The House Is Burning: Assessment of Habitat Loss Due to Wildfires in Central Mexico
Abstract
1. Introduction
2. Materials and Methods
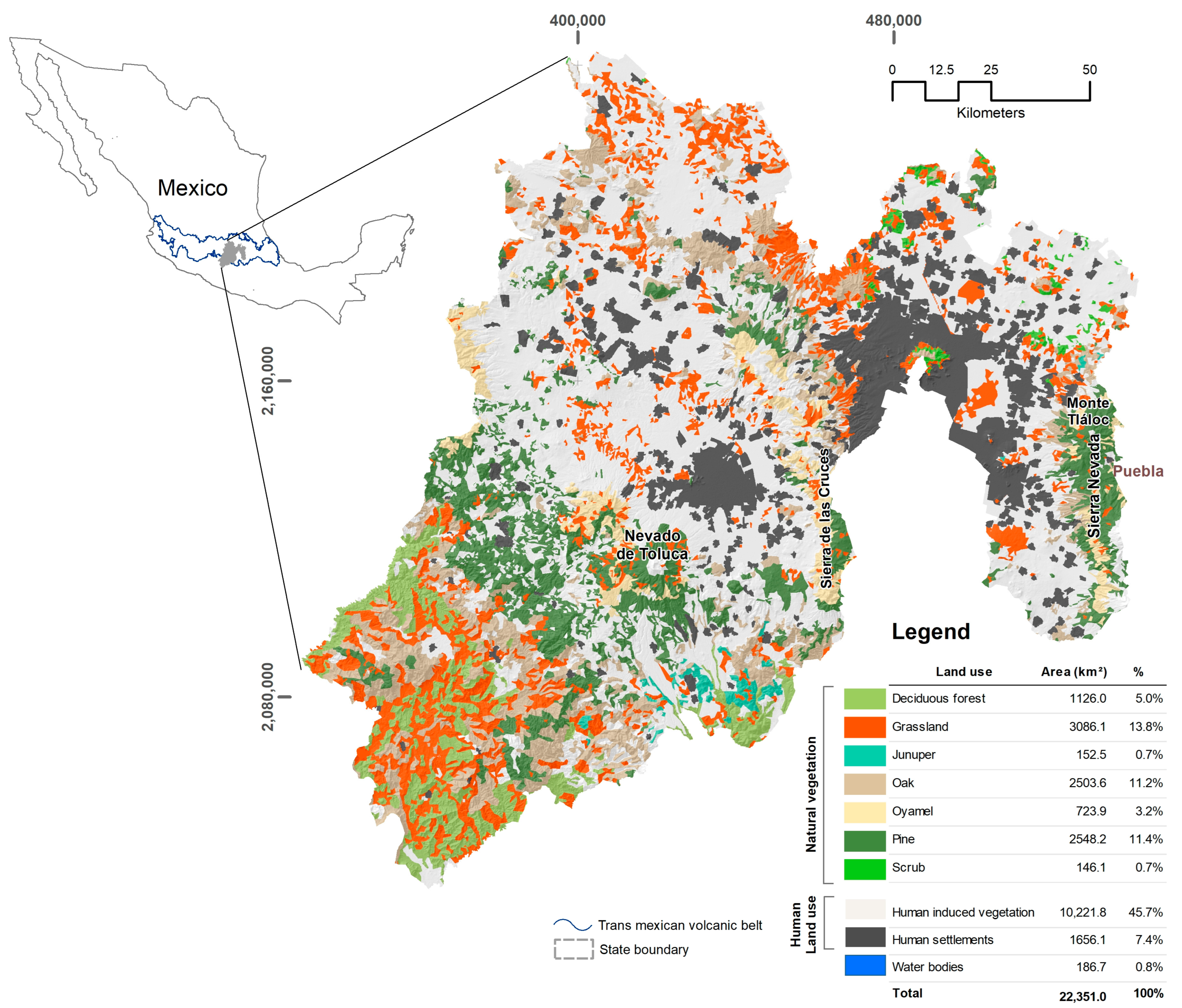
2.1. Study Area
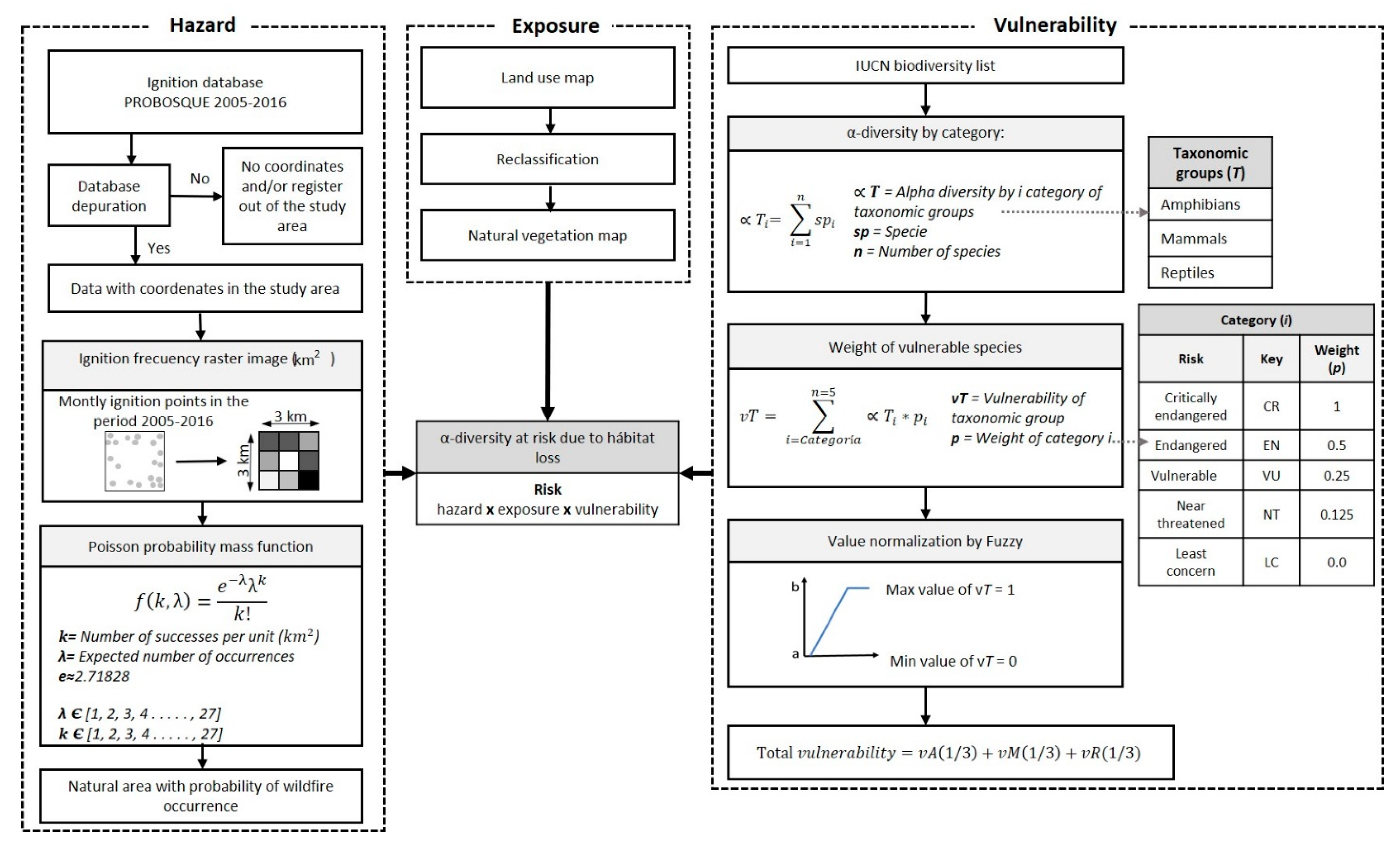
2.2. Methodology to Determine Biodiversity at Risk
2.2.1. Hazard
2.2.2. Exposure
2.2.3. Vulnerability
2.2.4. α-Diversity at Risk due to Wildfires Habitat Loss
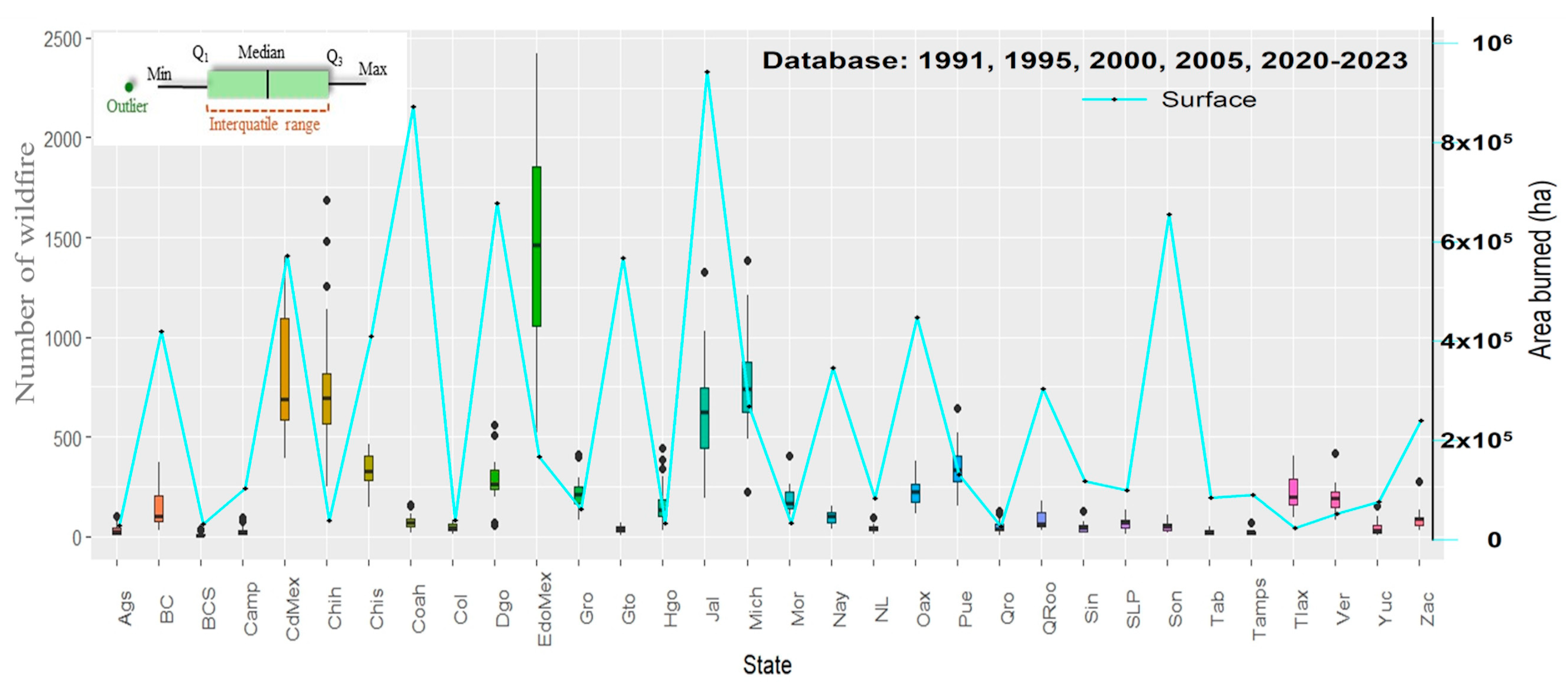
3. Results
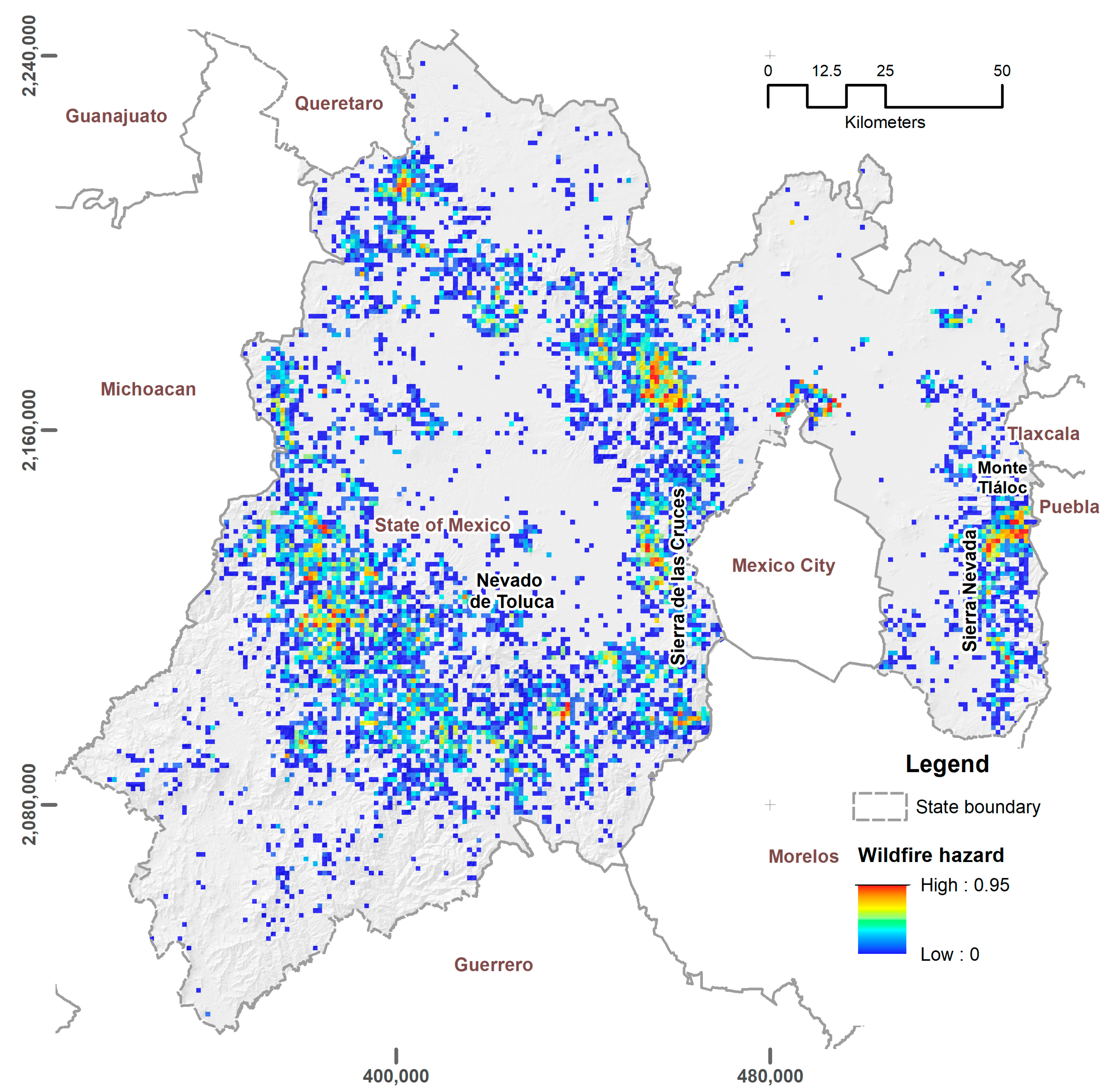
3.1. Exposure Zones and Hazard Analysis
3.2. Vulnerability of Amphibians, Mammals and Reptiles
3.3. α-Diversity at Risk due to Wildfires Habitat Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, S.P.; Marlon, J.R.; Bartlein, P.J. Fire in the Earth System; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 90-481-8715-X. [Google Scholar]
- Haque, M.K.; Azad, M.A.K.; Hossain, M.Y.; Ahmed, T.; Uddin, M.; Hossain, M.M. Wildfire in Australia during 2019–2020, Its Impact on Health, Biodiversity and Environment with Some Proposals for Risk Management: A Review. J. Environ. Prot. 2021, 12, 391–414. [Google Scholar] [CrossRef]
- Bowman, D.M.; Williamson, G.J.; Abatzoglou, J.T.; Kolden, C.A.; Cochrane, M.A.; Smith, A.M. Human Exposure and Sensitivity to Globally Extreme Wildfire Events. Nat. Ecol. Evol. 2017, 1, 0058. [Google Scholar] [CrossRef]
- Moretti, M.; Obrist, M.K.; Duelli, P. Arthropod Biodiversity after Forest Fires: Winners and Losers in the Winter Fire Regime of the Southern Alps. Ecography 2004, 27, 173–186. [Google Scholar] [CrossRef]
- Foster, C.; Barton, P.; Robinson, N.; MacGregor, C.; Lindenmayer, D.B. Effects of a Large Wildfire on Vegetation Structure in a Variable Fire Mosaic. Ecol. Appl. 2017, 27, 2369–2381. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Belcher, C.M.; Lamont, B.B.; Lim, S.L. A 350-million-year Legacy of Fire Adaptation among Conifers. J. Ecol. 2016, 104, 352–363. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of Invasive Alien Plants on Fire Regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Di Virgilio, G.; Evans, J.P.; Blake, S.A.; Armstrong, M.; Dowdy, A.J.; Sharples, J.; McRae, R. Climate Change Increases the Potential for Extreme Wildfires. Geophys. Res. Lett. 2019, 46, 8517–8526. [Google Scholar] [CrossRef]
- McWethy, D.B.; Schoennagel, T.; Higuera, P.E.; Krawchuk, M.; Harvey, B.J.; Metcalf, E.C.; Schultz, C.; Miller, C.; Metcalf, A.L.; Buma, B. Rethinking Resilience to Wildfire. Nat. Sustain. 2019, 2, 797–804. [Google Scholar] [CrossRef]
- Littell, J.S.; Peterson, D.L.; Riley, K.L.; Liu, Y.; Luce, C.H. A Review of the Relationships between Drought and Forest Fire in the United States. Glob. Chang. Biol. 2016, 22, 2353–2369. [Google Scholar] [CrossRef]
- Ruffault, J.; Curt, T.; St-Paul, N.M.; Moron, V.; Trigo, R.M. Extreme Wildfire Occurrence in Response to Global Change Type Droughts in the Northern Mediterranean. Nat. Hazards Earth Syst. Sci. 2017, 1–21. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary Ecology of Resprouting and Seeding in Fire-prone Ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Le Breton, T.D.; Lyons, M.B.; Nolan, R.H.; Penman, T.; Williamson, G.J.; Ooi, M.K. Megafire-induced Interval Squeeze Threatens Vegetation at Landscape Scales. Front. Ecol. Environ. 2022, 20, 327–334. [Google Scholar] [CrossRef]
- EM-DAT; CRED; UCLouvain, Brussels EM-DAT CRED. Available online: https://www.emdat.be (accessed on 20 January 2024).
- Linley, G.D.; Jolly, C.J.; Doherty, T.S.; Geary, W.L.; Armenteras, D.; Belcher, C.M.; Bliege Bird, R.; Duane, A.; Fletcher, M.; Giorgis, M.A. What Do You Mean,‘Megafire’? Glob. Ecol. Biogeogr. 2022, 31, 1906–1922. [Google Scholar] [CrossRef]
- Singleton, M.P.; Thode, A.E.; Meador, A.J.S.; Iniguez, J.M. Increasing Trends in High-Severity Fire in the Southwestern USA from 1984 to 2015. For. Ecol. Manag. 2019, 433, 709–719. [Google Scholar] [CrossRef]
- Weber, K.T.; Yadav, R. Spatiotemporal Trends in Wildfires across the Western United States (1950–2019). Remote Sens. 2020, 12, 2959. [Google Scholar] [CrossRef]
- Collins, L.; Bradstock, R.A.; Clarke, H.; Clarke, M.F.; Nolan, R.H.; Penman, T.D. The 2019/2020 Mega-Fires Exposed Australian Ecosystems to an Unprecedented Extent of High-Severity Fire. Environ. Res. Lett. 2021, 16, 044029. [Google Scholar] [CrossRef]
- Castellnou, M.; Guiomar, N.; Rego, F.; Fernandes, P.M. Fire Growth Patterns in the 2017 Mega Fire Episode of October 15, Central Portugal. Adv. For. Fire Res. 2018, 447–453. [Google Scholar]
- Pliscoff, P.; Folchi, M.; Aliste, E.; Cea, D.; Simonetti, J.A. Chile Mega-Fire 2017: An Analysis of Social Representation of Forest Plantation Territory. Appl. Geogr. 2020, 119, 102226. [Google Scholar] [CrossRef]
- Troumbis, A.Y.; Kalabokidis, K.; Palaiologou, P. Diverging Rationalities between Forest Fire Management Services and the General Public after the 21st-Century Mega-Fires in Greece. J. For. Res. 2022, 33, 553–564. [Google Scholar] [CrossRef]
- Fidelis, A.; Alvarado, S.T.; Barradas, A.C.S.; Pivello, V.R. The Year 2017: Megafires and Management in the Cerrado. Fire 2018, 1, 49. [Google Scholar] [CrossRef]
- Pereira, M.G.; Fernandes, L.S.; Carvalho, S.; Santos, R.B.; Caramelo, L.; Alencoao, A. Modelling the Impacts of Wildfires on Runoff at the River Basin Ecological Scale in a Changing Mediterranean Environment. Environ. Earth Sci. 2016, 75, 392. [Google Scholar] [CrossRef]
- Pastor, A.V.; Nunes, J.P.; Ciampalini, R.; Koopmans, M.; Baartman, J.; Huard, F.; Calheiros, T.; Le-Bissonnais, Y.; Keizer, J.J.; Raclot, D. Projecting Future Impacts of Global Change Including Fires on Soil Erosion to Anticipate Better Land Management in the Forests of NW Portugal. Water 2019, 11, 2617. [Google Scholar] [CrossRef]
- Kastridis, A.; Margiorou, S.; Sapountzis, M. Check-Dams and Silt Fences: Cost-Effective Methods to Monitor Soil Erosion under Various Disturbances in Forest Ecosystems. Land 2022, 11, 2129. [Google Scholar] [CrossRef]
- Pingree, M.R.; Kobziar, L.N. The Myth of the Biological Threshold: A Review of Biological Responses to Soil Heating Associated with Wildland Fire. For. Ecol. Manag. 2019, 432, 1022–1029. [Google Scholar] [CrossRef]
- Neary, D.G.; Ryan, K.C.; DeBano, L.F. Wildland Fire in Ecosystems: Effects of Fire on Soils and Water; Gen. Tech. Rep. RMRS-GTR-42-vol. 4; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2005; Volume 42, 250p. [Google Scholar]
- Bond, W.J.; Woodward, F.I.; Midgley, G.F. The Global Distribution of Ecosystems in a World without Fire. New Phytol. 2005, 165, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.M.; Louzada, J.; Barlow, J.; Andrade, R.; Mestre, L.; Solar, R.; Lacau, S.; Cochrane, M.A. A Multi-taxa Assessment of Biodiversity Change after Single and Recurrent Wildfires in a Brazilian Amazon Forest. Biotropica 2016, 48, 170–180. [Google Scholar] [CrossRef]
- Adams, M.A. Mega-Fires, Tipping Points and Ecosystem Services: Managing Forests and Woodlands in an Uncertain Future. For. Ecol. Manag. 2013, 294, 250–261. [Google Scholar] [CrossRef]
- Caon, L.; Vallejo, V.R.; Ritsema, C.J.; Geissen, V. Effects of Wildfire on Soil Nutrients in Mediterranean Ecosystems. Earth-Sci. Rev. 2014, 139, 47–58. [Google Scholar] [CrossRef]
- Slingsby, J.A.; Moncrieff, G.R.; Rogers, A.J.; February, E.C. Altered Ignition Catchments Threaten a Hyperdiverse Fire-dependent Ecosystem. Glob. Chang. Biol. 2020, 26, 616–628. [Google Scholar] [CrossRef]
- DeBano, L.F.; Neary, D.G.; Ffolliott, P.F. Fire Effects on Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Southwell, D.; Legge, S.; Woinarski, J.; Lindenmayer, D.; Lavery, T.; Wintle, B. Design Considerations for Rapid Biodiversity Reconnaissance Surveys and Long-term Monitoring to Assess the Impact of Wildfire. Divers. Distrib. 2022, 28, 559–570. [Google Scholar] [CrossRef]
- Gade, M.R.; Gould, P.R.; Peterman, W.E. Habitat-Dependent Responses of Terrestrial Salamanders to Wildfire in the Short-Term. For. Ecol. Manag. 2019, 449, 117479. [Google Scholar] [CrossRef]
- Bradstock, R.A. Effects of Large Fires on Biodiversity in South-Eastern Australia: Disaster or Template for Diversity? Int. J. Wildland Fire 2008, 17, 809. [Google Scholar] [CrossRef]
- Legge, S.; Rumpff, L.; Woinarski, J.C.; Whiterod, N.S.; Ward, M.; Southwell, D.G.; Scheele, B.C.; Nimmo, D.G.; Lintermans, M.; Geyle, H.M. The Conservation Impacts of Ecological Disturbance: Time-bound Estimates of Population Loss and Recovery for Fauna Affected by the 2019–2020 Australian Megafires. Glob. Ecol. Biogeogr. 2022, 31, 2085–2104. [Google Scholar] [CrossRef]
- Jolly, C.J.; Dickman, C.R.; Doherty, T.S.; van Eeden, L.M.; Geary, W.L.; Legge, S.M.; Woinarski, J.C.; Nimmo, D.G. Animal Mortality during Fire. Glob. Chang. Biol. 2022, 28, 2053–2065. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.C.; Block, W.M. Effects of Wildfire Severity on Small Mammals in Northern Arizona Ponderosa Pine Forests. In Fire and Forest Ecology: Innovative Silviculture and Vegetation Management. Tall Timbers Fire EcologyConference Proceedings, No. 21; Tall Timbers Research Station: Tallahassee, FL, USA, 2000; Volume 21, pp. 163–168. [Google Scholar]
- Pastro, L.A.; Dickman, C.R.; Letnic, M. Burning for Biodiversity or Burning Biodiversity? Prescribed Burn vs. Wildfire Impacts on Plants, Lizards, and Mammals. Ecol. Appl. 2011, 21, 3238–3253. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Armenteras, D.; Bennett, A.F.; Brotons, L.; Clarke, M.F.; Doherty, T.S.; Haslem, A.; Kelly, L.T.; Sato, C.F.; Sitters, H. How Fire Interacts with Habitat Loss and Fragmentation. Biol. Rev. 2021, 96, 976–998. [Google Scholar] [CrossRef] [PubMed]
- Cunillera-Montcusí, D.; Gascón, S.; Tornero, I.; Sala, J.; Àvila, N.; Quintana, X.D.; Boix, D. Direct and Indirect Impacts of Wildfire on Faunal Communities of Mediterranean Temporary Ponds. Freshw. Biol. 2019, 64, 323–334. [Google Scholar] [CrossRef]
- Ayars, J.; Kramer, H.A.; Jones, G.M. The 2020 to 2021 California Megafires and Their Impacts on Wildlife Habitat. Proc. Natl. Acad. Sci. USA 2023, 120, e2312909120. [Google Scholar] [CrossRef] [PubMed]
- González, T.M.; González-Trujillo, J.D.; Muñoz, A.; Armenteras, D. Effects of Fire History on Animal Communities: A Systematic Review. Ecol. Process. 2022, 11, 11. [Google Scholar] [CrossRef]
- Hossack, B.R.; Pilliod, D.S. Amphibian Responses to Wildfire in the Western United States: Emerging Patterns from Short-Term Studies. Fire Ecol. 2011, 7, 129–144. [Google Scholar] [CrossRef]
- Chia, E.K.; Bassett, M.; Nimmo, D.G.; Leonard, S.W.; Ritchie, E.G.; Clarke, M.F.; Bennett, A.F. Fire Severity and Fire-Induced Landscape Heterogeneity Affect Arboreal Mammals in Fire-Prone Forests. Ecosphere 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Roberts, S.L.; Kelt, D.A.; Van Wagtendonk, J.W.; Miles, A.K.; Meyer, M.D. Effects of Fire on Small Mammal Communities in Frequent-Fire Forests in California. J. Mammal. 2015, 96, 107–119. [Google Scholar] [CrossRef]
- Bagne, K.E.; Finch, D.M. Response of Small Mammal Populations to Fuel Treatment and Precipitation in a Ponderosa Pine Forest, New Mexico. Restor. Ecol. 2010, 18, 409–417. [Google Scholar] [CrossRef]
- Converse, S.J.; White, G.C.; Block, W.M. Small Mammal Responses to Thinning and Wildfire in Ponderosa Pine–Dominated Forests of the Southwestern United States. J. Wildl. Manag. 2006, 70, 1711–1722. [Google Scholar] [CrossRef]
- Otto, C.R.V.; Kroll, A.J.; McKenny, H.C. Amphibian Response to Downed Wood Retention in Managed Forests: A Prospectus for Future Biomass Harvest in North America. For. Ecol. Manag. 2013, 304, 275–285. [Google Scholar] [CrossRef]
- Todd, B.D.; Luhring, T.M.; Rothermel, B.B.; Gibbons, J.W. Effects of Forest Removal on Amphibian Migrations: Implications for Habitat and Landscape Connectivity. J. Appl. Ecol. 2009, 46, 554–561. [Google Scholar] [CrossRef]
- Beranek, C.T.; Hamer, A.J.; Mahony, S.V.; Stauber, A.; Ryan, S.A.; Gould, J.; Wallace, S.; Stock, S.; Kelly, O.; Parkin, T. Severe Wildfires Promoted by Climate Change Negatively Impact Forest Amphibian Metacommunities. Divers. Distrib. 2023, 29, 785–800. [Google Scholar] [CrossRef]
- Hossack, B.R.; Eby, L.A.; Guscio, C.G.; Corn, P.S. Thermal Characteristics of Amphibian Microhabitats in a Fire-Disturbed Landscape. For. Ecol. Manag. 2009, 258, 1414–1421. [Google Scholar] [CrossRef]
- Santos, X.; Belliure, J.; Gonçalves, J.F.; Pausas, J.G. Resilience of Reptiles to Megafires. Ecol. Appl. 2022, 32, e2518. [Google Scholar] [CrossRef]
- Wilgers, D.J.; Horne, E.A. Effects of Different Burn Regimes on Tallgrass Prairie Herpetofaunal Species Diversity and Community Composition in the Flint Hills, Kansas. J. Herpetol. 2006, 40, 73–84. [Google Scholar] [CrossRef]
- Barrile, G.M.; Chalfoun, A.D.; Estes-Zumpf, W.A.; Walters, A.W. Wildfire Influences Individual Growth and Breeding Dispersal, but Not Survival and Recruitment in a Montane Amphibian. Ecosphere 2022, 13, e4212. [Google Scholar] [CrossRef]
- INEGI. Censo Población y Vivienda 2020; INEGI: Aguascalientes, Mexico, 2020.
- INEGI. Producto Interno Bruto por Entidad Federativa (PIBE); INEGI: Aguascalientes, Mexico, 2023; p. 12.
- Godinez-Tovar, A.G.; Lopez-Gutierrez, M.; Becerril-Piña, R.; Mastachi-Loza, C.A. Influencia Del Cambio Del Uso de Suelo Sobre La Dinámica de La Precipitación. Caso de Estudio: Curso Alto de La Cuenca Alta Del Río Lerma, México. In Geología Ambiental y Recursos Hídricos; Cromberger Editores e Impresores, S.A. de C.V.: Mexico City, Mexico, 2023; Volume 37, pp. 349–364. ISBN 978-607-589-210-8. [Google Scholar]
- Monroy-Vilchis, O.; Luna-Gil, A.A.; Endara-Agramont, A.R.; Zarco-González, M.M.; González-Desales, G.A. Nevado de Toluca: Habitat for Romerolagus Diazi? Anim. Biodivers. Conserv. 2020, 43, 115–121. [Google Scholar] [CrossRef]
- Aguilar, X.; Casas, G. Secretaría del Medio Ambiente Anfibios y Reptiles. In Biodiversidad del Estado de México: Estudio de Estado; Secretaría del Medio Ambiente, Ed.; Gobierno del Estado de México y Comisión para el Conocimiento y Uso de la Biodiversidad: Toluca, Mexico, 2009; pp. 125–130. [Google Scholar]
- INEGI. Conjunto de Datos Vectoriales de Uso de Suelo y Vegetación Escala 1:250 000, Serie V; NEGI: Aguascalientes, Mexico, 2013.
- Gonzalez-Fernandez, A.; Segarra, J.; Sunny, A.; Couturier, S. Forest Cover Loss in the Nevado de Toluca Volcano Protected Area (Mexico) after the Change to a Less Restrictive Category in 2013. Biodivers. Conserv. 2022, 31, 871–894. [Google Scholar] [CrossRef]
- SEMARNAT. Sistema Nacional de Información Ambiental y de Recursos Naturales; SNIARN: Mexico City, Mexico, 2024. [Google Scholar]
- Crichton, D. The Risk Triangle in Natural Disaster Management; Tudor Rose: London, UK, 1999. [Google Scholar]
- Crichton, D. UK and Global Insurance Responses to Flood Hazard. Water Int. 2002, 27, 119–131. [Google Scholar] [CrossRef]
- Katti, S.K.; Rao, A.V. Handbook of the Poisson Distribution; Taylor & Francis Group: Abingdon, UK, 1968. [Google Scholar]
- CONABIO. Sistema Nacional de Información Sobre Biodiversidad (SNIB); Registros de Ejemplares; CONABIO: Mexico City, Mexico, 2024. [Google Scholar]
- Lange, H.J.D.; Lahr, J.; Van der Pol, J.J.; Wessels, Y.; Faber, J.H. Ecological Vulnerability in Wildlife: An Expert Judgment and Multicriteria Analysis Tool Using Ecological Traits to Assess Relative Impact of Pollutants. Environ. Toxicol. Chem. Int. J. 2009, 28, 2233–2240. [Google Scholar] [CrossRef]
- Leverington, F.; Costa, K.L.; Pavese, H.; Lisle, A.; Hockings, M. A Global Analysis of Protected Area Management Effectiveness. Environ. Manag. 2010, 46, 685–698. [Google Scholar] [CrossRef] [PubMed]
- IUCN. IUCN Red List Categories and Criteria, Version 3.1, 2nd ed.; IUCN: Gland, Switzerland, 2012; ISBN 978-2-8317-1435-6. [Google Scholar]
- CONABIO; CONANP; PNUD. Corredores Bioclimáticos Para La Conservación de La Biodiversidad. 2019. Mexico City, Mexico. Available online: http://www.conabio.gob.mx/informacion/gis/?vns=gis_root/region/prioridad/clccrecgw (accessed on 3 April 2024).
- Jung, M. LecoS—A Python Plugin for Automated Landscape Ecology Analysis. Ecol. Inform. 2016, 31, 18–21. [Google Scholar] [CrossRef]
- QGIS Development Team QGIS Geographic Information System. Open source Geospatial Foundation Project. Available online: http://qgis.org (accessed on 25 March 2020).
- Mastachi-Loza, C.A.; Becerril-Piña, R.; Gómez-Albores, M.A.; Díaz-Delgado, C.; Romero-Contreras, A.T.; Garcia-Aragon, J.A.; Vizcarra-Bordi, I. Regional Analysis of Climate Variability at Three Time Scales and Its Effect on Rainfed Maize Production in the Upper Lerma River Basin, Mexico. Agric. Ecosyst. Environ. 2016, 225, 1–11. [Google Scholar] [CrossRef]
- Luna-Vega, I.; Alcántara-Ayala, O.; García-Morales, L.J.; Espinosa, D.; Ramírez-Martínez, J.C.; Contreras-Medina, R. Threatened Trees Characteristic of Mexican Tropical Montane Cloud Forests. Diversity 2022, 15, 42. [Google Scholar] [CrossRef]
- Strachinis, I. The Herpetofauna of the Peri-Urban Forest Seich Sou (Kedrinos Lofos), Thessaloniki, Greece. Ecol. Balk. 2023, 15, 1–7. [Google Scholar]
- Glickman, D.; Babbitt, B. Urban Wildland Interface Communities within the Vicinity of Federal Lands That Are at High Risk from Wildfire. Fed. Regist. 2001, 66, 751–777. [Google Scholar]
- Cobos, E.P. Zona Metropolitana Del Valle de México: Neoliberalismo y Contradicciones Urbanas. Sociologias 2016, 18, 54–89. [Google Scholar] [CrossRef]
- CITES. Estado de Conservación, Uso, Gestión y Comercio de Las Especies Del Género Abronia Que Se Distribuyen En México; Vigésimo Séptima Reunión Del Comité de Fauna Veracruz: Veracruz, Mexico, 2014; Volume 23. [Google Scholar]
- Cruz-Sáenz, D.; Vázquez, S.G.; Lazcano, D. Notes on the Herpetofauna of Western Mexico 13: Effects of Wildfires on the Reptile Community in the Natural Protected Area “La Primavera,” in Jalisco, Mexico. Bull. Chic. Herp. Soc. 2015, 50, 96–100. [Google Scholar]
- Moreira, F.; Russo, D. Modelling the Impact of Agricultural Abandonment and Wildfires on Vertebrate Diversity in Mediterranean Europe. Landsc. Ecol. 2007, 22, 1461–1476. [Google Scholar] [CrossRef]
- Pianka, E.R.; Goodyear, S.E. Lizard Responses to Wildfire in Arid Interior Australia: Long-Term Experimental Data and Commonalities with Other Studies. Austral Ecol. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- SEMARNAT. Programa de Acción Para La Conservación de Las Especies Abronia (Abronia spp) En México; SNIARN: Mexico City, Mexico, 2018. [Google Scholar]
- Setser, K.; Cavitt, J.F. Effects of Burning on Snakes in Kansas, USA, Tallgrass Prairie. Nat. Areas J. 2003, 23, 315–319. [Google Scholar]
- Woinarski, J.C.; Armstrong, M.; Price, O.; McCartney, J.; Griffiths, A.D.; Fisher, A. The Terrestrial Vertebrate Fauna of Litchfield National Park, Northern Territory: Monitoring over a 6-Year Period and Response to Fire History. Wildl. Res. 2005, 31, 587–596. [Google Scholar] [CrossRef]
- Hossack, B.R.; Lowe, W.H.; Corn, P.S. Rapid Increases and Time-Lagged Declines in Amphibian Occupancy after Wildfire. Conserv. Biol. 2013, 27, 219–228. [Google Scholar] [CrossRef]
- García, S.; Monroy-Vilchis, O.; Fajardo, V.; Aguilera-Reyes, U. Genetic Diversity and Structure of an Endemic and Critically Endangered Stream River Salamander (Caudata: Ambystoma Leorae) in Mexico. Conserv. Genet. 2014, 15, 49–59. [Google Scholar]
- Hossack, B.R.; Lowe, W.H.; Honeycutt, R.K.; Parks, S.A.; Corn, P.S. Interactive Effects of Wildfire, Forest Management, and Isolation on Amphibian and Parasite Abundance. Ecol. Appl. 2013, 23, 479–492. [Google Scholar] [CrossRef]
- Monroy Vilchis, O.; Zarco Gonzalez, M.; Dominguez Vega, H.; Garcia Aguilar, A.S. Ambystoma Leorae (Taylor, 1943). New Records, Natural History Notes and Threat Status. Short Note Herpetozoa 2015, 30, 166–168. [Google Scholar]
- Romo-Vázquez, E.; León-Paniagua, L.; Sánchez, O. A New Species of Habromys (Rodentia: Neotominae) from México. Proc. Biol. Soc. Wash. 2005, 118, 605–618. [Google Scholar] [CrossRef]
- Velázquez, A.; Romero, F.J.; León, L.V.I. Fragmentación Del Hábitat Del Conejo Zacatuche. In Ecología y Conservación Del Conejo Zacatuche y Su Hábitat; Velázquez, A., Romero, F.J., López-Paniagua, Y.J., Eds.; Universidad Nacional Autónoma de México-Fondo de Cultura Económica: Mexico City, Mexico, 1996; pp. 73–86. [Google Scholar]
- Granados, H. Basic Information on the Vokano Rabbit Ln: Proceedings of the World Lagomorph Conference, Guelph 1979 (Fcis K. Myers and CA Maclnnes); University of Guelph: Guelph, ON, Canada, 1981. [Google Scholar]
- Cervantes, F.A.; Barrera, C.B. Estudios Sobre La Biología de Roedores Silvestres Mexicanos; Universidad Nacional Autónoma de México: Ciudad de México, México, 2012. [Google Scholar]
- Rizo-Aguilar, A.; Guerrero, J.A.; Hidalgo-Mihart, M.G.; González-Romero, A. Relationship between the Abundance of the Endangered Volcano Rabbit Romerolagus Diazi and Vegetation Structure in the Sierra Chichinautzin Mountain Range, Mexico. Oryx 2015, 49, 360–365. [Google Scholar] [CrossRef]
- Matthews, J.M. Effects of Wildfire Intensity on Invasives, Stand Structure and Fuel Loading in Shenandoah National Park. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2004. [Google Scholar]
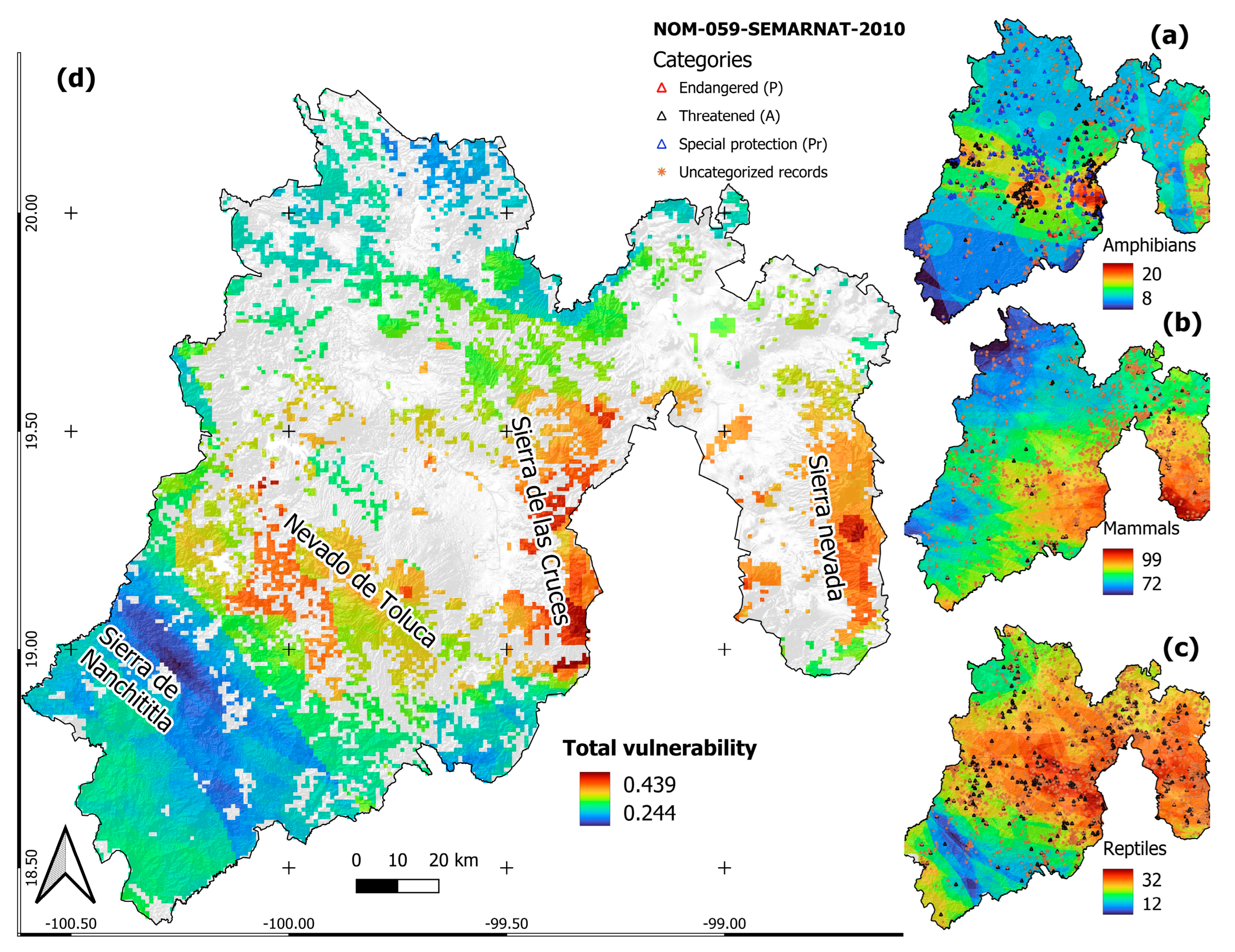
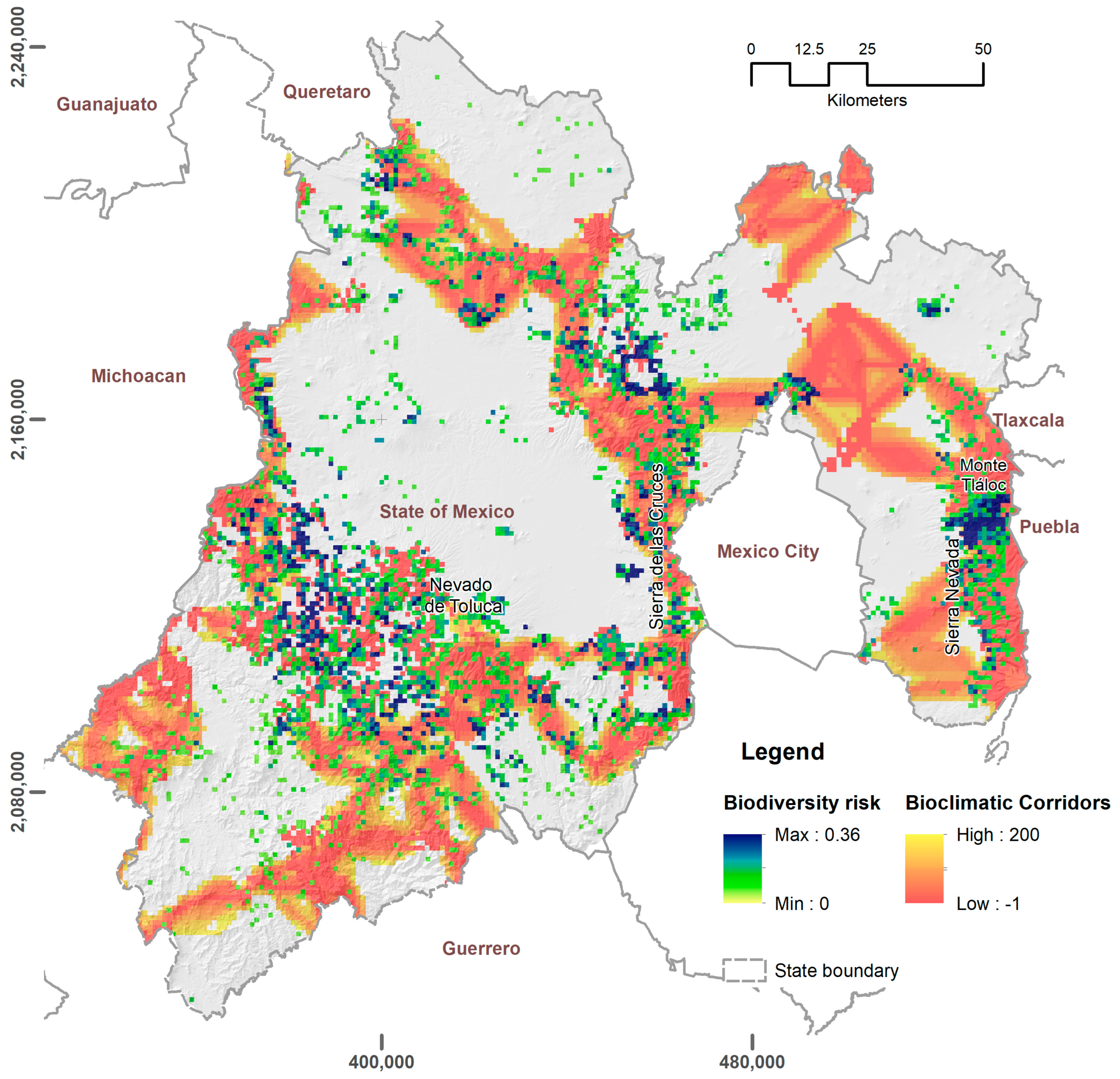
| Category | Name | Last Record | Records in the Last 30 Years | Natural Inhabited Area (km2) | Natural Area Burned (km2) | % Natural Area Burned | |
|---|---|---|---|---|---|---|---|
| Amphibians | CR | Ambystoma bombypellum | 2016 | 3 | 131.24 | 0.69 | 0.52 |
| Ambystoma granulosum | 1997 | 73 | 74.30 | 3.04 | 4.09 | ||
| Ambystoma leorae | 2018 | 6 | 426.57 | 52.79 | 12.37 | ||
| Lithobates tlaloci | -- | -- | 45.70 | 1.84 | 4.02 | ||
| Pseudoeurycea robertsi | 2018 | 20 | 318.43 | 22.70 | 7.13 | ||
| EN | Ambystoma altamirani | 2019 | 57 | 565.18 | 42.69 | 7.55 | |
| Ambystoma lermaense | 2011 | 64 | 8.97 | 0.00 | 0.00 | ||
| Ambystoma ordinarium | -- | -- | 863.92 | 63.12 | 7.31 | ||
| Craugastor hobartsmithi | 1997 | 7 | 94.84 | 3.33 | 3.51 | ||
| Plectrohyla pentheter | -- | -- | 1345.64 | 14.01 | 1.04 | ||
| Pseudoeurycea longicauda | 2014 | 47 | 110.57 | 3.80 | 3.44 | ||
| Pseudoeurycea tlilicxitl | 2015 | 2 | 742.45 | 65.41 | 8.81 | ||
| VU | Chiropterotriton orculus | 2019 | 21 | 1201.87 | 8.89 | 8.89 | |
| Isthmura bellii | 2019 | 7 | 6782.87 | 343.60 | 5.07 | ||
| NT | Aquiloeurycea cephalica | -- | -- | 1221.26 | 95.33 | 7.81 | |
| Lithobates neovolcanicus | 2018 | 7 | 257.67 | 9.96 | 3.87 | ||
| Mammals | CR | Habromys schmidlyi | 2006 | 2 | 4.02 | 0.0 | 0.0 |
| EN | Leptonycteris nivalis | 2000 | 18 | 10,238.87 | 395.51 | 3.86 | |
| Romerolagus diazi | 2018 | 23 | 978.95 | 109.16 | 11.15 | ||
| VU | Leptonycteris yerbabuenae | 2019 | 28 | 10,238.87 | 395.51 | 3.86 | |
| Sigmodon alleni | -- | -- | 220.18 | 0.45 | 0.20 | ||
| NT | Choeronycteris mexicana | 2019 | 1 | 10,238.87 | 395.51 | 3.86 | |
| Corynorhinus mexicanus | 2009 | 11 | 9981.36 | 395.06 | 3.96 | ||
| Leopardus wiedii | -- | -- | 1435.27 | 10.55 | 0.74 | ||
| Lepus callotis | -- | -- | 10,237.84 | 395.51 | 3.86 | ||
| Microtus quasiater | -- | -- | 363.36 | 47.72 | 13.13 | ||
| Reptiles | EN | Abronia deppii | 2019 | 5 | 3093.42 | 174.57 | 5.64 |
| Barisia herrerae | 2016 | 1 | 105.17 | 3.14 | 2.99 | ||
| Barisia rudicollis | 2018 | 12 | 1437.02 | 98.24 | 6.84 | ||
| Thamnophis melanogaster | 2019 | 32 | 3473.83 | 191.94 | 5.53 | ||
| VU | Thamnophis scaliger | 2019 | 56 | 673.97 | 44.45 | 6.60 | |
| NT | Agkistrodon bilineatus | -- | -- | 218.79 | 17.13 | 7.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastachi-Loza, C.A.; Paredes-Tavares, J.; Becerril-Piña, R.; Ruiz-Gómez, M.d.L.; Rangel Patiño, C.A.; Diaz-Delgado, C. The House Is Burning: Assessment of Habitat Loss Due to Wildfires in Central Mexico. Fire 2024, 7, 134. https://doi.org/10.3390/fire7040134
Mastachi-Loza CA, Paredes-Tavares J, Becerril-Piña R, Ruiz-Gómez MdL, Rangel Patiño CA, Diaz-Delgado C. The House Is Burning: Assessment of Habitat Loss Due to Wildfires in Central Mexico. Fire. 2024; 7(4):134. https://doi.org/10.3390/fire7040134
Chicago/Turabian StyleMastachi-Loza, Carlos Alberto, Jorge Paredes-Tavares, Rocio Becerril-Piña, María de Lourdes Ruiz-Gómez, Carlos Alejandro Rangel Patiño, and Carlos Diaz-Delgado. 2024. "The House Is Burning: Assessment of Habitat Loss Due to Wildfires in Central Mexico" Fire 7, no. 4: 134. https://doi.org/10.3390/fire7040134
APA StyleMastachi-Loza, C. A., Paredes-Tavares, J., Becerril-Piña, R., Ruiz-Gómez, M. d. L., Rangel Patiño, C. A., & Diaz-Delgado, C. (2024). The House Is Burning: Assessment of Habitat Loss Due to Wildfires in Central Mexico. Fire, 7(4), 134. https://doi.org/10.3390/fire7040134






