Kinetics Investigation of Copper Ore Oxygen Carrier for Chemical Looping Combustion
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Conversion Determination
2.3. Pre-Experiment in TGA
2.4. Formal Experiment in TGA
2.5. Isothermal Kinetic Analysis Method
3. Results and Discussion
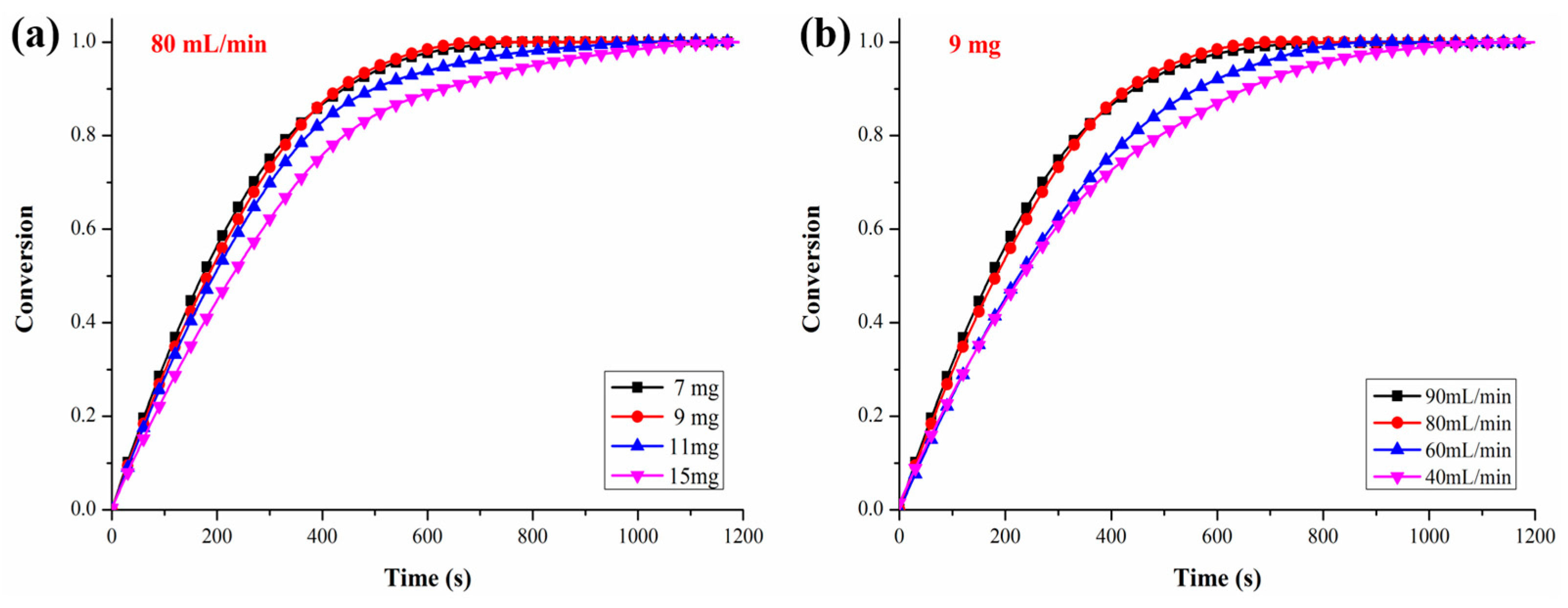
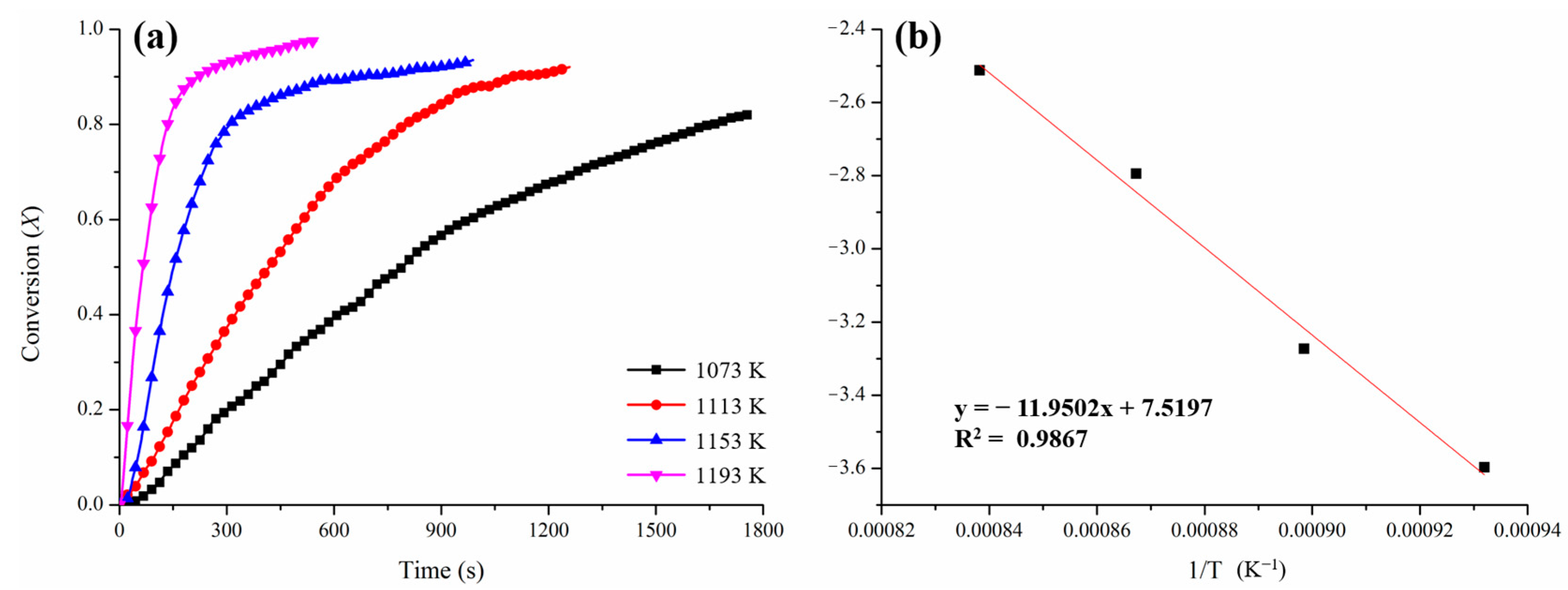
3.1. Kinetic Determination for the Oxygen Releasing Process
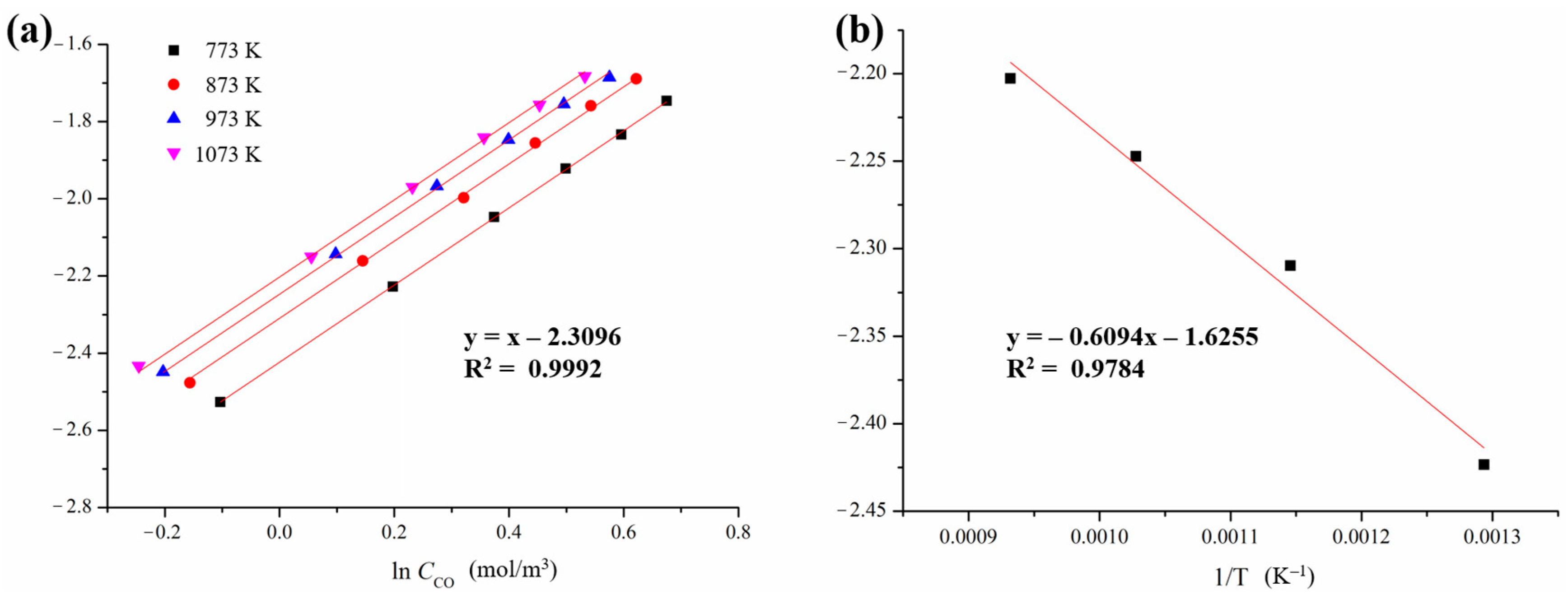
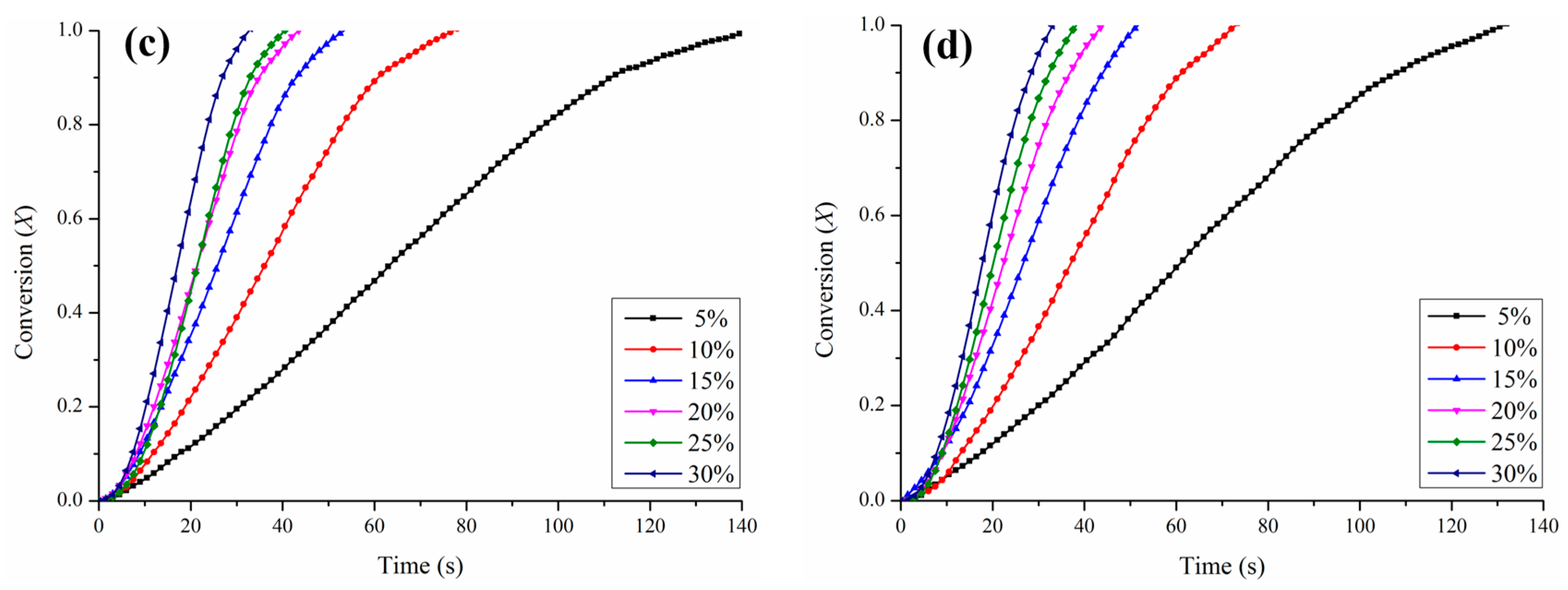
3.2. Kinetic Determination for the Reduction of CO
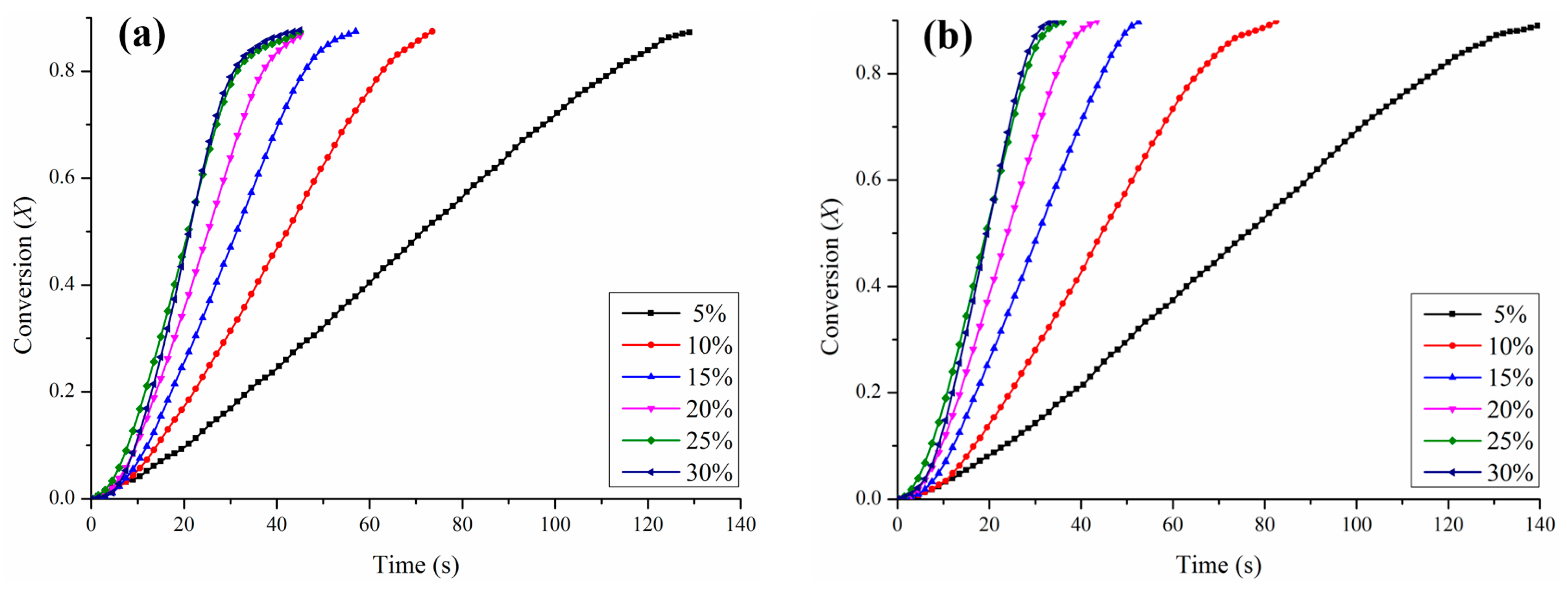
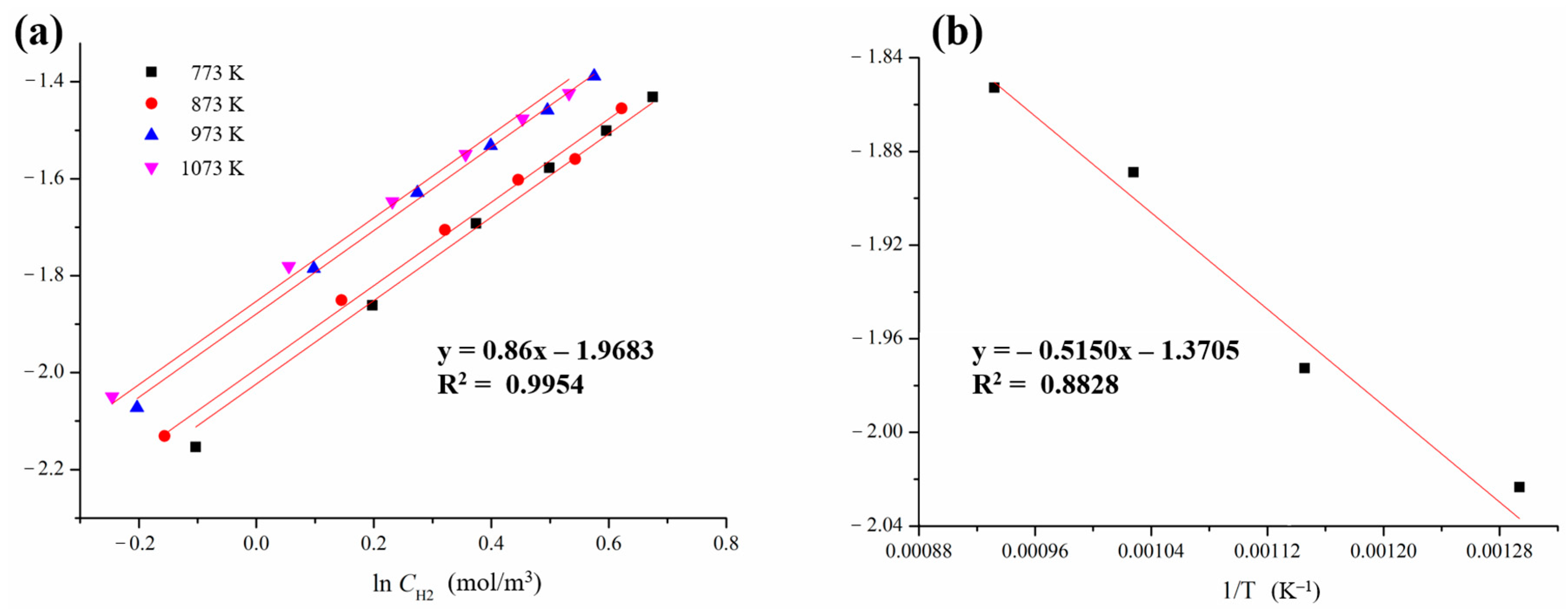
3.3. Kinetic Determination for the Reduction with H2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mongo, M.; Belaïd, F.; Ramdani, B. The effects of environmental innovations on CO2 emissions: Empirical evidence from Europe. Environ. Sci. Policy 2021, 118, 1–9. [Google Scholar] [CrossRef]
- Shan, Y.; Huang, Q.; Guan, D. Hubacek, China CO2 emission accounts 2016–2017. Sci. Data 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Gür, T.M. Carbon dioxide emissions, capture, storage and utilization: Review of materials, processes and technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Al Baroudi, H.; Awoyomi, A.; Patchigolla, K.; Jonnalagadda, K.; Anthony, E.J. A review of large-scale CO2 shipping and marine emissions management for carbon capture, utilisation and storage. Appl. Energy 2021, 287, 116510. [Google Scholar] [CrossRef]
- Ishida, M.; Zheng, D.; Akehata, T. Evaluation of a chemical-looping-combustion power-generation system by graphic exergy analysis. Energy 1987, 12, 147–154. [Google Scholar] [CrossRef]
- Production of Pure Carbon Dioxide. US2665971A, 12 January 1954.
- Richter, H.J.; Knoche, K.F. Reversibility of combustion process. In Efficiency and Costing: Second Law Analysis of Processes; ACS Symposium Series, 235; Gaggioli, R.A., Ed.; ACS Publications: Washington, DC, USA, 1983; pp. 71–85. [Google Scholar]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A. Chemical-looping combustion: Status and research needs. Proc. Combust. Inst. 2019, 37, 4303–4317. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, X.; Ma, J.; Chen, X.; Su, M.; Zheng, C.; Wang, Y. Chemical looping combustion of coal in China: Comprehensive progress, remaining challenges, and potential opportunities. Energy Fuels 2020, 34, 6696–6734. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H.; Okamoto, T. A Fundamental Study of a New Kind of Medium Material for Chemical-Looping Combustion. Energy Fuels 1996, 10, 958–963. [Google Scholar] [CrossRef]
- Ishida, M.; Hongguang, J. A new advanced power-generation system using chemical-looping combustion. Energy 1994, 19, 415–422. [Google Scholar] [CrossRef]
- Mattisson, T.; Garcia-Labiano, F.; Kronberger, B.; Lyngfelt, A.; Adanez, J.; Hofbauer, H. Chemical-looping combustion using syngas as fuel. Int. J. Greenh. Gas Control 2007, 1, 158–169. [Google Scholar] [CrossRef]
- Arjmand, M.; Azad, A.-M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Evaluation of CuAl2O4 as an oxygen carrier in chemical-looping combustion. Ind. Eng. Chem. Res. 2012, 51, 13924–13934. [Google Scholar] [CrossRef]
- Shulman, A.; Cleverstam, E.; Mattisson, T.; Lyngfelt, A. Lyngfelt, Manganese/iron, manganese/nickel, and manganese/silicon oxides used in chemical-looping with oxygen uncoupling (CLOU) for combustion of methane. Energy Fuels 2009, 23, 5269–5275. [Google Scholar] [CrossRef]
- Han, T.; Hong, H.; He, F.; Jin, H. Reactivity study on oxygen carriers for solar-hybrid chemical-looping combustion of di-methyl ether. Combust. Flame 2012, 159, 1806–1813. [Google Scholar] [CrossRef]
- Mattisson, T.; Jerndal, E.; Linderholm, C.; Lyngfelt, A. Reactivity of a spray-dried NiO/NiAl2O4 oxygen carrier for chemical-looping combustion. Chem. Eng. Sci. 2011, 66, 4636–4644. [Google Scholar] [CrossRef]
- Wen, Y.-Y.; Li, Z.-S.; Xu, L.; Cai, N.-S. Experimental study of natural Cu ore particles as oxygen carriers in chemical looping with oxygen uncoupling (CLOU). Energy Fuels 2012, 26, 3919–3927. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, H.; Ma, J. Cement bonded fine hematite and copper ore particles as oxygen carrier in chemical looping combustion. Appl. Energy 2017, 204, 242–253. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Y.; Du, H.; Ma, J.; Zheng, Y.; Zhao, H. Using copper ore and hematite fine particles as raw materials of an oxygen carrier for chemical looping combustion of coal: Spray drying granulation and performance evaluation. Energy Fuels 2020, 34, 8587–8599. [Google Scholar] [CrossRef]
- Gu, Z.; Zhang, L.; Lu, C.; Qing, S.; Li, K. Enhanced performance of copper ore oxygen carrier by red mud modification for chemical looping combustion. Appl. Energy 2020, 277, 115590. [Google Scholar] [CrossRef]
- Liu, X.; Bu, H.; Zou, G.; Wu, X.; Zheng, C.; Ma, J.; Zhao, H. Industrial-Scale Preparation of Biore Oxygen Carriers for Chemical Looping Combustion via a Hydroforming Method. Energy Fuels 2024, 38, 6156–6170. [Google Scholar] [CrossRef]
- Tian, X.; Su, M.; Zhao, H. Kinetics of redox reactions of CuO@TiO2–Al2O3 for chemical looping combustion and chemical looping with oxygen uncoupling. Combust. Flame 2020, 213, 255–267. [Google Scholar] [CrossRef]
- Abad, A.; Cabello, A.; Gayán, P.; García-Labiano, F.; De Diego, L.; Mendiara, T.; Adánez, J. Kinetics of CaMn0.775Ti0.125Mg0.1O2.9-δ perovskite prepared at industrial scale and its implication on the performance of chemical looping combustion of methane. Chem. Eng. J. 2020, 394, 124863. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Li, Z.; Larring, Y.; Cai, N. Heterogeneous reaction kinetics of a perovskite oxygen carrier for chemical looping combustion coupled with oxygen uncoupling. Chem. Eng. J. 2021, 417, 128054. [Google Scholar] [CrossRef]
- Liu, L.; Li, Z.; Cai, N. Oxidization and reduction kinetics of a manganese oxygen carrier granulated with the spray drying method at a tonnage scale for chemical looping combustion. Fuel 2021, 303, 121267. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, K.; Zhao, H. Reduction Kinetics of Low-Cost Cu/Fe-Based Oxygen Carriers in Chemical Looping Mode. Energy Fuels 2023, 37, 16716–16728. [Google Scholar] [CrossRef]
- Wang, K.; Yu, Q.; Qin, Q. Reduction kinetics of Cu-based oxygen carriers for chemical looping air separation. Energy Fuels 2013, 27, 5466–5474. [Google Scholar] [CrossRef]
- Garcia-Labiano, F.; de Diego, L.F.; Adanez, J.; Abad, A.; Gayan, P. Reduction and oxidation kinetics of a copper-based oxygen carrier prepared by impregnation for chemical-looping combustion. Ind. Eng. Chem. Res. 2004, 43, 8168–8177. [Google Scholar] [CrossRef]
- Moghtaderi, B.; Song, H. Reduction Properties of Physically Mixed Metallic Oxide Oxygen Carriers in Chemical Looping Combustion. Energy Fuels 2010, 24, 5359–5368. [Google Scholar] [CrossRef]
- Abad, A.; Garcia-Labiano, F.; de Diego, L.F.; Gayan, P.; Adanez, J. Reduction kinetics of Cu-, Ni-, and Fe-based oxygen carriers using syngas (CO + H2) for chemical-looping combustion. Energy Fuels 2007, 21, 1843–1853. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Dennis, J.S.; Hayhurst, A.N.; Scott, S.A. Kinetics of the chemical looping oxidation of CO by a co-precipitated mixture of CuO and Al2O3. Proc. Combust. Inst. 2009, 32, 2633–2640. [Google Scholar] [CrossRef]
- Chuang, S.; Dennis, J.; Hayhurst, A.; Scott, S. Kinetics of the chemical looping oxidation of H2 by a co-precipitated mixture of CuO and Al2O3. Chem. Eng. Res. Des. 2011, 89, 1511–1523. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P. Modeling of the chemical-looping combustion of methane using a Cu-based oxygen-carrier. Combust. Flame 2010, 157, 602–615. [Google Scholar] [CrossRef]
- Goldstein, E.A.; Mitchell, R.E. Chemical kinetics of copper oxide reduction with carbon monoxide. Proc. Combust. Inst. 2011, 33, 2803–2810. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Gayán, P.; Abad, A.; García-Labiano, F.; De Diego, L.; Adánez, J. Kinetic analysis of a Cu-based oxygen carrier: Relevance of temperature and oxygen partial pressure on reduction and oxidation reactions rates in Chemical Looping with Oxygen Uncoupling (CLOU). Chem. Eng. J. 2014, 256, 69–84. [Google Scholar] [CrossRef]
- Clayton, C.K.; Whitty, K.J. Measurement and modeling of decomposition kinetics for copper oxide-based chemical looping with oxygen uncoupling. Appl. Energy 2014, 116, 416–423. [Google Scholar] [CrossRef]
- Pio, M.S.; Martini, M.; Gallucci, F.; Roghair, I.; van Sint Annaland, M. Kinetics of CuO/SiO2 and CuO/Al2O3 oxygen carriers for chemical looping combustion. Chem. Eng. Sci. 2018, 175, 56–71. [Google Scholar]
- Chuang, S.; Dennis, J.; Hayhurst, A.; Scott, S. Kinetics of the oxidation of a co-precipitated mixture of Cu and Al2O3 by O2 for chemical-looping combustion. Energy Fuels 2010, 24, 3917–3927. [Google Scholar] [CrossRef]
- Su, M.; Zhao, H.; Tian, X.; Zhang, P.; Du, B.; Liu, Z. Intrinsic reduction kinetics investigation on a hematite oxygen carrier by CO in chemical looping combustion. Energy Fuels 2017, 31, 3010–3018. [Google Scholar] [CrossRef]

| Oxygen Carrier | Testing Condition | Kinetics Parameters | Ref. |
|---|---|---|---|
| CuO/Al2O3 | 523–1173 K 1.1–9.77% CO | DRM n = 1.0, Ea = 28–52 kJ/mol | [34] |
| CuO/Al2O3 | 523–1173 K 1.1–9.77% H2 | DRM n = 1.0, Ea = 58 kJ/mol | [35] |
| CuO/Al2O3 | 723–1073 K 5–70% CH4 5–70% H2 5–70% CO | SCM n = 0.4, Ea = 60 kJ/mol n = 0.6, Ea = 33 kJ/mol n = 0.8, Ea = 15 kJ/mol | [31] |
| CuO/Al2O3 | 873–1073 K 5–70% CH4 5–70% CO 5–70% H2 | SCM n = 0.5, Ea = 106 kJ/mol n = 0.8, Ea = 11 kJ/mol n = 0.5, Ea = 20 kJ/mol | [36] |
| CuO | 473–773 K 1.6–5% CO | DRM n = 0.7, Ea = 20–25 kJ/mol | [37] |
| CuO/Al2O3 | 773–1073 K 20–70% H2 20–70% CO | SCM n = 0.55, Ea = 30 kJ/mol n = 0.8, Ea = 16 kJ/mol | [32] |
| CuO/MgAl2O4 | 0–9% O2, 1148–1273 K 2.5–21% O2, 1123–1273 K | NNGM, n = 0.5, Ea = 270 kJ/mol L-H, n = 1.2, Ea = 32 kJ/mol | [38] |
| CuO/ZrO2 | 0–3.4% O2, 1048–1198 K | First order, n = 1.0, Ea = 58 kJ/mol | [39] |
| CuO/TiO2 | 0–3.4% O2, 1073–1173 K | First order, n = 1.0, Ea = 67 kJ/mol | [39] |
| CuO/SiO2 | 973–1173 K 0–0.875% O2 | SCM Ea = 249 kJ/mol | [40] |
| CuO/Al2O3 | 573–1023 K 1.22–7.5% O2 | DRM n = 1.0, Ea = 40–60 kJ/mol | [41] |
| CuO/Al2O3 | 773–1073 K 5–21% O2 | SCM n = 1.0, Ea = 15 kJ/mol | [31] |
| CuO@TiO2-Al2O3 | 0–1.0% O2, 1083–1163 K 5.2–21% O2, 793–873 K 5–35% H2, 498–598 K 5–35% CO, 573–673 K 5–20% CH4, 948–1048 K | NNGM, n = 0.5, Ea = 217.2 kJ/mol CRM, n = 0.2, Ea = 87.5 kJ/mol SCM, n = 0.8, Ea = 44.5 kJ/mol SCM, n = 1.0, Ea = 40.1 kJ/mol NNGM, n = 0.6, Ea = 112.2 kJ/mol | [25] |
| Chemical Content (wt. %) | Ultimate Analysis (wt. %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CuO | CuFe2O4 | SiO2 | CaSO4 | Al2O3 | Cu | Fe | Si | Ca | S | Al |
| 21.04 | 70.05 | 5.53 | 2.29 | 1.08 | 48.88 | 45.00 | 7.07 | 1.31 | 1.47 | 1.63 |
| Reaction Model | Symbol | f(X) | G(X) |
|---|---|---|---|
| nucleation and nuclei growth (n = 1) | A1(X) | ||
| nucleation and nuclei growth (n = 2) | A2(X) | ||
| nucleation and nuclei growth (n = 3) | A3(X) | ||
| 2D-diffusion model | D2(X) | ||
| 3D-diffusion model (Jander) | D3(X) | ||
| 3D-diffusion model (Grinstling) | D4(X) | ||
| zero order contraction model | R1(X) | ||
| 2D-contraction model | R2(X) | ||
| 3D-contraction model | R3(X) |
| Kinetics Model Equation | n (Dimensionless) | E (kJ/mol) | A (atm−n·s−1) | |
|---|---|---|---|---|
| N2 | - | 99.35 | 1.84 × 103 | |
| CO | 1 | 5.08 | 1.96 × 10−1 | |
| H2 | 0.86 | 4.82 | 2.54 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Su, M.; Zhao, H. Kinetics Investigation of Copper Ore Oxygen Carrier for Chemical Looping Combustion. Fire 2024, 7, 245. https://doi.org/10.3390/fire7070245
Tian X, Su M, Zhao H. Kinetics Investigation of Copper Ore Oxygen Carrier for Chemical Looping Combustion. Fire. 2024; 7(7):245. https://doi.org/10.3390/fire7070245
Chicago/Turabian StyleTian, Xin, Mingze Su, and Haibo Zhao. 2024. "Kinetics Investigation of Copper Ore Oxygen Carrier for Chemical Looping Combustion" Fire 7, no. 7: 245. https://doi.org/10.3390/fire7070245
APA StyleTian, X., Su, M., & Zhao, H. (2024). Kinetics Investigation of Copper Ore Oxygen Carrier for Chemical Looping Combustion. Fire, 7(7), 245. https://doi.org/10.3390/fire7070245







