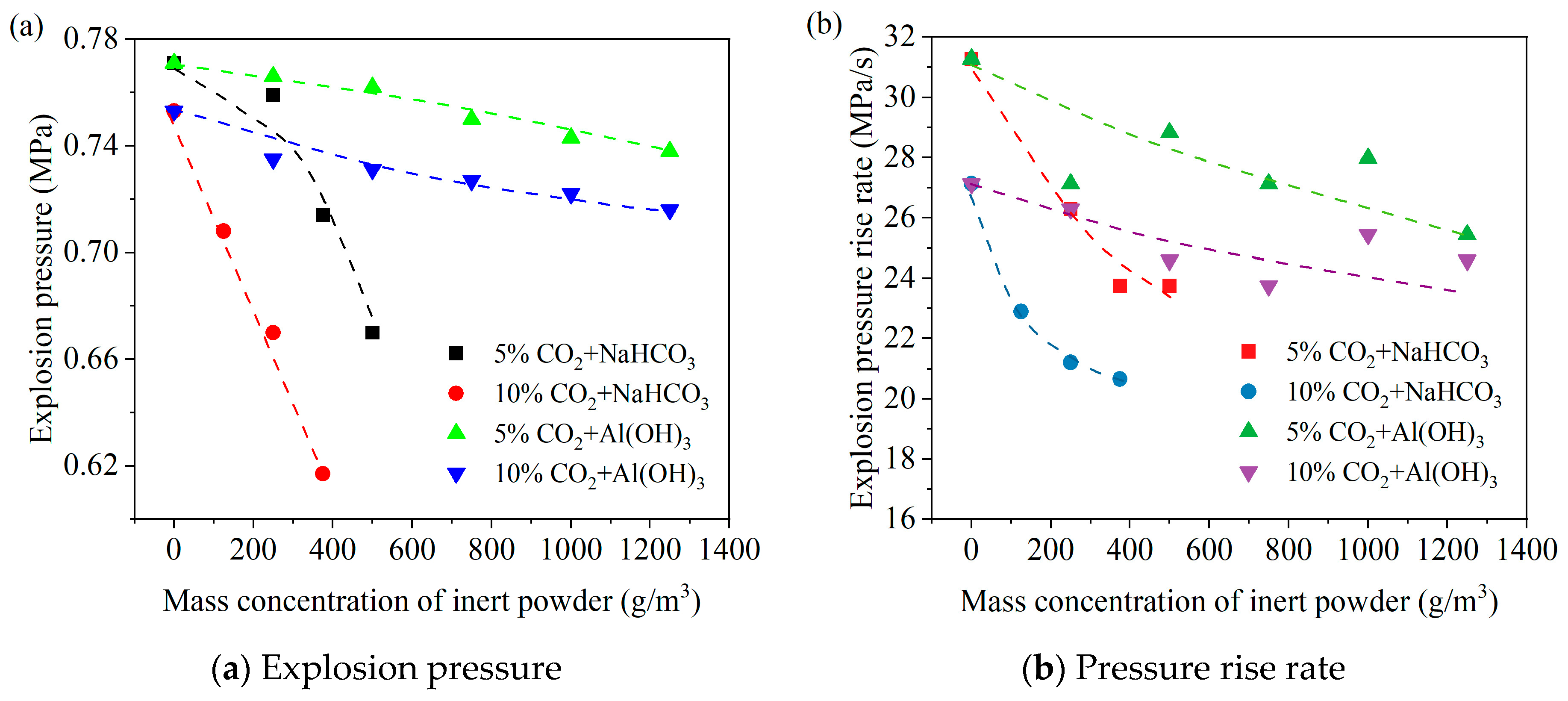
3.1. Comparison of Explosion Suppression Effects of Different Inert Powders
3.2. Synergistic Explosion Suppression Effect of CO2 with Inert Powders
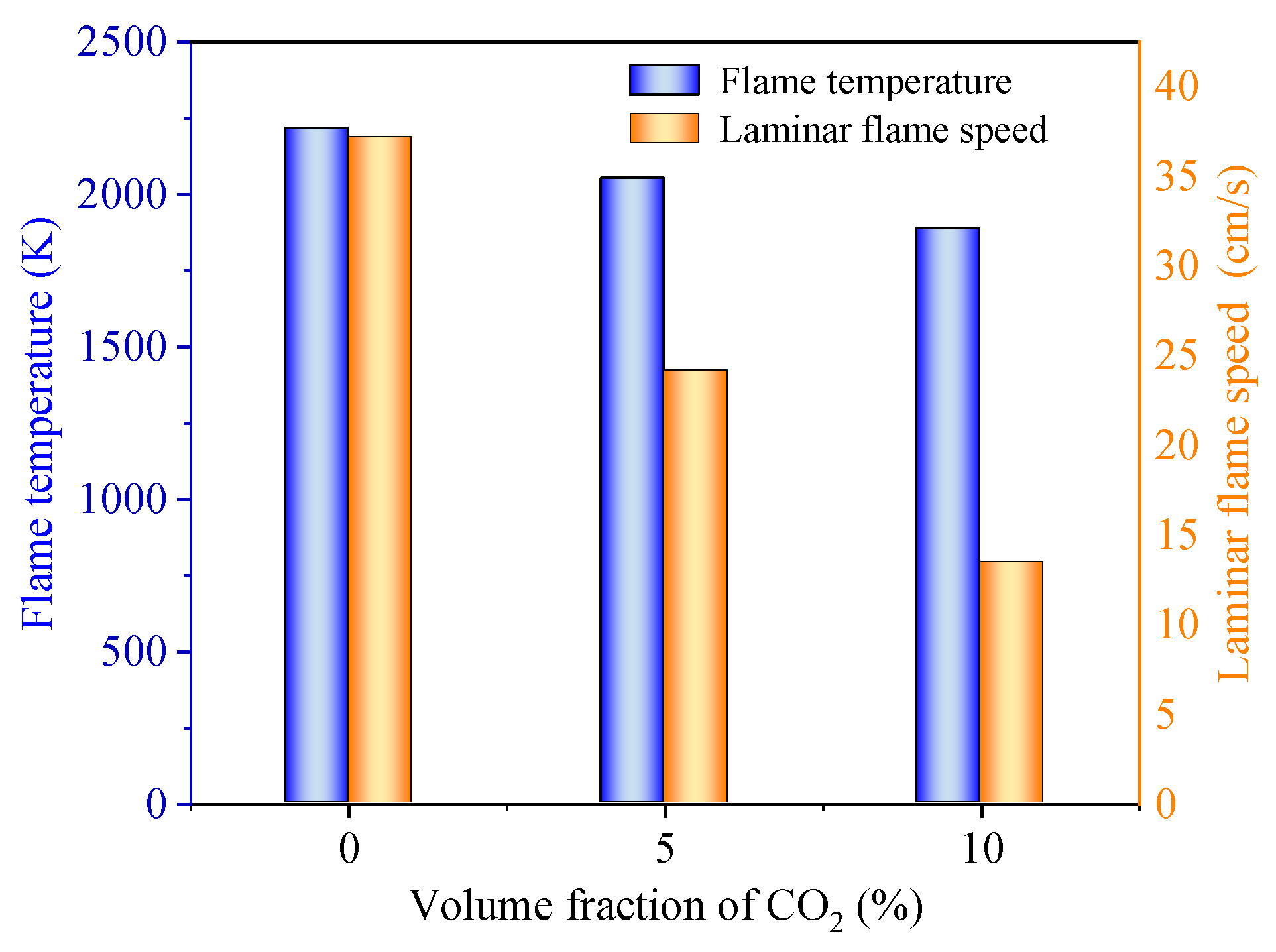
3.3. Mechanism Analysis of Explosion Suppression
4. Conclusions
- The inert powders of NaHCO3 and Al(OH)3 both have inhibitory effects on LPG explosions. As the inert powder content increases, the explosion pressure gradually decreases. The inhibitory effect on explosions is better for NaHCO3 than for Al(OH)3.
- The explosion suppression effects of both gas/solid two-phase inhibitors increase with an increasing CO2 volume fraction or NaHCO3 and Al(OH)3 mass concentrations. Among them, CO2/NaHCO3 exhibits better synergistic explosion suppression than CO2/Al(OH)3, and the synergistic suppression effect becomes more pronounced with higher CO2 volume fractions.
- The addition of both inert powders and inert gases reduces the flame propagation speed of LPG explosions and weakens the flame brightness. CO2 generated from the thermal decomposition of inert powders can decrease the O2 concentration in the explosion reaction system, and the generated H2O absorbs the heat released by the explosion reaction system. The resulting solid salts are non-flammable inorganic substances that hinder combustion reactions. CO2 can reduce the combustion rate and flame temperature of LPG, weakening the intensity of combustion explosions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, G.; Kong, Y.; Yu, J.; Zhang, Q.; Li, R.; Wang, D.; Fan, T.; Cui, Y.; Li, Z. Experimental study on the flame-dual field overpressure coupling evolution characteristics of LPG/DME blended gas explosion venting. J. Clean. Prod. 2024, 444, 141220. [Google Scholar] [CrossRef]
- Lyu, S.; Zhang, S.; Huang, X.; Peng, S.; Li, J. Investigation and modeling of the LPG tank truck accident in Wenling, China. Process Saf. Environ. Prot. 2022, 157, 493–508. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, R.; Zhang, Q.; Yuan, M.; Zhao, Y. Cause Analysis of the Large-Scale LPG Explosion Accident Based on Key Investigation Technology: A Case Study. ACS Omega 2021, 6, 20644–20656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qian, X.; Fu, L.; Yuan, M.; Chen, Y. Shock wave evolution and overpressure hazards in partly premixed gas deflagration of DME/LPG blended multi-clean fuel. Fuel 2020, 268, 117368. [Google Scholar] [CrossRef]
- Zhang, S.; Wen, X.; Guo, Z.; Zhang, S.; Ji, W. Experimental study on the multi-level suppression of N2 and CO2 on hydrogen-air explosion. Process Saf. Environ. Prot. 2023, 169, 970–981. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Ji, W.; Pei, B.; Lin, C.; Feng, H.; Zheng, L. The inhibition effect of gas–solid two-phase inhibitors on methane explosion. Energies 2019, 12, 12030398. [Google Scholar] [CrossRef]
- Wu, Y.; Meng, X.; Zhang, Y.; Shi, L.; Wu, Q.; Liu, L.; Wang, Z.; Liu, J.; Yan, K.; Wang, T. Experimental study on the suppression of coal dust explosion by silica aerogel. Energy 2023, 267, 126372. [Google Scholar] [CrossRef]
- Cheng, C.; Si, R.; Wang, L.; Jia, Q.; Xin, C. Explosion and explosion suppression of gas/deposited coal dust in a realistic environment. Fuel 2024, 357, 129710. [Google Scholar] [CrossRef]
- Huang, Z.; Si, R.; Wen, G.; Jin, S.; Xue, S. Experimental Study on the Isolation Effect of an Active Flame-Proof Device on a Gas Explosion in an Underground Coal Mine. Fire 2023, 6, 6120468. [Google Scholar] [CrossRef]
- Mitu, M.; Prodan, M.; Giurcan, V.; Razus, D.; Oancea, D. Influence of inert gas addition on propagation indices of methane-air deflagrations. Process Saf. Environ. Prot. 2016, 102, 513–522. [Google Scholar] [CrossRef]
- Luo, Z.; Wei, C.; Wang, T.; Su, B.; Cheng, F.; Liu, C.; Wang, Y. Effects of N2 and CO2 dilution on the explosion behavior of liquefied petroleum gas (LPG)-air mixtures. J. Hazard. Mater. 2021, 403, 123843. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, Y.; Zhao, Z. Effect of N2 and CO2 on explosion behavior of H2-Liquefied petroleum gas-air mixtures in a confined space. Int. J. Hydrogen Energy 2022, 47, 23887–23897. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lin, N.K.; Shu, C.M. Effects of flammability characteristics of methane with three inert gases. Process Saf. Prog. 2010, 29, 349–352. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Wang, C.; Wang, B. Thermal and kinetics mechanism of explosion mitigation of methane-air mixture by N2/CO2 in a closed compartment. Fuel 2019, 255, 115747. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Meng, X.; Yan, K.; Wang, Z.; Liu, J.; Wang, Z.; Yang, P.; Dai, W.; Li, F. Research on flame propagation and explosion overpressure of oil shale dust explosion suppression by NaHCO3. Fuel 2022, 314, 122778. [Google Scholar] [CrossRef]
- Yang, P.P.; Meng, X.; Zhang, Y.S.; Liu, J.Q.; Yan, K.; Li, F.; Wang, Z.; Liu, Y.; Dai, W.; Wang, Z. Experimental study and mechanism analysis on the suppression of flour explosion by NaCl and NaHCO3. Combust. Sci. Technol. 2023, 195, 4053–4068. [Google Scholar]
- Jiang, H.; Bi, M.; Peng, Q.; Gao, W. Suppression of pulverized biomass dust explosion by NaHCO3 and NH4H2PO4. Renewable Energy 2020, 147, 2046–2055. [Google Scholar] [CrossRef]
- Wang, Q.H.; Wen, H.; Wang, Q.S.; Sun, J.H. Inhibiting effect of Al(OH)3 and Mg(OH)2 dust on the explosions of methane-air mixtures in closed vessel. Sci. China Technol. Sci. 2012, 55, 1371–1375. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.; Qian, J.; Zhao, H.; Ali, M.; Gu, Z. Inertant effects and mechanism of Al(OH)3 powder on polyethylene dust explosions based on flame propagation behavior and thermal analysis. Fire Saf. J. 2021, 124, 103392. [Google Scholar] [CrossRef]
- Meng, X.; Yang, P.; Zhang, Y.; Li, F.; Liu, J. Effect and mechanism of Aluminium hydroxide and Magnesium hydroxide powder on flame suppression of flour explosion. Combust. Sci. Technol. 2024, 196, 981–996. [Google Scholar] [CrossRef]
- Yu, X.; Chen, J.; Meng, X.; Zhu, Y.; Li, Y.; Qin, Z.; Wu, Y.; Yan, K.; Song, S. Polyethylene deflagration characterization and kinetic mechanism analysis. Energy 2024, 303, 131990. [Google Scholar] [CrossRef]
- Nan, F.; Luo, Z.; Cheng, F.; Xiao, Y.; Li, R.; Su, B.; Wang, T. Research progress and development trends of hydrogen explosion suppression materials and mechanisms. Process Saf. Environ. Prot. 2024, 184, 1318–1331. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, X.; Li, R.; Zhou, G.; Sun, Y.; Ma, Y.; Kong, Y. Explosion characteristics and chemical kinetics of blended LPG/DME clean fuel based on pyrolysis and oxidation mechanism model. Fuel 2022, 320, 123896. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, Y.; Kong, Y.; Zhang, Q.; Sun, Y.; Wang, Y.; Ding, J. Study on explosion dynamics and kinetic mechanism of DME/H2 blended gas at typical fuel-lean/rich concentrations. Case Stud. Therm. Eng. 2022, 40, 10244. [Google Scholar] [CrossRef]
- Pei, B.; Han, Y.; Chen, L.; Hu, Z.; Wu, Z.; Lv, H.; Ji, W. Study on the synergistic suppression effect and mechanism of N2/ultrafine water mist on liquefied petroleum gas explosion. ACS Omega 2024, 9, 14539–14550. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.; Chu, X.; Tang, Y.; Chen, D. Investigation of the effects of N2 and CO2 on the overpressure hazards, flame behaviors and reaction kinetics of LPG/air explosions. Energy Sources Part A 2023, 46, 918–929. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, E.; Liu, Z.; Lin, S.; Chu, X. Experimental Study on Explosion Characteristics of LPG/Air Mixtures Suppressed by CO2 Synergistic Inert Powder. Fire 2024, 7, 275. https://doi.org/10.3390/fire7080275
Zhao E, Liu Z, Lin S, Chu X. Experimental Study on Explosion Characteristics of LPG/Air Mixtures Suppressed by CO2 Synergistic Inert Powder. Fire. 2024; 7(8):275. https://doi.org/10.3390/fire7080275
Chicago/Turabian StyleZhao, Enlai, Zhentang Liu, Song Lin, and Xiaomeng Chu. 2024. "Experimental Study on Explosion Characteristics of LPG/Air Mixtures Suppressed by CO2 Synergistic Inert Powder" Fire 7, no. 8: 275. https://doi.org/10.3390/fire7080275
APA StyleZhao, E., Liu, Z., Lin, S., & Chu, X. (2024). Experimental Study on Explosion Characteristics of LPG/Air Mixtures Suppressed by CO2 Synergistic Inert Powder. Fire, 7(8), 275. https://doi.org/10.3390/fire7080275









