Cooling Performance of a Nano Phase Change Material Emulsions-Based Liquid Cooling Battery Thermal Management System for High-Capacity Square Lithium-Ion Batteries
Abstract
1. Introduction
2. Experiment and Method
2.1. Preparation and Characterization of the NCPMEs
2.1.1. Preparation
2.1.2. Characterization
2.2. Liquid Cooling Battery Thermal Management System
2.3. Description of the Test Setup
3. Results and Discussion
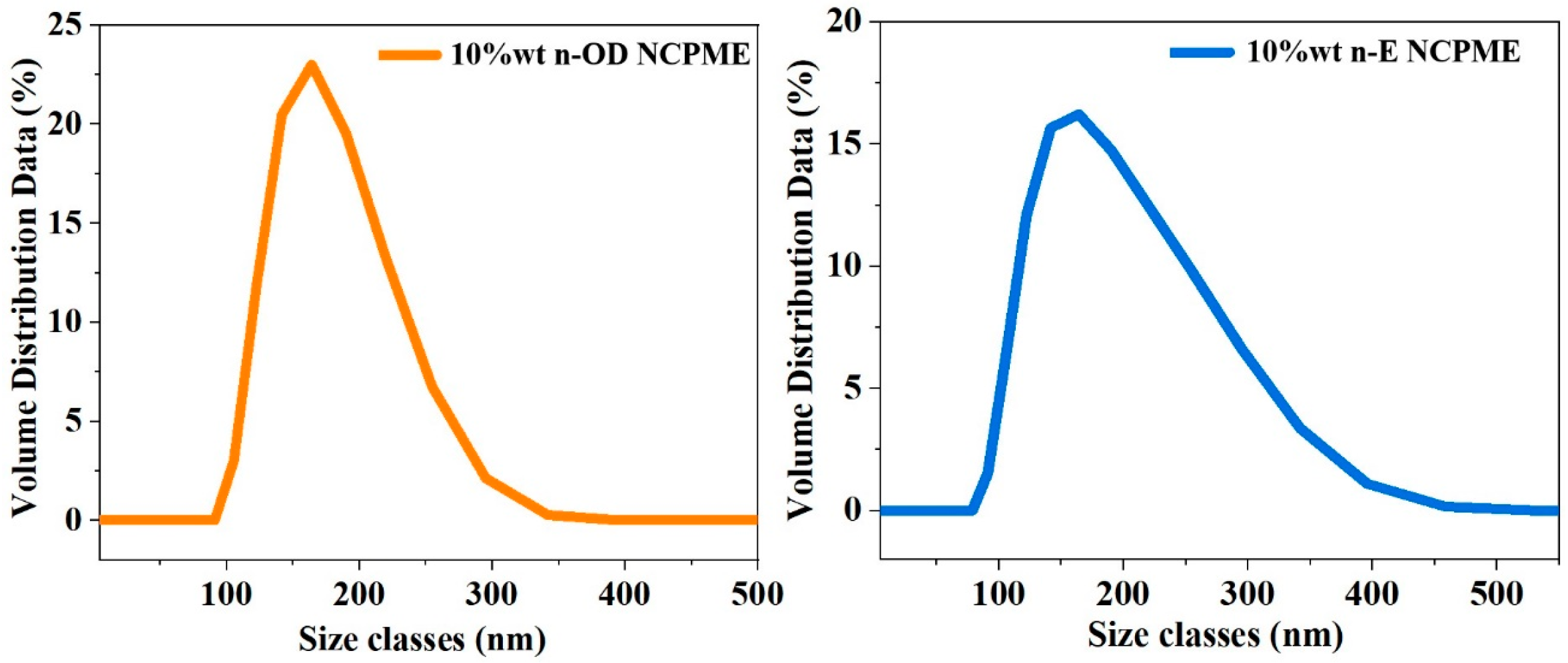
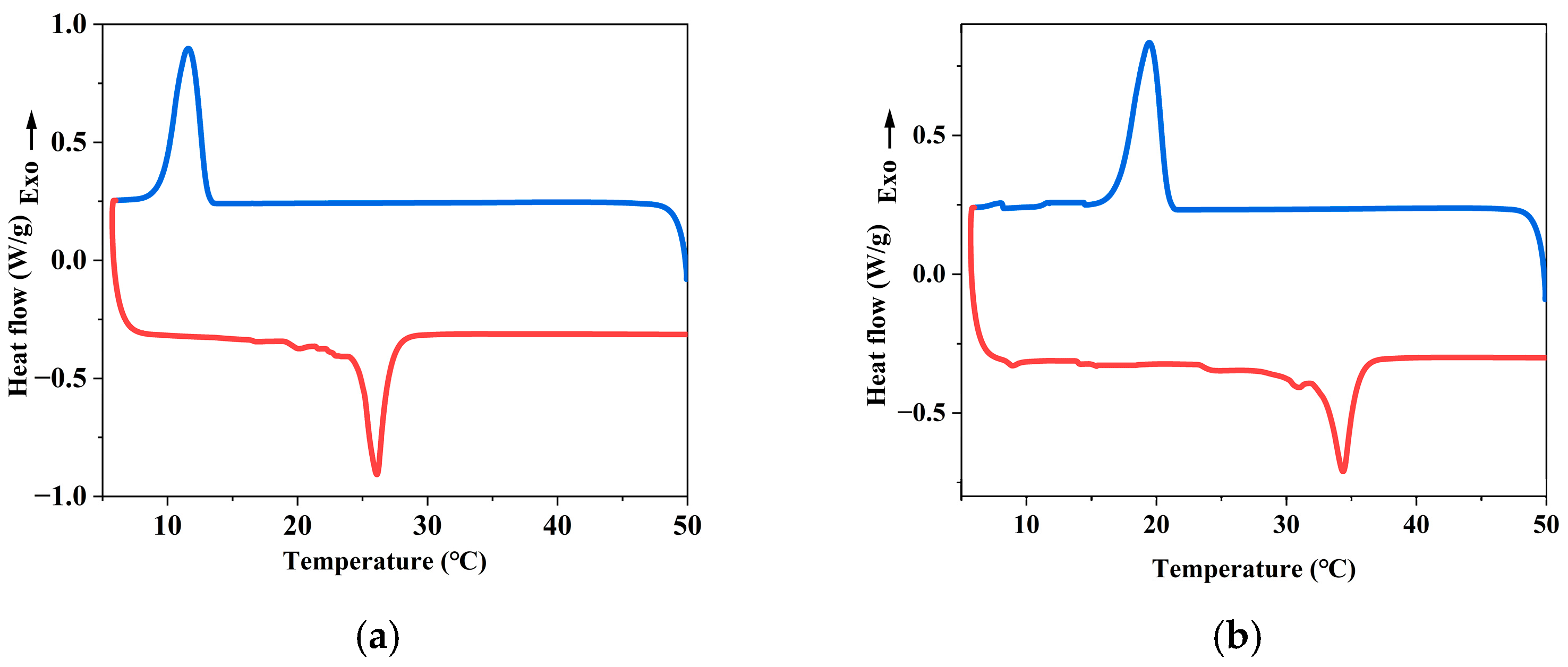
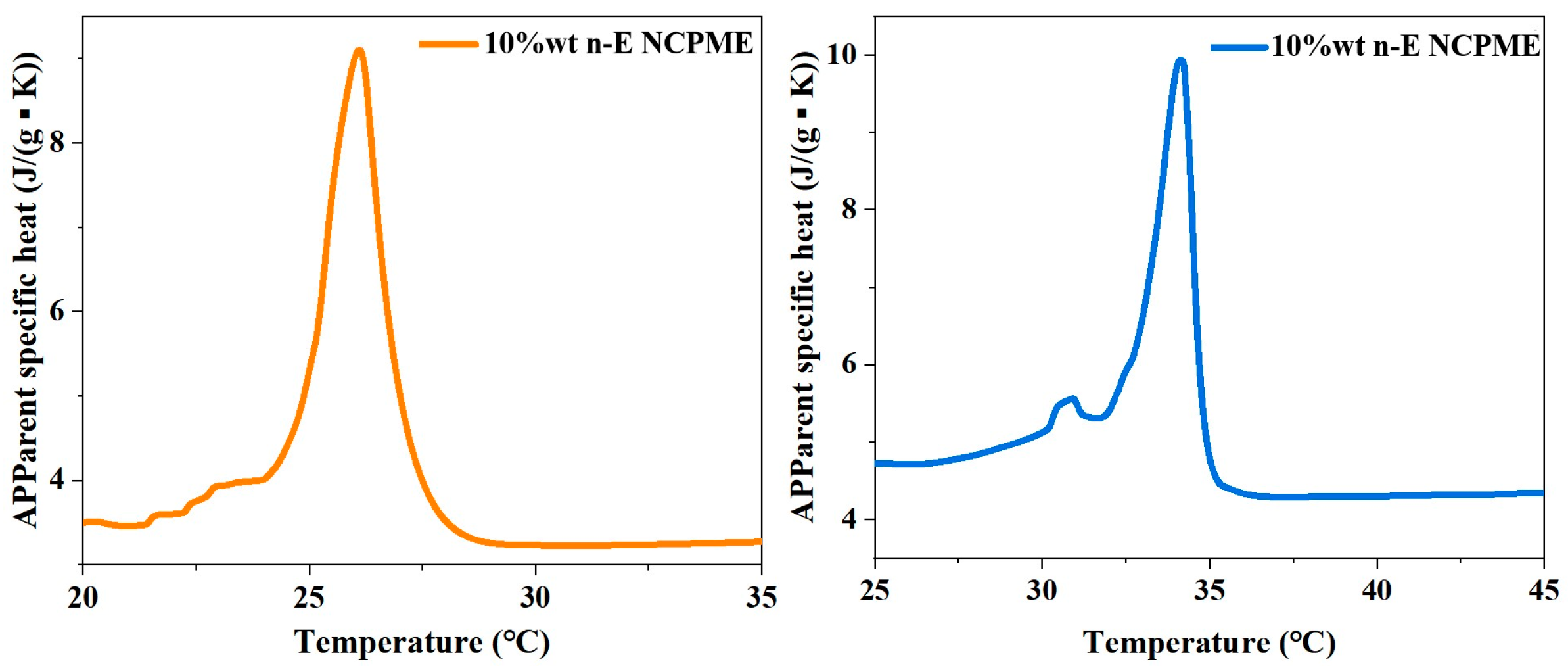
3.1. Thermo-Physical Properties of the NPCMEs
3.2. Cooling Performance of NPCMEs
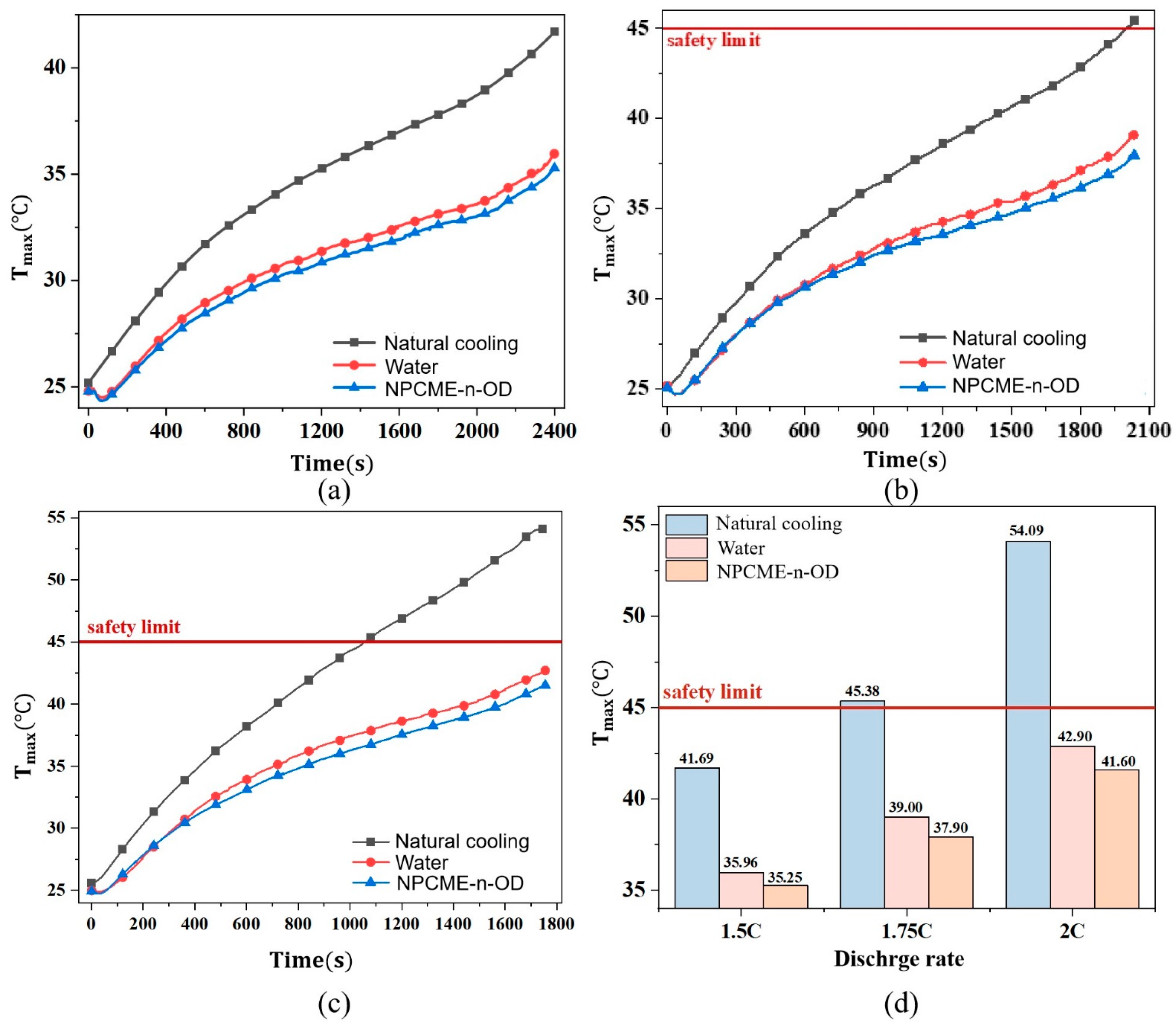
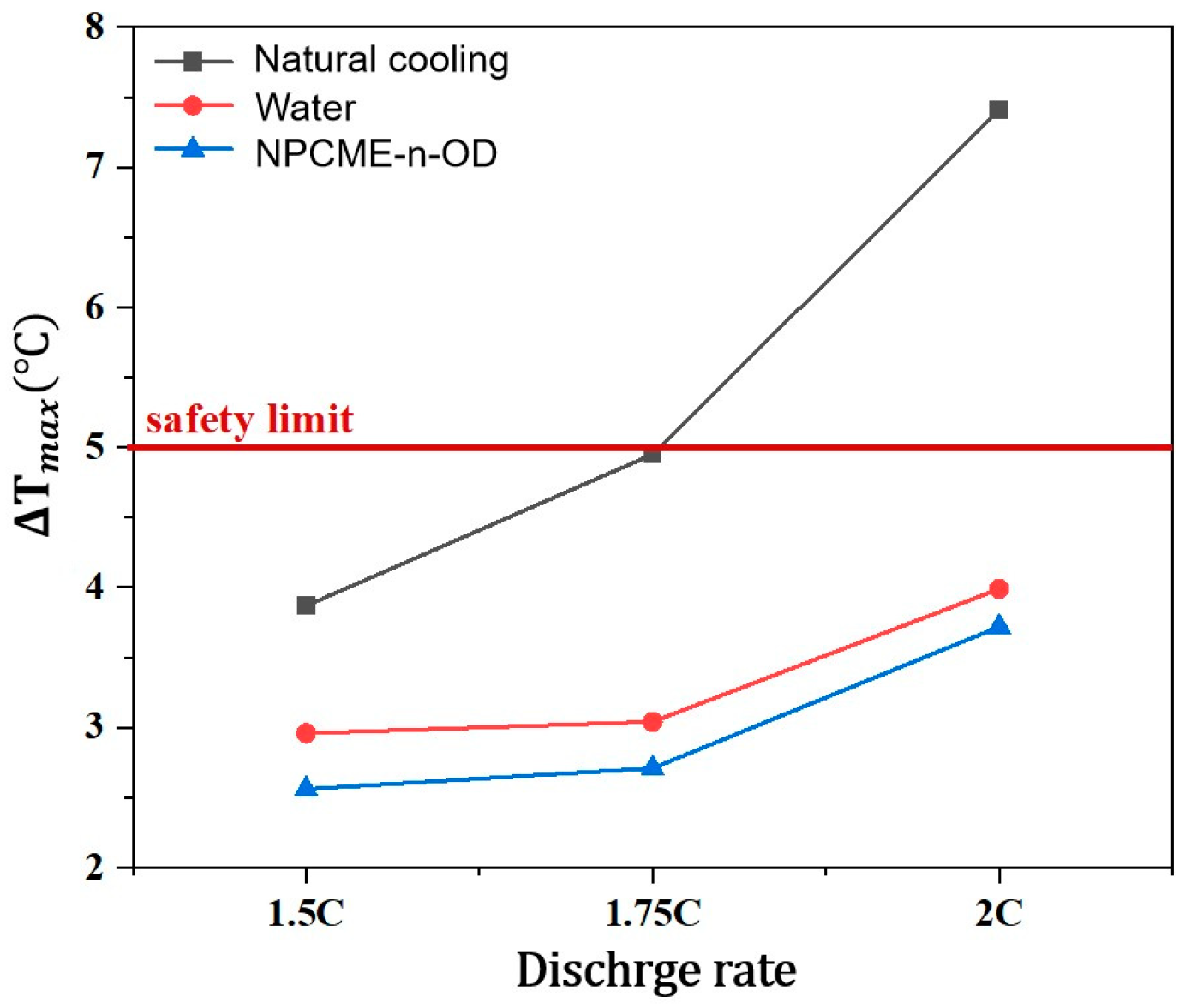
3.2.1. Thermal Management Performance at Various Discharge Rates
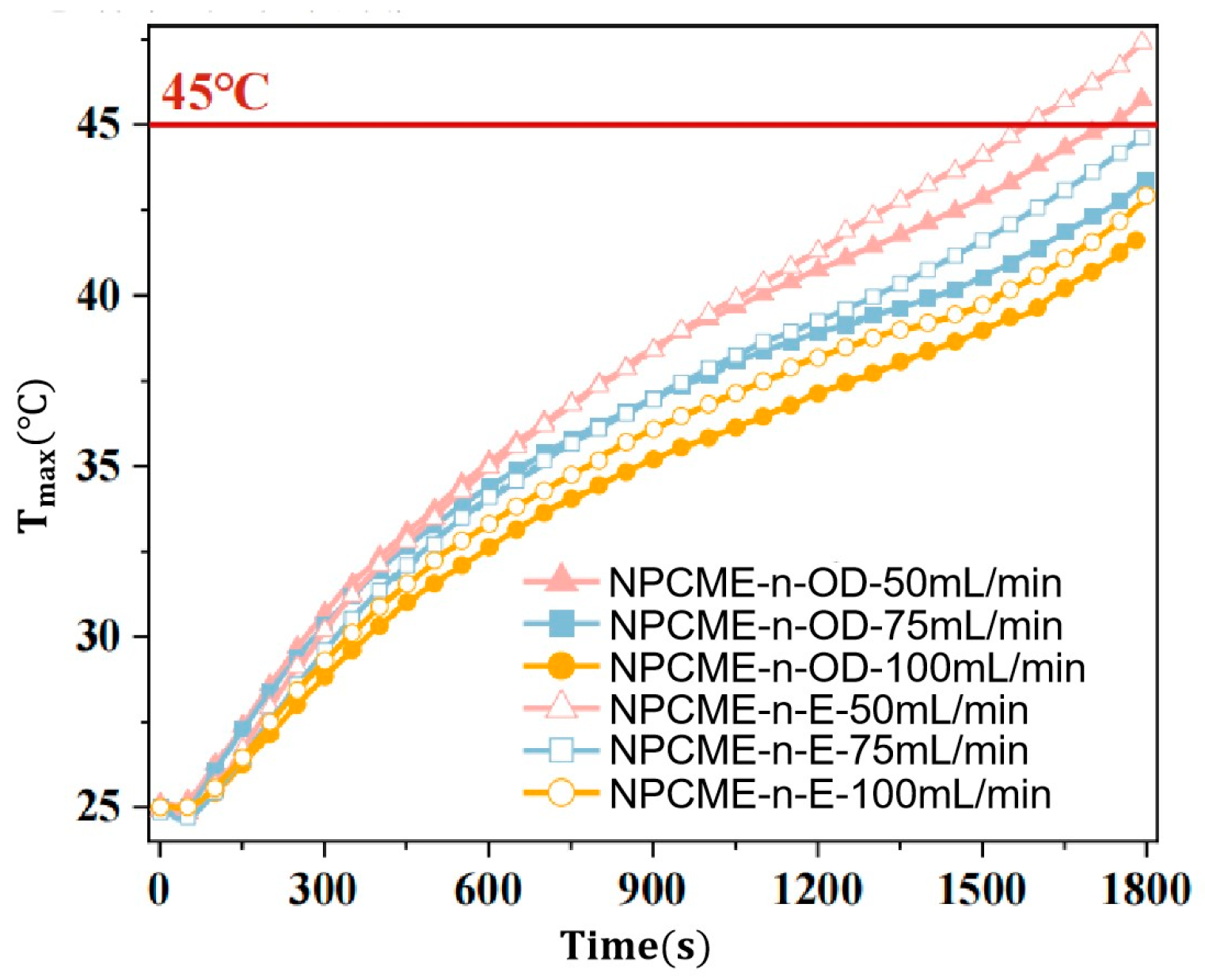
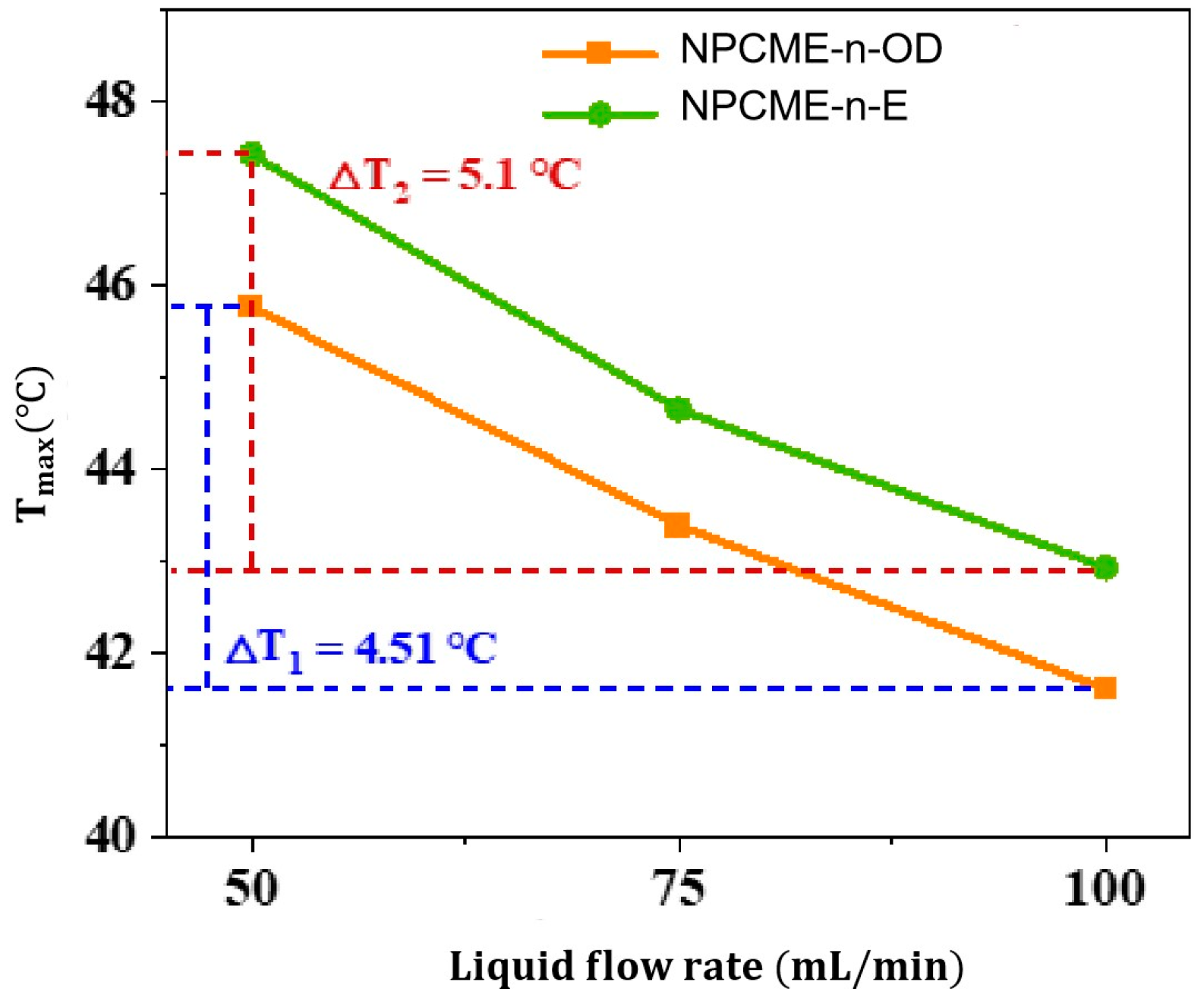
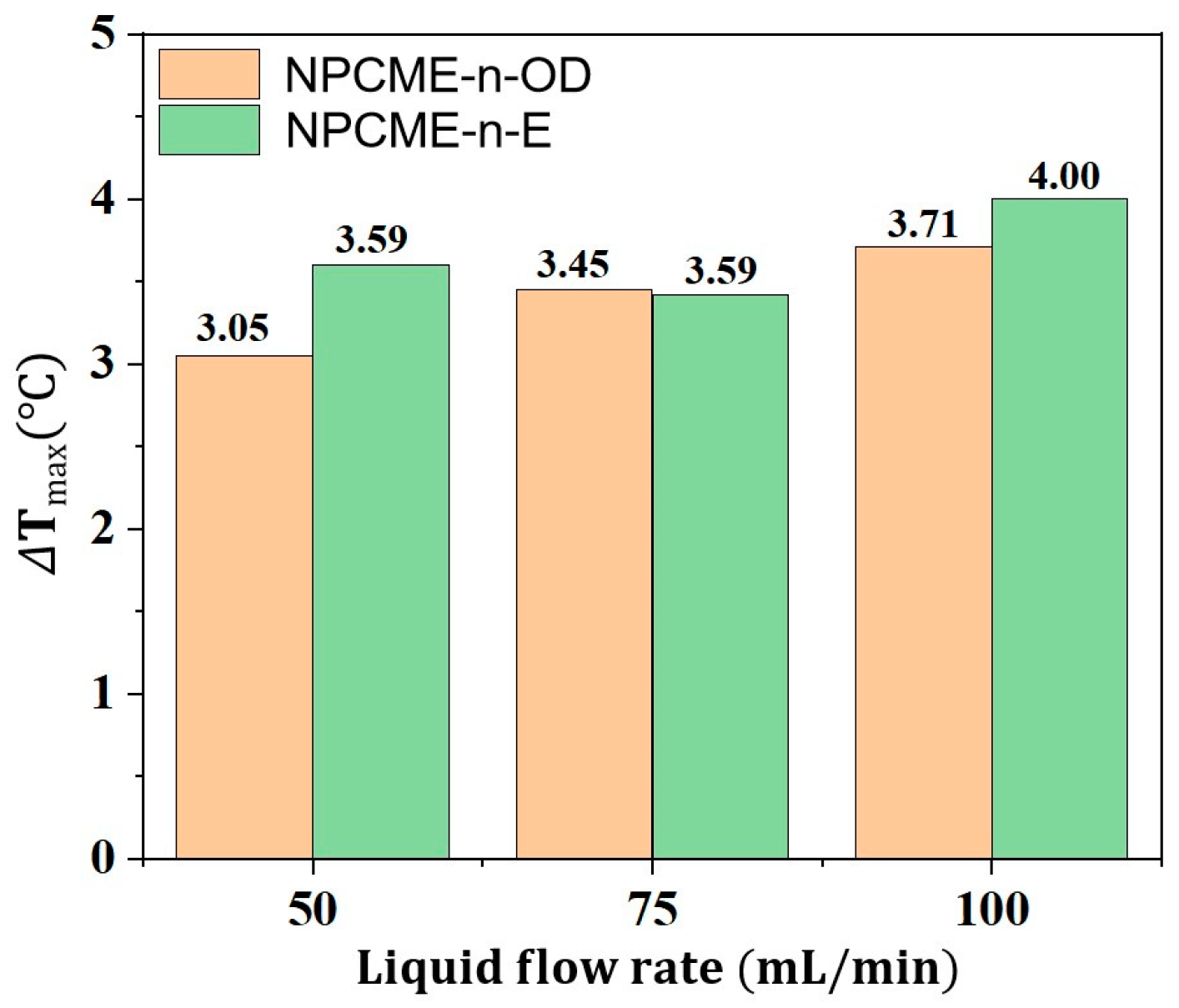
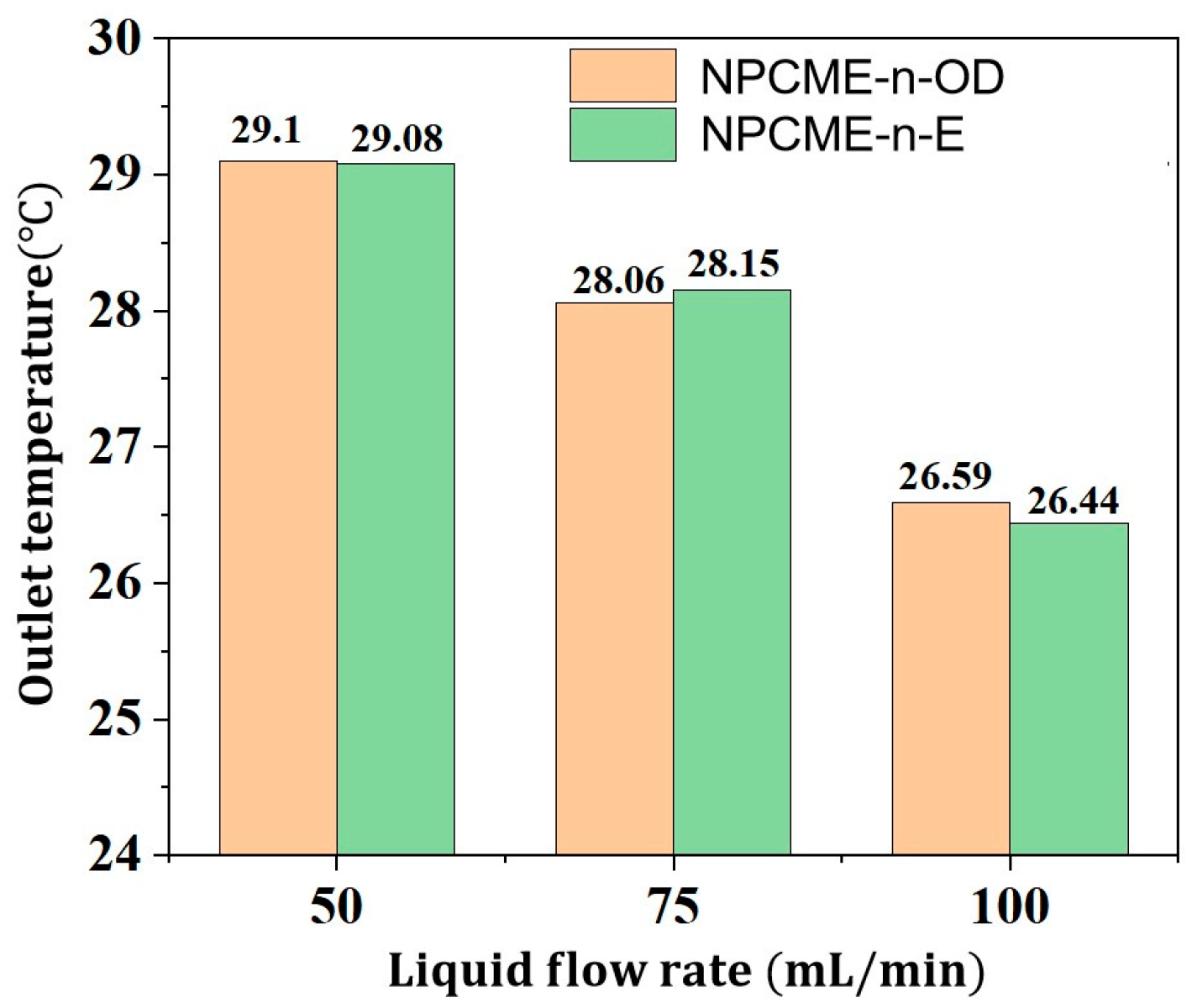
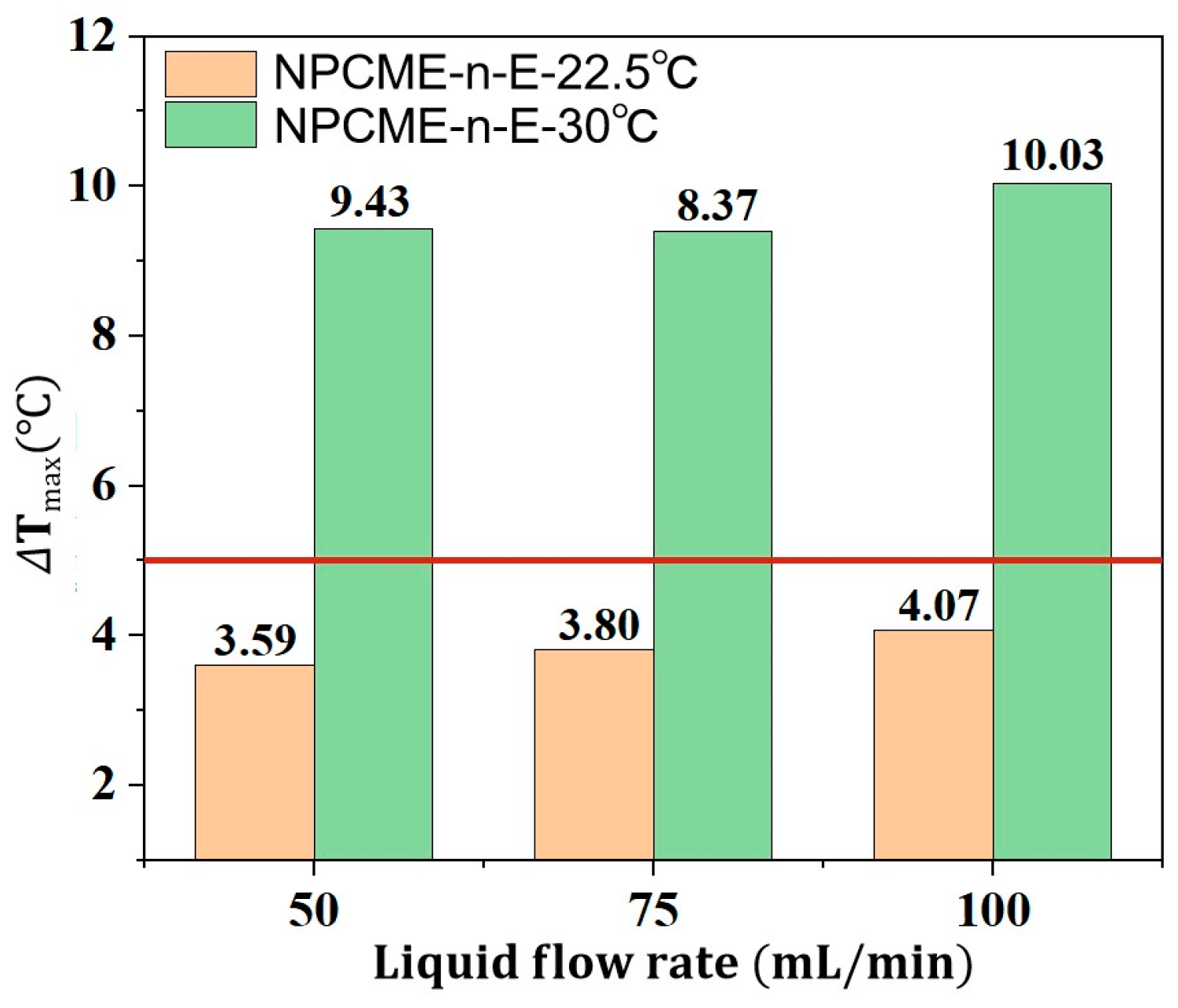
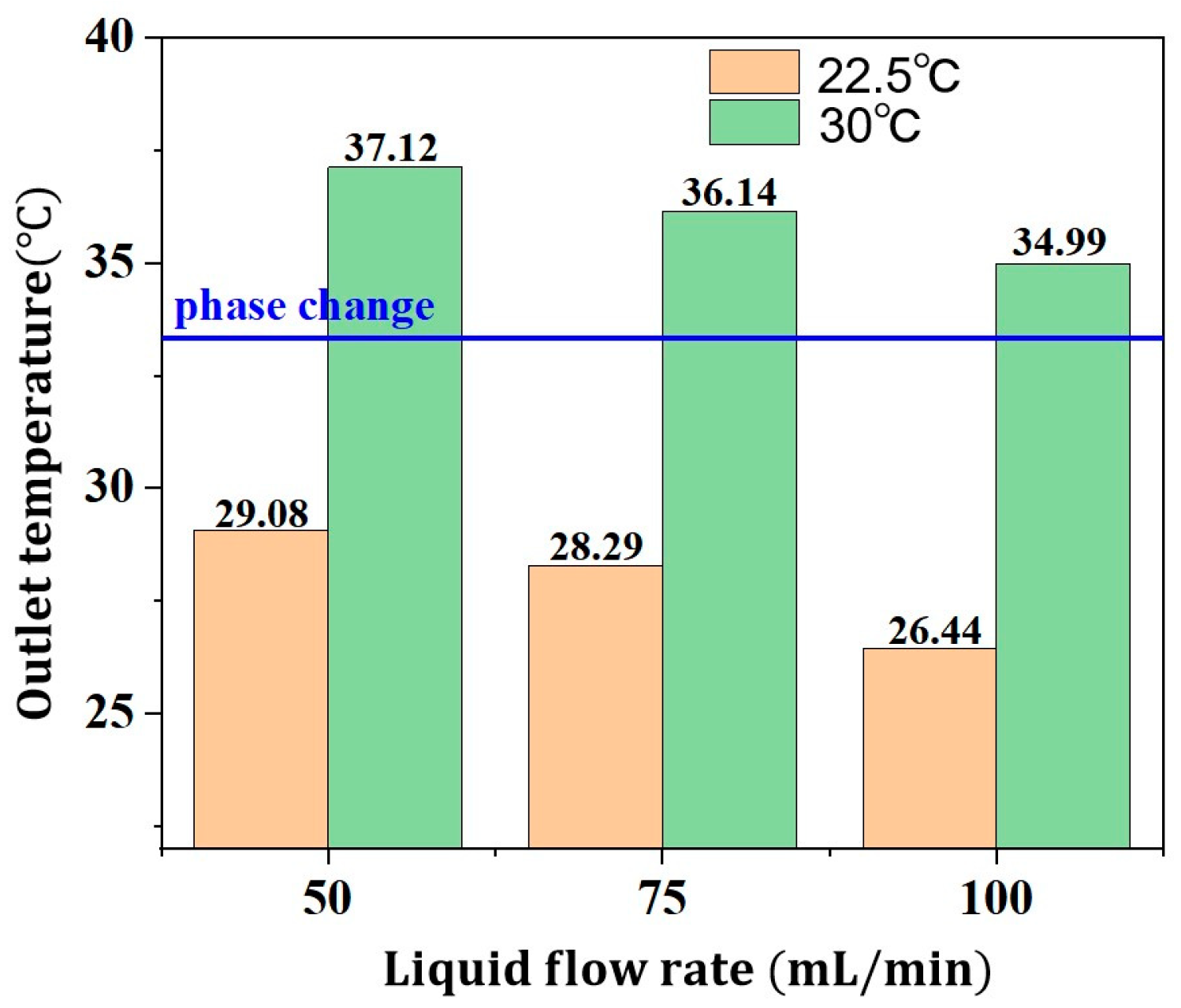
3.2.2. Thermal Management Performance of NPCMEs at Various Liquid Flow Rates
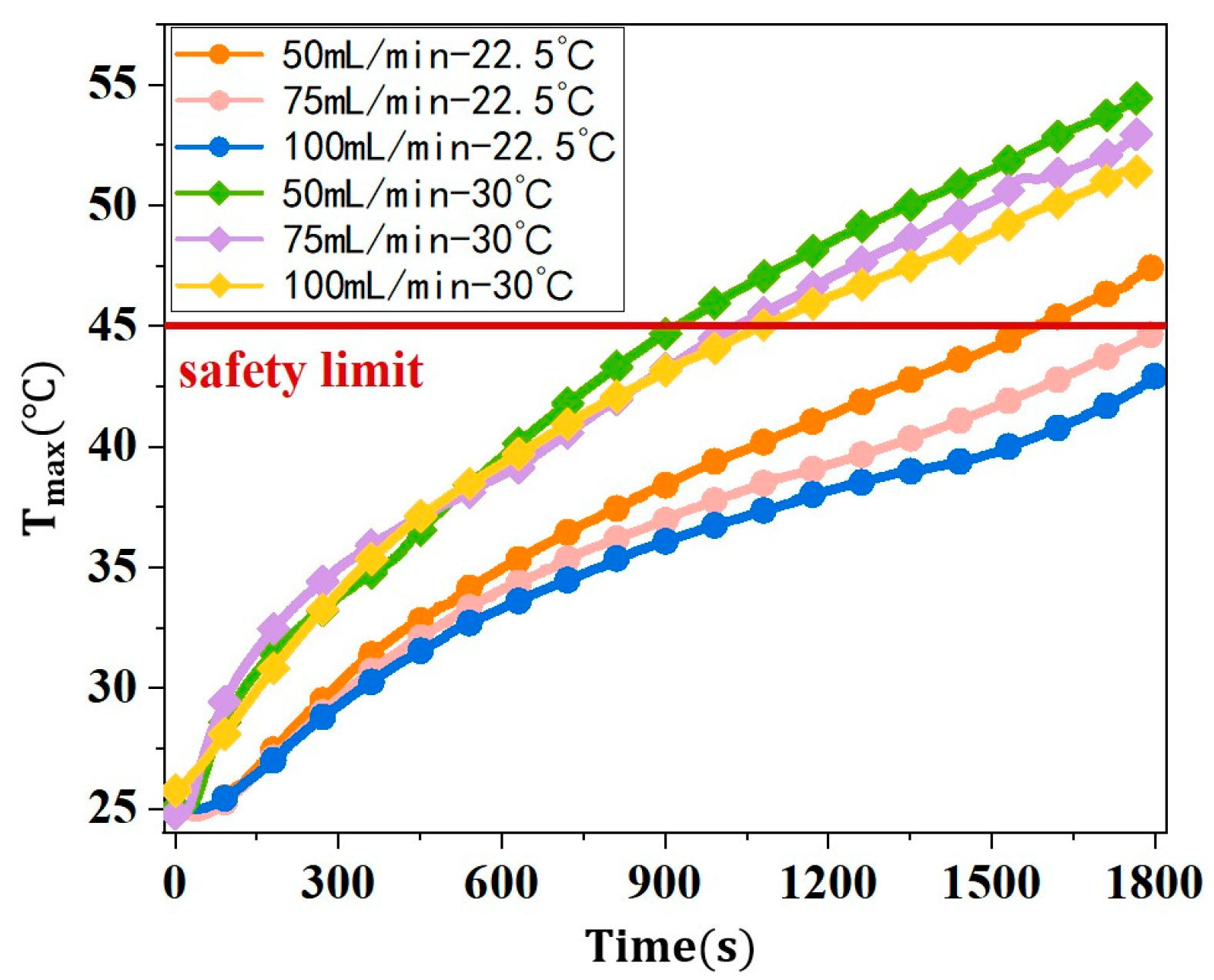
3.2.3. Thermal Management Performance of NPCMEs at Different Inlet Temperatures and Flow Rates
4. Conclusions
- The prepared NPCME-n-OD and NPCME-n-E have phase change onset temperatures of approximately 25.5 °C and 32.5 °C, respectively, with particle sizes mainly distributed between 100 and 400 nm and melting enthalpies of 16.9 J/g and 18.4 J/g, respectively. Test results show that the apparent specific heat capacities of the NPCME-n-OD and the NPCME-n-E are significantly higher than that of water, being 2.1 and 2.4 times that of water, respectively.
- The phase change temperature significantly affects the temperature control performance of NPCMEs, especially when it is close to the ambient temperature, where their performance is superior to water. For example, the NPCME-n-OD with a phase change temperature of 25–28 °C, at an inlet temperature of 22.5 °C, achieves a maximum temperature (Tmax) of 37.9 °C during a 1.75 C discharge rate, which is significantly lower than that of natural convection and water cooling. As the discharge rate increases, the cooling effect of NPCMEs becomes more pronounced due to the increased utilization rate of latent heat.
- With the increase in the NPCME’s flow rate, although the temperature of the battery pack is reduced, the utilization efficiency of the latent heat of the nano emulsion decreases, and the system energy consumption increases. Increasing the flow rate can enhance the convective heat transfer effect, which, to some extent, compensates for the loss of thermal management performance due to the reduction of phase change heat absorption. However, rapid flow also means higher energy consumption. Therefore, when using nano emulsions for thermal management, it is necessary to balance the dual impact of flow rate on thermal performance and energy consumption.
- For nano emulsions with higher phase change temperatures, such as the NPCME-n-E, increasing the inlet temperature can induce phase change and utilize latent heat, but the overall thermal control performance remains suboptimal. While an inlet temperature below the ambient temperature does not readily cause phase change, effective liquid cooling can still maintain battery temperatures within the desired range. Conversely, raising the inlet temperature to near the phase change point can result in a poor cooling efficiency due to the higher initial battery temperature, potentially compromising battery safety. Therefore, when the phase change temperature significantly exceeds the initial battery temperature, it is not advisable to rely on increased inlet temperatures to enhance latent heat utilization. Instead, consider adjusting the contact time between the nano emulsion and the battery at lower inlet temperatures to increase heat absorption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, N.; Zhang, Y.; Liu, X.; Luo, R.; Liu, Y.; Ma, C. Thermal performance and structural optimization of a hybrid thermal management system based on MHPA/PCM/liquid cooling for lithium-ion battery. Appl. Therm. Eng. 2023, 235, 121341. [Google Scholar] [CrossRef]
- Xu, B.; Lee, J.; Kwon, D.; Kong, L.; Pecht, M. Mitigation strategies for Li-ion battery thermal runaway: A review. Renew. Sustain. Energy Rev. 2021, 150, 111437. [Google Scholar] [CrossRef]
- Ramadass, P.H.B.W.R.P.B.; Haran, B.; White, R.; Popov, B.N. Capacity fade of Sony 18650 cells cycled at elevated temperatures Part I. Cycling performance. J. Power Sources 2002, 112, 602–613. [Google Scholar] [CrossRef]
- Ramadass, P.H.B.W.R.P.B.; Haran, B.; White, R.; Popov, B.N. Capacity fade of Sony 18650 cells cycled at elevated temperatures Part II. Capacity fade analysis. J. Power Sources 2002, 112, 614–620. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Jaguemont, J.; Kalogiannis, T.; Karimi, D.; He, J.; Jin, L.; Berecibar, M. A novel liquid cooling plate concept for thermal management of lithium-ion batteries in electric vehicles. Energy Convers. Manag. 2021, 231, 113862. [Google Scholar] [CrossRef]
- Yue, Q.L.; He, C.X.; Wu, M.C.; Zhao, T.S. Advances in thermal management systems for next-generation power batteries. Int. J. Heat Mass Transf. 2021, 181, 121853. [Google Scholar] [CrossRef]
- Hasan, H.A.; Togun, H.; Abed, A.M.; I Mohammed, H.; Biswas, N. A novel air-cooled Li-ion battery (LIB) array thermal management system–a numerical analysis. Int. J. Therm. Sci. 2023, 190, 108327. [Google Scholar] [CrossRef]
- Ye, J.; Aldaher, A.Y.M.; Tan, G. Thermal performance analysis of 18,650 battery thermal management system integrated with liquid-cooling and air-cooling. J. Energy Storage 2023, 72, 108766. [Google Scholar] [CrossRef]
- Sahin, R.C.; Gocmen, S.; Cetkin, E. Thermal management system for air-cooled battery packs with flow-disturbing structures. J. Power Sources 2022, 551, 232214. [Google Scholar] [CrossRef]
- Roe, C.; Feng, X.; White, G.; Li, R.; Wang, H.; Rui, X.; Li, C.; Zhang, F.; Null, V.; Parkes, M.; et al. Immersion cooling for lithium-ion batteries–A review. J. Power Sources 2022, 525, 231094. [Google Scholar] [CrossRef]
- Wang, H.; Tao, T.; Xu, J.; Shi, H.; Mei, X.; Gou, P. Thermal performance of a liquid-immersed battery thermal management system for lithium-ion pouch batteries. J. Energy Storage 2022, 46, 103835. [Google Scholar] [CrossRef]
- Weng, J.; Xiao, C.; Yang, X.; Ouyang, D.; Chen, M.; Zhang, G.; Waiming, E.L.; Yuen, R.K.K.; Wang, J. An energy-saving battery thermal management strategy coupling tubular phase-change-material with dynamic liquid cooling under different ambient temperatures. Renew. Energy 2022, 195, 918–930. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Zhang, G.; Wang, Y.; Guo, J.; Wang, Y.; Huang, Q.; Xiao, C.; Zhong, Z. Characterization and experimental investigation of aluminum nitride-based composite phase change materials for battery thermal management. Energy Convers. Manag. 2020, 204, 112319. [Google Scholar] [CrossRef]
- Yang, X.; Huang, R.; Yao, Z.; Zhang, G. Modular assembly strategy to construct lightweight thermal management system by developing high-stable phase change material units. Chem. Eng. Sci. 2024, 299, 120529. [Google Scholar] [CrossRef]
- Weragoda, D.M.; Tian, G.; Burkitbayev, A.; Lo, K.-H.; Zhang, T. A comprehensive review on heat pipe based battery thermal management systems. Appl. Therm. Eng. 2023, 224, 120070. [Google Scholar] [CrossRef]
- Mohammed, A.G.; Elfeky, K.E.; Wang, Q. Recent advancement and enhanced battery performance using phase change materials based hybrid battery thermal management for electric vehicles. Renew. Sustain. Energy Rev. 2022, 154, 111759. [Google Scholar] [CrossRef]
- Wu, W.; Ye, G.; Zhang, G.; Yang, X. Composite phase change material with room-temperature-flexibility for battery thermal management. Chem. Eng. J. 2022, 428, 131116. [Google Scholar] [CrossRef]
- Yao, Z.; Xie, J.; Fu, T.; Luo, Y.; Yang, X. One-pot preparation of phase change material employing nano-scaled resorcinol-furfural frameworks. Chem. Eng. J. 2024, 484, 149553. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; Ma, C.; Zhang, Y. Efficient purification of metal-organic framework and its trigger strategy in battery thermal management system. Energy Convers. Manag. 2023, 292, 117416. [Google Scholar] [CrossRef]
- Sabbah, R.; Kizilel, R.; Selman, J.R.; Al-Hallaj, S. Active (air-cooled) vs. passive (phase change material) thermal management of high power lithium-ion packs: Limitation of temperature rise and uniformity of temperature distribution. J. Power Sources 2008, 182, 630–638. [Google Scholar] [CrossRef]
- Widyantara, R.D.; Naufal, M.A.; Sambegoro, P.L.; Nurprasetio, I.P.; Triawan, F.; Djamari, D.W.; Nandiyanto, A.B.D.; Budiman, B.A.; Aziz, M. Low-Cost Air-Cooling System Optimization on Battery Pack of Electric Vehicle. Energies 2021, 14, 7954. [Google Scholar] [CrossRef]
- Zeng, W.; Niu, Y.; Li, S.; Hu, S.; Mao, B.; Zhang, Y. Cooling performance and optimization of a new hybrid thermal management system of cylindrical battery. Appl. Therm. Eng. 2022, 217, 119171. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, G.; Yang, X. Thermal performance analysis of battery thermal management system utilizing bionic liquid cooling plates with differentiated velocity distribution strategy. Appl. Therm. Eng. 2024, 249, 123351. [Google Scholar] [CrossRef]
- Patil, M.S.; Seo, J.-H.; Panchal, S.; Jee, S.-W.; Lee, M.-Y. Investigation on thermal performance of water-cooled Li-ion pouch cell and pack at high discharge rate with U-turn type microchannel cold plate. Int. J. Heat Mass Transf. 2020, 155, 119728. [Google Scholar] [CrossRef]
- Wang, F.; Lin, W.; Ling, Z.; Fang, X. A comprehensive review on phase change material emulsions: Fabrication, characteristics, and heat transfer performance. Sol. Energy Mater. Sol. Cells 2019, 191, 218–234. [Google Scholar] [CrossRef]
- Yu, Q.; Tchuenbou-Magaia, F.; Al-Duri, B.; Zhang, Z.; Ding, Y.; Li, Y. Thermo-mechanical analysis of microcapsules containing phase change materials for cold storage. Appl. Energy 2018, 211, 1190–1202. [Google Scholar] [CrossRef]
- Mikkola, V.; Puupponen, S.; Saari, K.; Ala-Nissila, T.; Seppälä, A. Thermal properties and convective heat transfer of phase changing paraffin nanofluids. Int. J. Therm. Sci. 2017, 117, 163–171. [Google Scholar] [CrossRef]
- Pey, C.M.; Maestro, A.; Solé, I.; González, C.; Solans, C.; Gutiérrez, J.M. Optimization of nano-emulsions prepared by low-energy emulsification methods at constant temperature using a factorial design study. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 144–150. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Wu, J.-Y. Development and characterization of novel and stable silicon nanoparticles-embedded PCM-in-water emulsions for thermal energy storage. Appl. Energy 2019, 238, 1407–1416. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P. Preparation and characterization of nano-sized phase change emulsions as thermal energy storage and transport media. Appl. Energy 2017, 190, 868–879. [Google Scholar] [CrossRef]
- Xiang, N.; Yuan, Y.; Sun, L.; Cao, X.; Zhao, J. Simultaneous decrease in supercooling and enhancement of thermal conductivity of paraffin emulsion in medium temperature range with graphene as additive. Thermochim. Acta 2018, 664, 16–25. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, Z.; Cui, G.; Dou, B.; Lu, W.; Yan, X. Fabrication of a novel nano phase change material emulsion with low supercooling and enhanced thermal conductivity. Renew. Energy 2020, 151, 542–550. [Google Scholar] [CrossRef]
- Zhang, C.; Ji, J.; Zhang, X.; Cai, S. Development of highly stable low supercooling paraffin nano phase change emulsions for thermal management systems. J. Mol. Liq. 2024, 413, 125905. [Google Scholar] [CrossRef]
- Wang, F.; Cao, J.; Ling, Z.; Zhang, Z.; Fang, X. Experimental and simulative investigations on a phase change material nano-emulsion-based liquid cooling thermal management system for a lithium-ion battery pack. Energy 2020, 207, 118215. [Google Scholar] [CrossRef]
- Cao, J.; He, Y.; Feng, J.; Lin, S.; Ling, Z.; Zhang, Z.; Fang, X. Mini-channel cold plate with nano phase change material emulsion for Li-ion battery under high-rate discharge. Appl. Energy 2020, 279, 115808. [Google Scholar] [CrossRef]
- Mitra, A.; Kumar, R.; Singh, D.K. Thermal management of lithium-ion batteries using carbon-based nanofluid flowing through different flow channel configurations. J. Power Sources 2023, 555, 232251. [Google Scholar] [CrossRef]
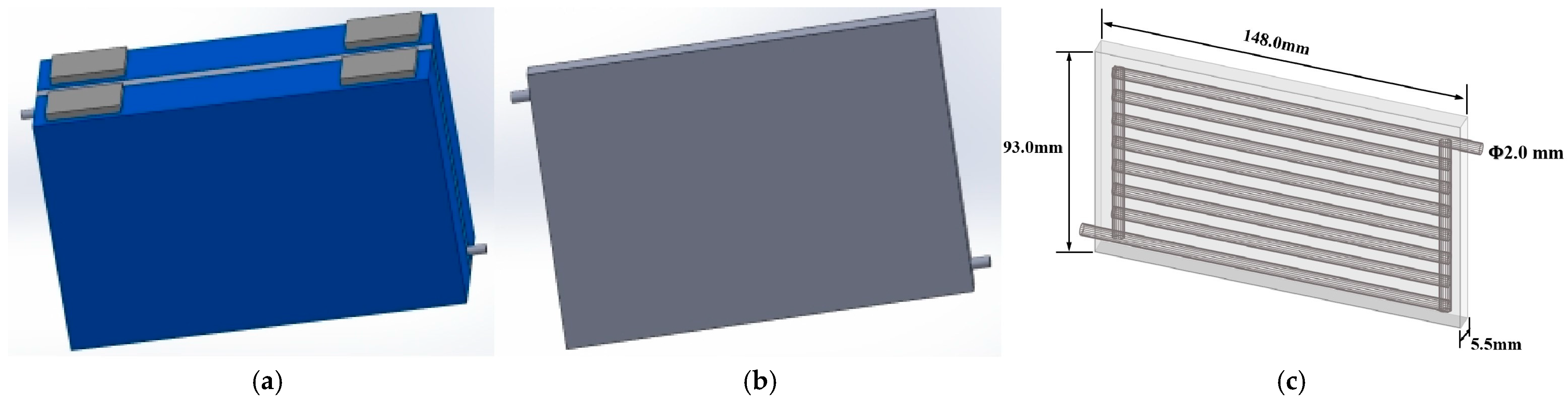

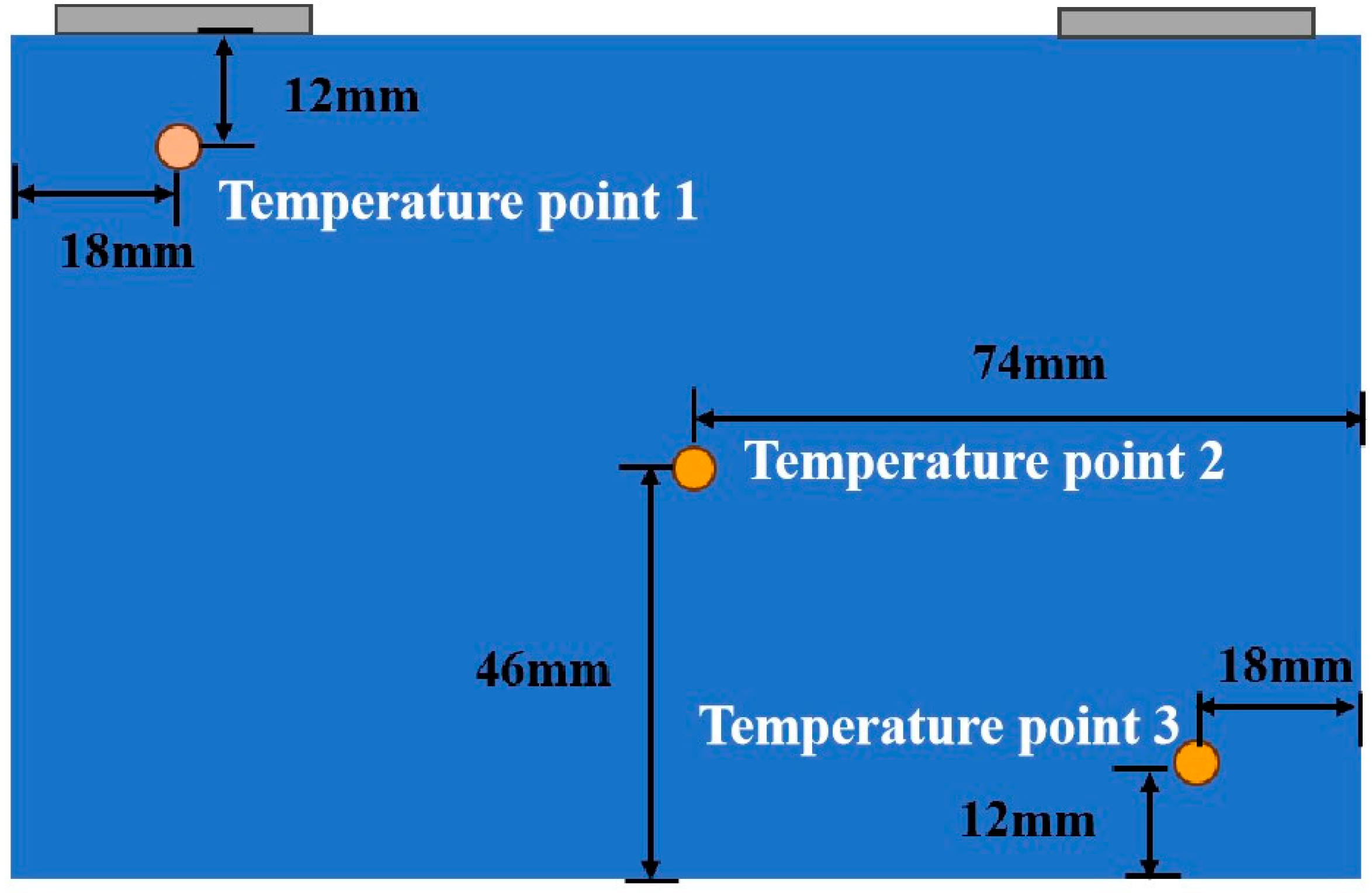

| Parameter | Value |
|---|---|
| Nominal capacity | 58 Ah |
| Nominal voltage | 3.62 V |
| Operating voltage range | 4.25 V |
| Discharge cut-off voltage | 2.8 V |
| Standard charging current | 1 C |
| Standard discharge current | 1 C |
| Height | 92.8 mm |
| Width | 148.66 mm |
| Thickness | 26.72 mm |
| Weight | 860 g |
| Device | Type | Accuracy |
|---|---|---|
| Environmental cabinet | SC-1000-CB-3, Guangdong Sanwood (Dongguan, China) | ±0.5 °C (Temperature) |
| Battery test system | CE-6002n-100V200A-H, Neware (Shenzhen, China) | ±0.02% (Current and Voltage) |
| Thermocouple | K-type (Shanghai, China) | ±0.75% |
| Data acquisition | LR8400-21, HIOKI (Shanghai, China) | ±0.6 °C |
| Water pump | ST600FC, RONGBAI (Baoding, China) | ±0.1 °C |
| Thermostatic water bath | SC-30, SCIENTZ (Xinzhi, China) | 0.001 mL/min |
| NO. | Influencing Factors | Coolant | Discharge Rate | Inlet Temperature | Liquid Flow Rate | Environmental Temperature |
|---|---|---|---|---|---|---|
| 1 | Discharge rate | Water | 1.5 C | 22.5 °C ± 0.3 °C | 100 mL/min | 25 °C |
| 2 | 1.75 C | |||||
| 3 | 2 C | |||||
| 4 | n-OD | 1.5 C | ||||
| 5 | 1.75 C | |||||
| 6 | 2 C | |||||
| 7 | Natural cooling | 1.5 C | ||||
| 1.75 C | ||||||
| 2 C | ||||||
| 8 | Liquid flow rate | n-OD | 2 C | 22.5 °C ± 0.3 °C | 50 mL/min | |
| 9 | 75 mL/min | |||||
| 10 | 100 mL/min | |||||
| 11 | n-E | 2 C | 22.5 °C ± 0.3 °C | 50 mL/min | ||
| 12 | 75 mL/min | |||||
| 13 | 100 mL/min | |||||
| 14 | Inlet temperature | n-OD | 2 C | 22.5 °C ± 0.3 °C | 50 mL/min | |
| 15 | 75 mL/min | |||||
| 16 | 100 mL/min | |||||
| 17 | 30 °C ± 0.3 °C | 50 mL/min | ||||
| 18 | 75 mL/min | |||||
| 19 | 100 mL/min |
| Materials | Melting Point (°C) | ΔH (J/g) | Apparent Specific Heat Capacity (J g−1 K−1) | Apparent Specific Thermal Conductivities (W m−1 K−1) |
|---|---|---|---|---|
| n-OD | 26.19 | 195.4 | ||
| n-E | 35.81 | 210.8 | ||
| 10 wt% NPCME-n-OD | 25 | 16.9 | 9.1 | 0.5414 |
| 10 wt% NPCME-n-E | 32 | 18.4 | 9.9 | 0.8918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Chen, G.; Li, P.; Xie, Z.; Li, Y.; Luo, T. Cooling Performance of a Nano Phase Change Material Emulsions-Based Liquid Cooling Battery Thermal Management System for High-Capacity Square Lithium-Ion Batteries. Fire 2024, 7, 371. https://doi.org/10.3390/fire7100371
Zhang G, Chen G, Li P, Xie Z, Li Y, Luo T. Cooling Performance of a Nano Phase Change Material Emulsions-Based Liquid Cooling Battery Thermal Management System for High-Capacity Square Lithium-Ion Batteries. Fire. 2024; 7(10):371. https://doi.org/10.3390/fire7100371
Chicago/Turabian StyleZhang, Guanghui, Guofeng Chen, Pan Li, Ziyi Xie, Ying Li, and Tuantuan Luo. 2024. "Cooling Performance of a Nano Phase Change Material Emulsions-Based Liquid Cooling Battery Thermal Management System for High-Capacity Square Lithium-Ion Batteries" Fire 7, no. 10: 371. https://doi.org/10.3390/fire7100371
APA StyleZhang, G., Chen, G., Li, P., Xie, Z., Li, Y., & Luo, T. (2024). Cooling Performance of a Nano Phase Change Material Emulsions-Based Liquid Cooling Battery Thermal Management System for High-Capacity Square Lithium-Ion Batteries. Fire, 7(10), 371. https://doi.org/10.3390/fire7100371







