Numerical Study on the Explosion Reaction Mechanism of Multicomponent Combustible Gas in Coal Mines
Abstract
1. Introduction
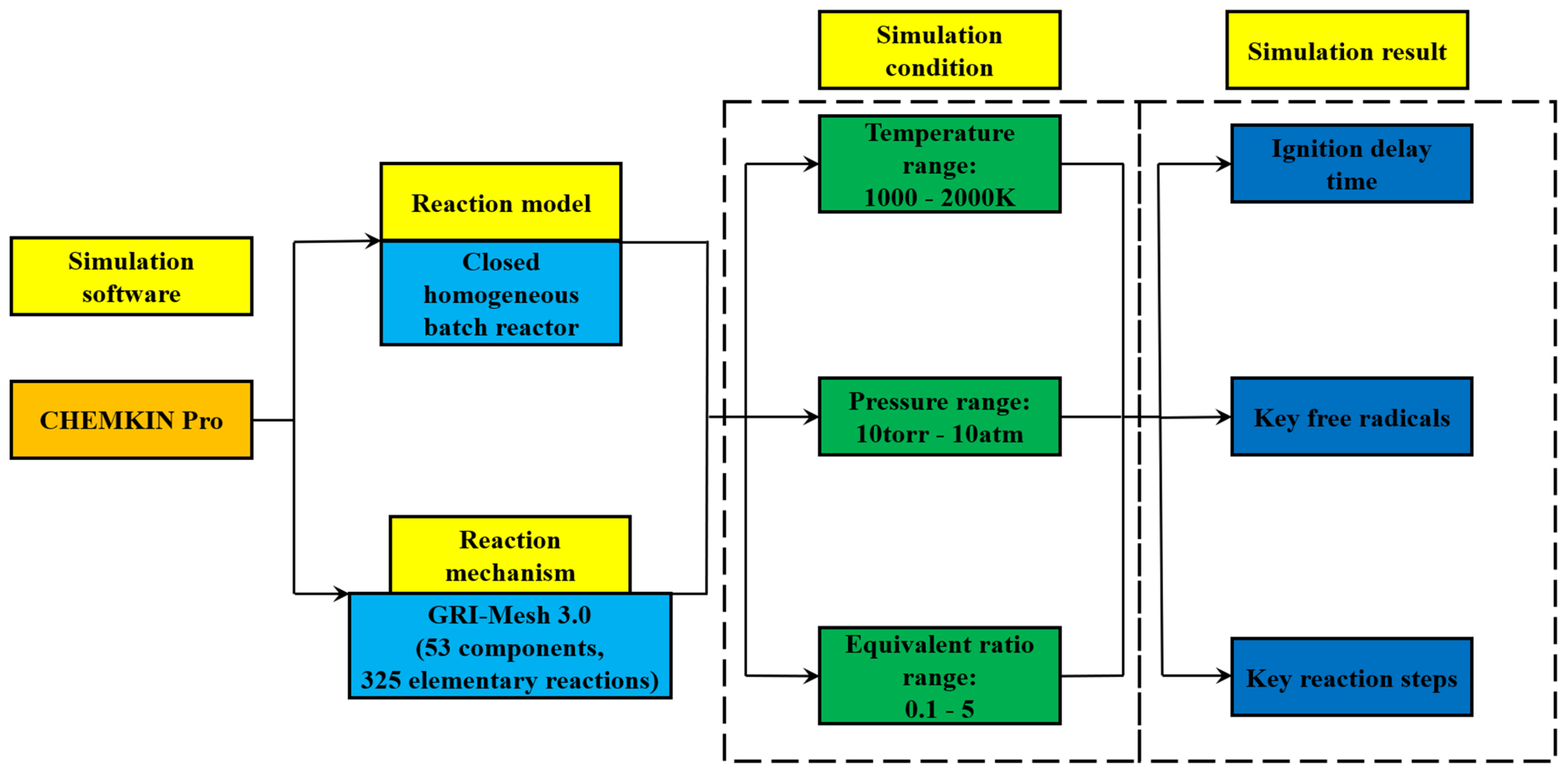
2. Methodology
2.1. Selection of Reaction Mechanism
2.2. Initial Computational Conditions
2.3. Sensitivity Analysis
3. Results and Analysis
3.1. Impact of Coal Self-Combustion Environmental Mixed Gas on the Ignition Delay Time of Gas Explosions
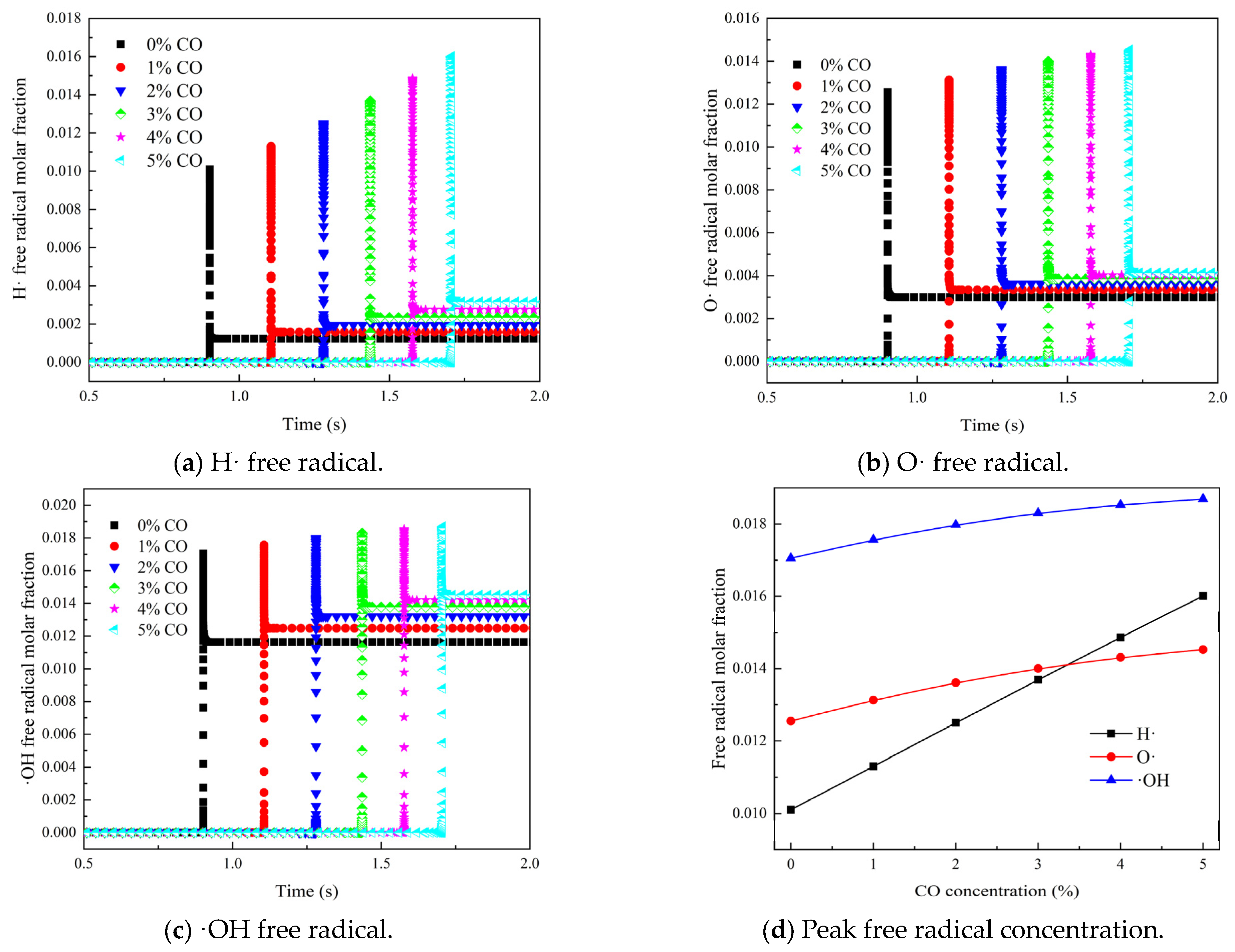
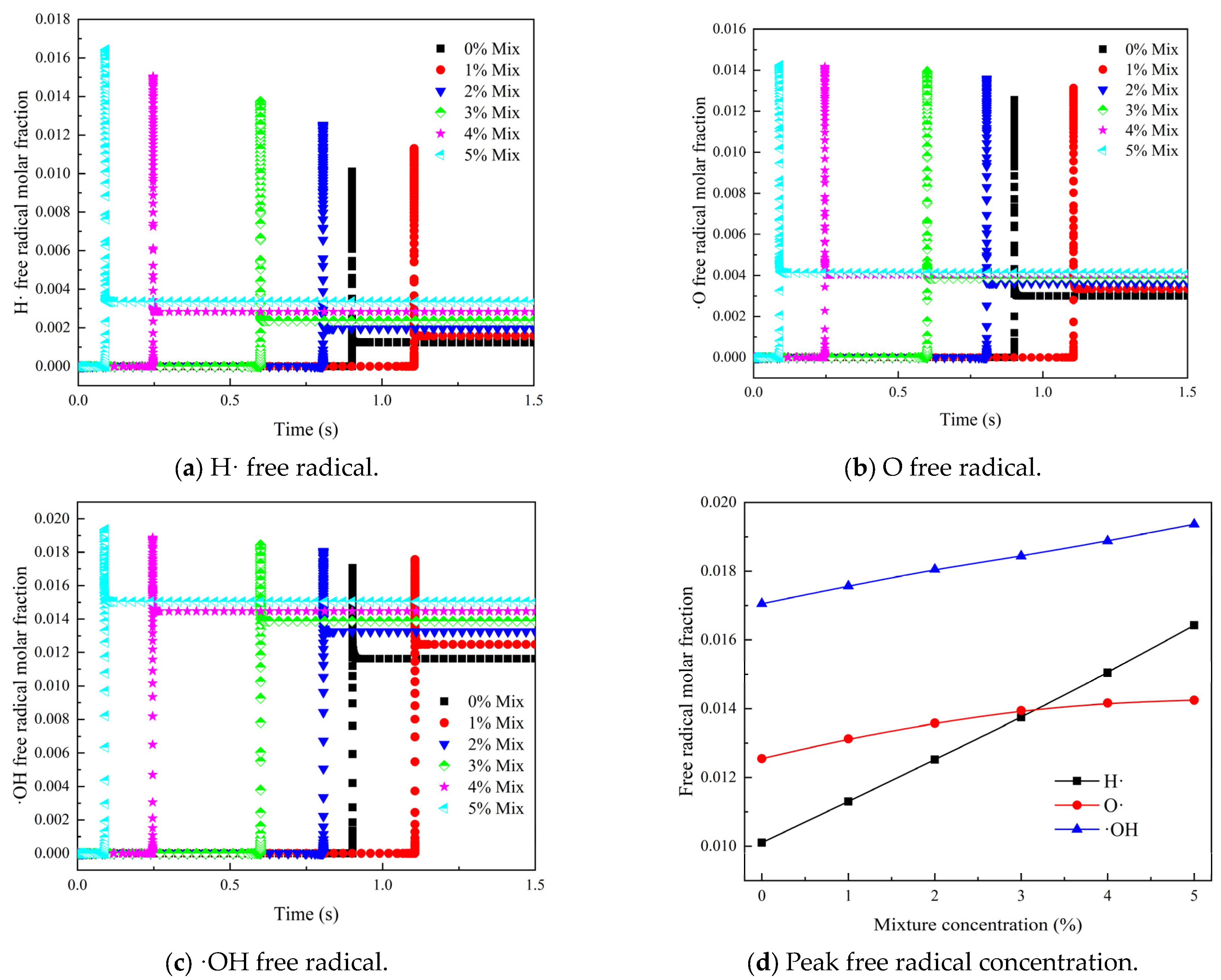
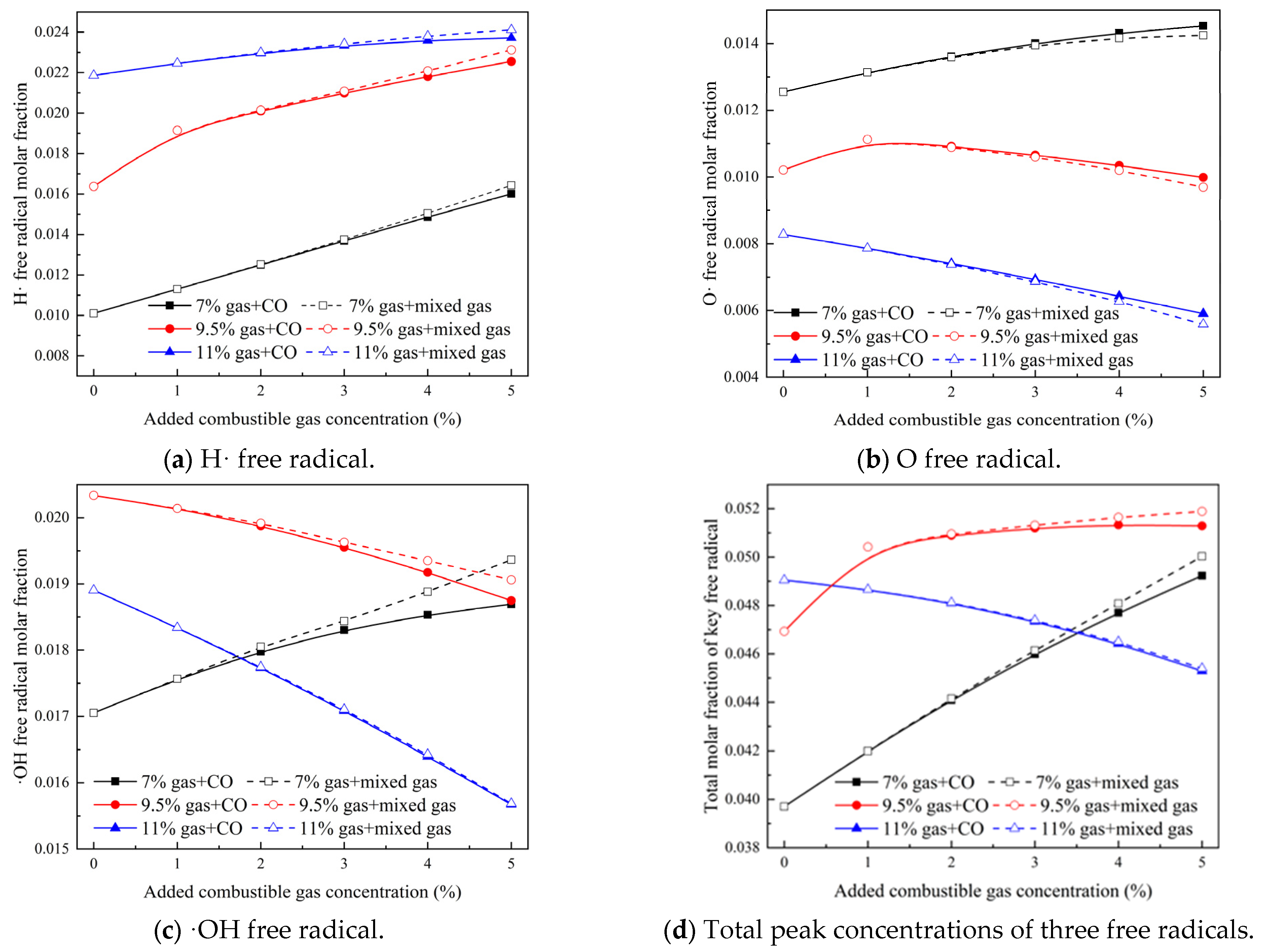
3.2. Effect of Gas Mixture in Coal Spontaneous Combustion Environment on Concentration of Key Free Radicals in Gas Explosion
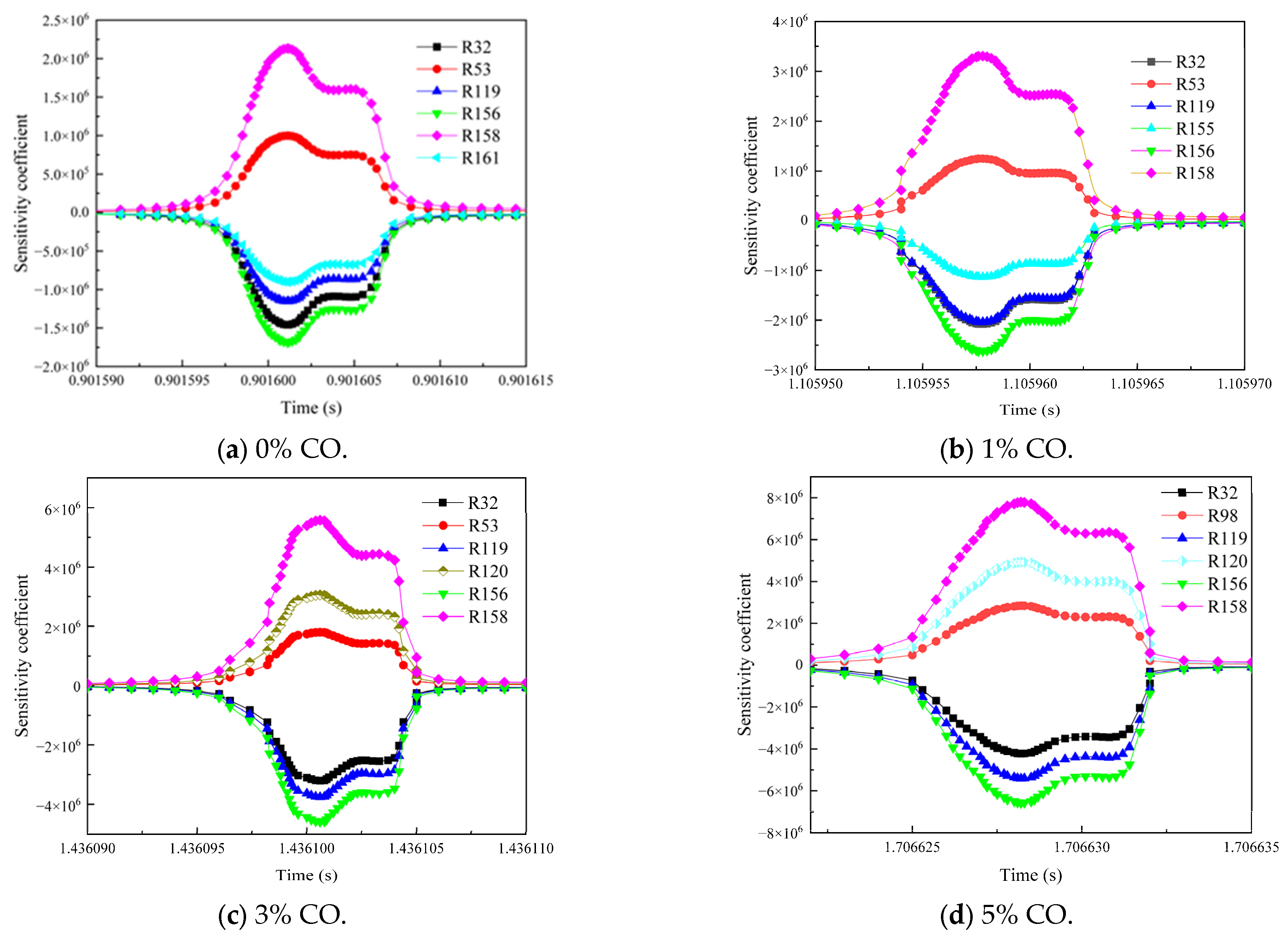
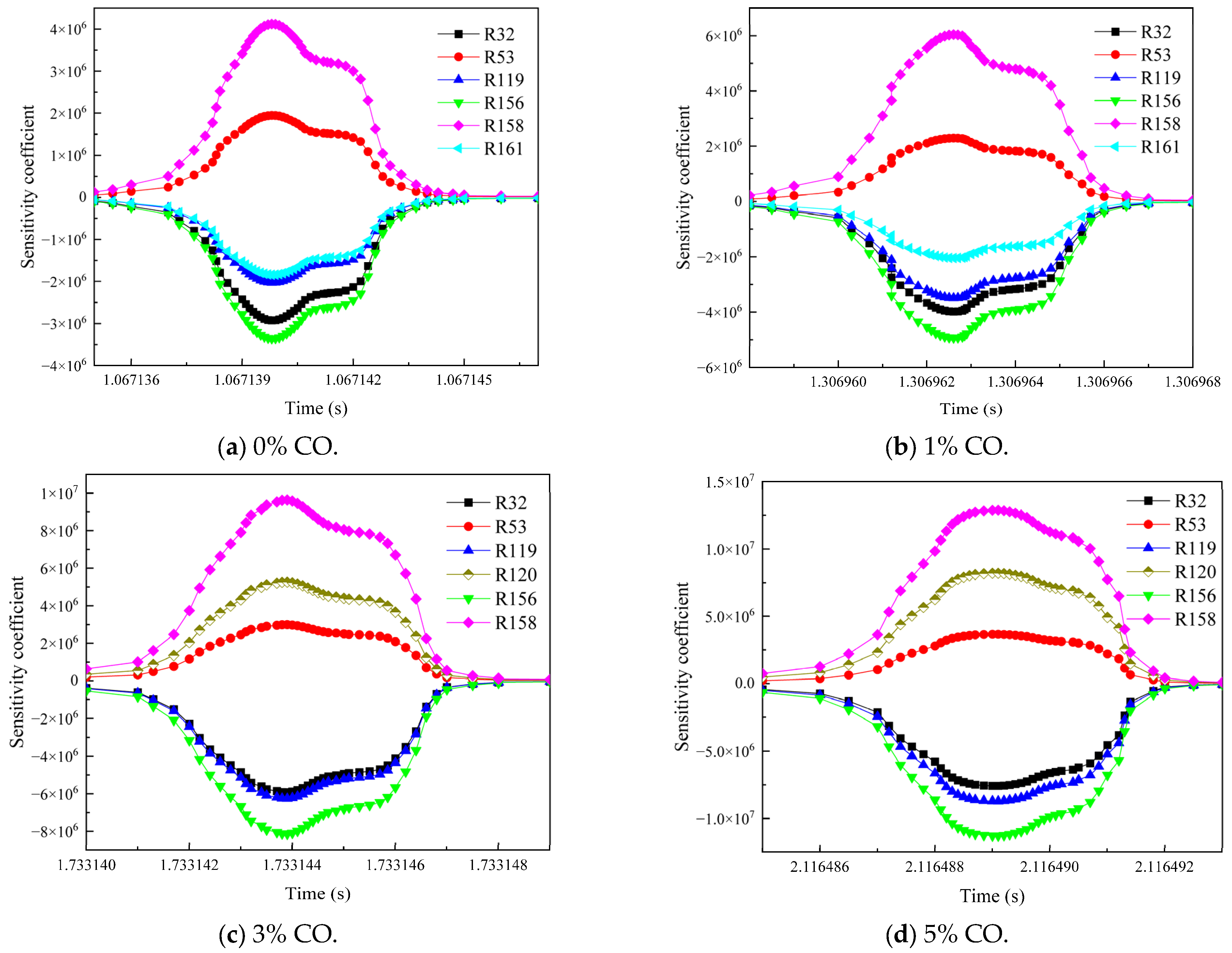
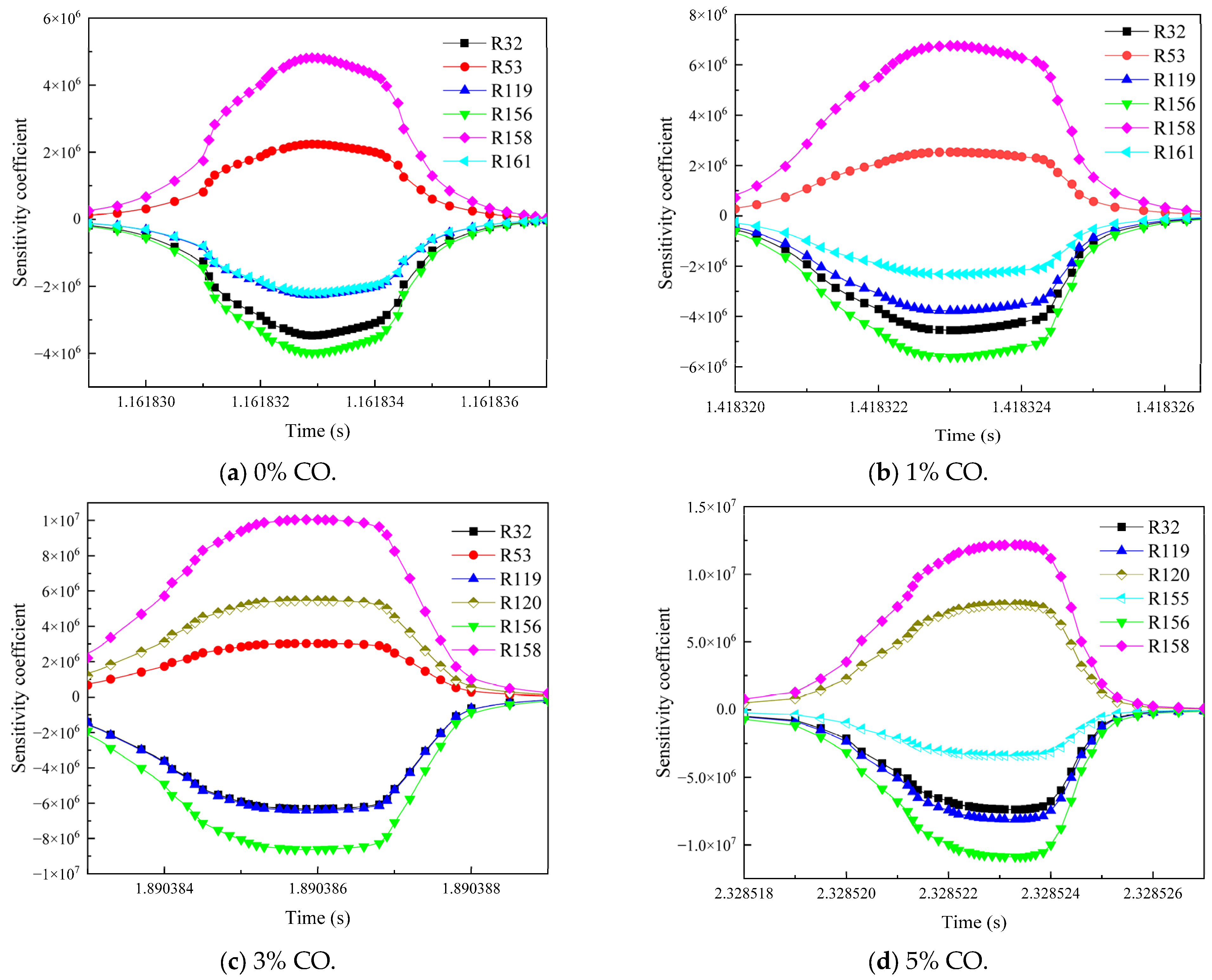
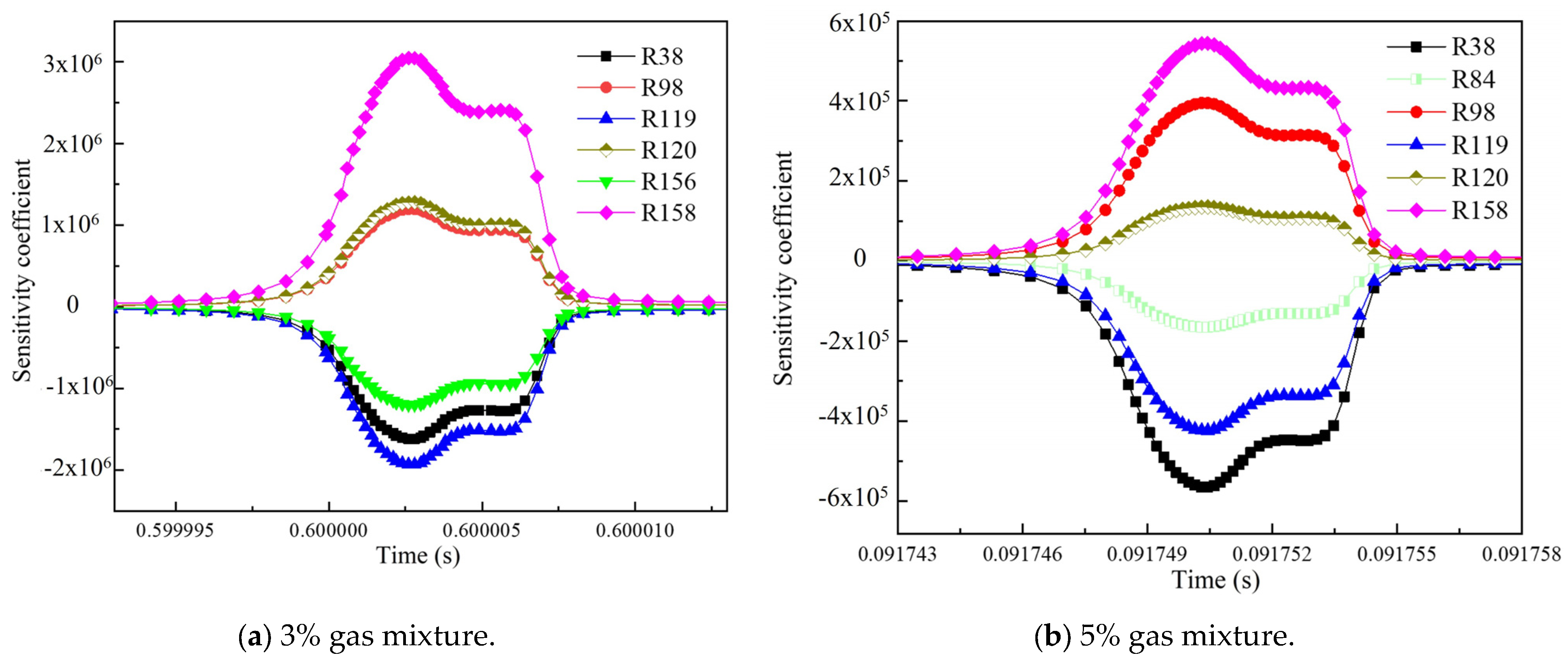
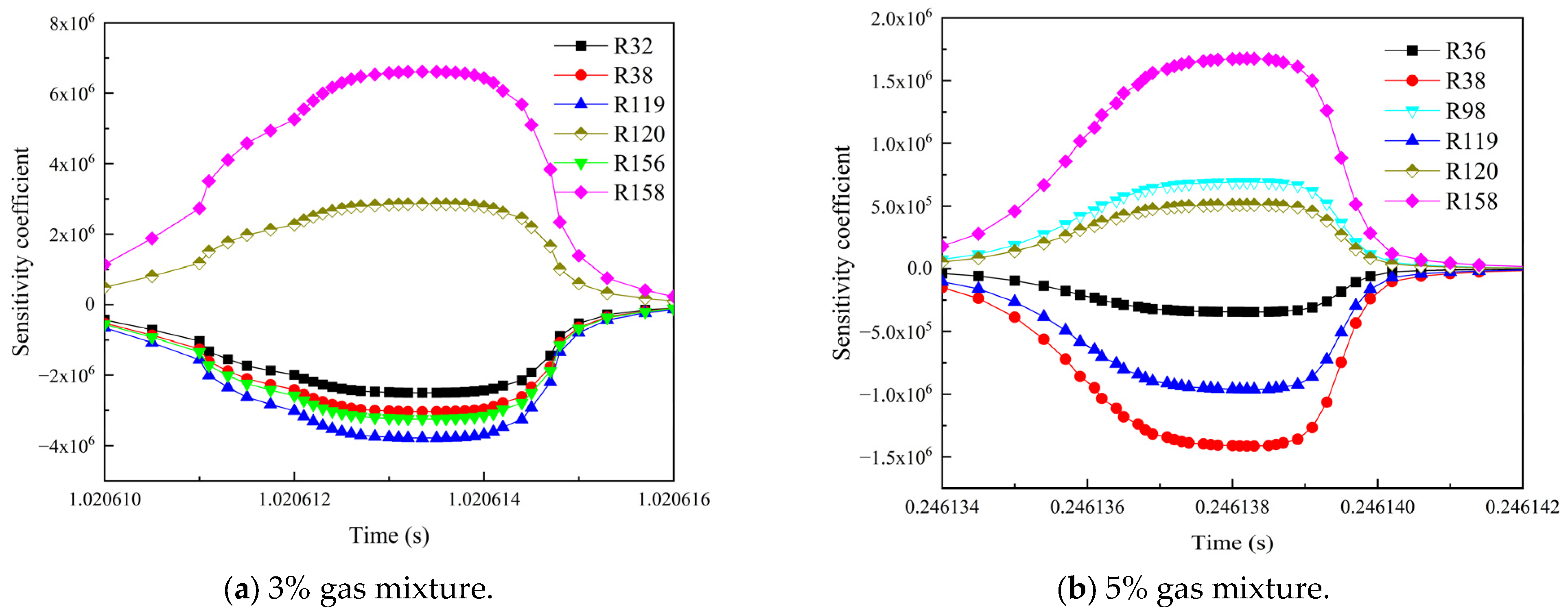
3.3. Influence of the Environmental Mixed Gas from Coal Self-Ignition on the Key Reaction Steps of Methane Consumption
4. Conclusions
- (1)
- At the CH4 concentrations of 7%, 9.5%, and 11%, the ignition delay time was significantly prolonged as the CO concentration increased from 1% to 5%. The presence of CO suppressed the initial ignition stage of the explosion and delayed the start of the combustion chain. When H2 was added, the result was completely the opposite, with a significant reduction in the ignition delay time. H2 had a significant acceleration effect in the mixed gases, where it greatly reduced the ignition time and increased the risk of explosion.
- (2)
- As the concentration of the mixed gas increased, the peak molar fractions of three critical free radicals—H·, O·, and ·OH—also showed an increasing trend, with their occurrence times advancing. The total peak molar fractions of these critical free radicals—H·, O·, and ·OH—in the mixed gas were greater than in the reactions that involved only CO, indicating that the integration of H2 into the mixed gas promoted the generation of key free radicals, which facilitated the occurrence of gas explosions.
- (3)
- As the concentration of CO increased, R119 and R156 became the dominant reaction steps, which promoted the gas explosion by generating more ·OH radicals. The addition of H2 changed the main reaction pathway, and the sensitivity of various elemental reactions that affected the gas consumption showed a general trend of weakening. R38 became the most critical reaction step. This reaction accelerated the explosive chain reaction by generating O· and ·OH radicals, which significantly enhanced the explosiveness of the mixed gas.
- (4)
- The explosive behavior of the multi-component mixed gases was significantly influenced by the concentrations of CO and H2. In actual coal spontaneous combustion zones, the presence of CO prolongs the reaction time of the explosion, and the addition of H2 significantly shortens the ignition delay time, accelerating the generation of free radicals and the explosion chain reaction. This discovery has important guiding significance for the prevention and control of gas explosions in actual coal mines. In practical applications, special attention must be paid to the presence of H2, as even small concentrations of H2 may significantly increase the risk of explosion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Qin, B.; Ma, D.; Zhuo, H.; Liang, H.; Gao, A. Unique spatial methane distribution caused by spontaneous coal combustion in coal mine goafs: An experimental study. Process Saf. Environ. Prot. 2018, 116, 199–207. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Q.; Zhu, P.; Li, X.; Zhang, G.; Ma, X.; Zhao, Y. Study on multi-field evolution and influencing factors of coal spontaneous combustion in goaf. Combust. Sci. Technol. 2023, 195, 247–264. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Zhu, C.; Lin, B.; Li, Q.; Li, B.; Huang, Z. Investigating the Influence of Flue Gas Induced by Coal Spontaneous Combustion on Methane Explosion Risk. Fire 2024, 7, 105. [Google Scholar] [CrossRef]
- Cui, G.; Yang, C.; Li, Z.-L.; Zhou, Z.; Li, J.-L. Experimental study and theoretical calculation of flammability limits of methane/air mixture at elevated temperatures and pressures. J. Loss Prev. Process Ind. 2016, 41, 252–258. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Q.; Shen, S. Theoretical estimation of the lower flammability limit of fuel-air mixtures at elevated temperatures and pressures. J. Loss Prev. Process Ind. 2015, 36, 13–19. [Google Scholar] [CrossRef]
- Ma, D.; Shi, Z.; Zhu, T.; Zhang, L.; Yin, C. Effect of flammable gases produced from coal smoldering on methane explosion limits under nitrogen dilution in coal mines. Fuel 2025, 380, 133164. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, L.; Gao, S.; Wang, T.; Su, B.; Wang, L.; Yang, Y.; Li, X. Simulation studies on the influence of other combustible gases on the characteristics of methane explosions at constant volume and high temperature. Arch. Min. Sci. 2021, 2021, 279–295. [Google Scholar]
- Wang, H.; Gu, S.; Chen, T. Experimental Investigation of the Impact of CO, C2H6, and H2 on the Explosion Characteristics of CH4. ACS Omega 2020, 5, 24684–24692. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, L.; Wang, H.; Zhang, Y.; Li, Y.; Qiu, D.; Chen, X. Effects of methane concentration on flame propagation mechanisms and dynamic characteristics of methane/coal dust explosions. Powder Technol. 2024, 439, 119744. [Google Scholar] [CrossRef]
- Deng, J.; Qu, J.; Wang, Q.-H.; Xiao, Y.; Cheng, Y.-C.; Shu, C.-M. Experimental data revealing explosion characteristics of methane, air, and coal mixtures. RSC Adv. 2019, 9, 24627–24637. [Google Scholar] [CrossRef]
- Cashdollar, K.L.; Zlochower, I.A.; Green, G.M.; Thomas, R.A.; Hertzberg, M. Flammability of methane, propane, and hydrogen gases. J. Loss Prev. Process Ind. 2000, 13, 327–340. [Google Scholar] [CrossRef]
- Cammarota, F.; Di Sarli, V.; Salzano, E.; Di Benedetto, A. Measurements of pressure and flame speed during explosions of CH4/O2/N2/CO2 mixtures. J. Loss Prev. Process Ind. 2016, 44, 771–774. [Google Scholar] [CrossRef]
- Salzano, E.; Cammarota, F.; Di Benedetto, A.; Di Sarli, V. Explosion behavior of hydrogen-methane/air mixtures. J. Loss Prev. Process Ind. 2012, 25, 443–447. [Google Scholar] [CrossRef]
- Pekalski, A.; Schildberg, H.; Smallegange, P.; Lemkowitz, S.; Zevenbergen, J.; Braithwaite, M.; Pasman, H. Determination of the explosion behaviour of methane and propene in air or oxygen at standard and elevated conditions. Process Saf. Environ. Prot. 2005, 83, 421–429. [Google Scholar] [CrossRef]
- Gieras, M.; Klemens, R.; Rarata, G.; Wolański, P. Determination of explosion parameters of methane-air mixtures in the chamber of 40 dm3 at normal and elevated temperature. J. Loss Prev. Process Ind. 2006, 19, 263–270. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, S.; Si, Z.; Huang, Z.; Zhang, K.; Jin, Z. High methane natural gas/air explosion characteristics in confined vessel. J. Hazard. Mater. 2014, 278, 520–528. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Pei, S.; Cui, G.; Zhang, L.; Ren, S.; Zhang, Z.; Wang, N. Effect of elevated pressure on the explosion and flammability limits of methane-air mixtures. Energy 2019, 186, 115840. [Google Scholar] [CrossRef]
- Takahashi, A.; Urano, Y.; Tokuhashi, K.; Nagai, H.; Kaise, M.; Kondo, S. Fusing ignition of various metal wires for explosion limits measurement of methane/air mixture. J. Loss Prev. Process Ind. 1998, 11, 353–360. [Google Scholar] [CrossRef]
- Abbas, Z.; Zinke, R.; Gabel, D.; Addai, E.K.; Darbanan, A.F.; Krause, U. Theoretical evaluation of lower explosion limit of hybrid mixtures. J. Loss Prev. Process Ind. 2019, 60, 296–302. [Google Scholar] [CrossRef]
- Liaw, H.-J.; Chen, C.-C.; Lin, N.-K.; Shu, C.-M.; Shen, S.-Y. Flammability limits estimation for fuel-air-diluent mixtures tested in a constant volume vessel. Process Saf. Environ. Prot. 2016, 100, 150–162. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Askar, E.; Markus, D.; Brandes, E.; El-Sayed, I.; Hassan, M.; Nour, M.; Stolz, T. Explosion regions of propane, isopropanol, acetone, and methyl acetate/inert gas/air mixtures. J. Loss Prev. Process Ind. 2016, 43, 669–675. [Google Scholar] [CrossRef]
- Kobiera, A.; Kindracki, J.; Zydak, P.; Wolanski, P. A new phenomenological model of gas explosion based on characteristics of flame surface. J. Loss Prev. Process Ind. 2007, 20, 271–280. [Google Scholar] [CrossRef]
- Maremonti, M.; Russo, G.; Salzano, E.; Tufano, V. Numerical simulation of gas explosions in linked vessels. J. Loss Prev. Process Ind. 1999, 12, 189–194. [Google Scholar] [CrossRef]
- Salzano, E.; Marra, F.; Russo, G.; Lee, J. Numerical simulation of turbulent gas flames in tubes. J. Hazard. Mater. 2002, 95, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-B.; Gao, J.-L.; Ren, J.-Z.; Wang, C.-X. Analysis of the Characteristics and Influencing Factors of Gas Explosion in Heading Face. Shock. Vib. 2020, 2020, 10709622. [Google Scholar] [CrossRef]
- Yang, A.; Liu, Y.; Gao, K.; Li, R.; Li, Q.; Li, S. Numerical Simulation of Gas Explosion with Non-uniform Concentration Distribution by Using OpenFOAM. ACS Omega 2023, 8, 48789–48812. [Google Scholar] [CrossRef]
- Qiu, J.; Jiang, B.; Tang, M.; Zhou, L.; Ren, B. Effect of Different Bend Pipes on the Propagation Characteristics of Premixed Methane-Air Explosion in Confined Spaces. Geofluids 2021, 2021, 6635156. [Google Scholar] [CrossRef]
- Coppens, F.; Deruyck, J.; Konnov, A. The effects of composition on burning velocity and nitric oxide formation in laminar premixed flames of CH4+H2+O2+N2. Combust. Flame 2007, 149, 409–417. [Google Scholar] [CrossRef]
- Berg, P.A.; Hill, D.A.; Noble, A.R.; Smith, G.P.; Jeffries, J.B.; Crosley, D.R. Absolute CH concentration measurements in low-pressure methane flames: Comparisons with model results. Combust. Flame 2000, 121, 223–235. [Google Scholar] [CrossRef]
- Sung, C.; Law, C.; Chen, J.-Y. Further Validation of an Augmented Reduced Mechanism for Methane Oxidation: Comparison of Global Parameters and Detailed Structure. Combust. Sci. Technol. 2000, 156, 201–220. [Google Scholar] [CrossRef]
- Wang, T.; Luo, Z.; Wen, H.; Cheng, F.; Liu, L.; Su, Y.; Liu, C.; Zhao, J.; Deng, J.; Yu, M. The explosion enhancement of methane-air mixtures by ethylene in a confined chamber. Energy 2021, 214, 119042. [Google Scholar] [CrossRef]


| CH4 Concentration (%) | Concentration of CO and H2 (%) | Ratio 1 (Excluding H2) | Ratio 2 | |||||
|---|---|---|---|---|---|---|---|---|
| CO (%) | O2 (%) | N2 (%) | CO (%) | H2 (%) | O2 (%) | N2 (%) | ||
| 7 | 0 | 0 | 19.53 | 73.47 | 0 | 0 | 19.53 | 73.47 |
| 1 | 1 | 19.32 | 79.68 | 1 | 0 | 19.32 | 79.68 | |
| 2 | 2 | 19.11 | 78.89 | 1.9 | 0.1 | 19.11 | 78.89 | |
| 3 | 3 | 18.9 | 78.1 | 2.8 | 0.2 | 18.9 | 78.1 | |
| 4 | 4 | 18.69 | 77.31 | 3.5 | 0.5 | 18.69 | 77.31 | |
| 5 | 5 | 18.48 | 76.52 | 4 | 1.0 | 18.48 | 76.52 | |
| 9.5 | 0 | 0 | 19.005 | 71.495 | 0 | 0 | 19.005 | 71.495 |
| 1 | 1 | 18.795 | 70.705 | 1 | 0 | 18.795 | 70.705 | |
| 2 | 2 | 18.585 | 69.915 | 1.9 | 0.1 | 18.585 | 69.915 | |
| 3 | 3 | 18.375 | 69.125 | 2.8 | 0.2 | 18.375 | 69.125 | |
| 4 | 4 | 18.165 | 68.335 | 3.5 | 0.5 | 18.165 | 68.335 | |
| 5 | 5 | 17.955 | 67.545 | 4 | 1.0 | 17.955 | 67.545 | |
| 11 | 0 | 0 | 18.69 | 70.31 | 0 | 0 | 18.69 | 70.31 |
| 1 | 1 | 18.48 | 69.52 | 1 | 0 | 18.48 | 69.52 | |
| 2 | 2 | 18.27 | 68.73 | 1.9 | 0.1 | 18.27 | 68.73 | |
| 3 | 3 | 18.06 | 67.94 | 2.8 | 0.2 | 18.06 | 67.94 | |
| 4 | 4 | 17.85 | 67.15 | 3.5 | 0.5 | 17.85 | 67.15 | |
| 5 | 5 | 17.64 | 66.36 | 4 | 1.0 | 17.64 | 66.36 | |
| CH4 Concentration (%) | Combustible Gas Concentration (%) | Ignition Delay Time (s) | |
|---|---|---|---|
| Add CO Gas | Add CO and H2 Gas Mixture | ||
| 7 | 0 | 0.902 | 0.902 |
| 1 | 1.106 | 1.106 | |
| 2 | 1.281 | 0.806 | |
| 3 | 1.436 | 0.600 | |
| 4 | 1.577 | 0.246 | |
| 5 | 1.707 | 0.092 | |
| 9.5 | 0 | 1.067 | 1.067 |
| 1 | 1.307 | 1.307 | |
| 2 | 1.527 | 1.060 | |
| 3 | 1.733 | 0.868 | |
| 4 | 1.929 | 0.428 | |
| 5 | 2.116 | 0.181 | |
| 11 | 0 | 1.162 | 1.162 |
| 1 | 1.418 | 1.418 | |
| 2 | 1.659 | 1.200 | |
| 3 | 1.890 | 1.020 | |
| 4 | 2.113 | 0.544 | |
| 5 | 2.328 | 0.246 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Zhang, L.; Han, G.; Zhu, T. Numerical Study on the Explosion Reaction Mechanism of Multicomponent Combustible Gas in Coal Mines. Fire 2024, 7, 368. https://doi.org/10.3390/fire7100368
Ma D, Zhang L, Han G, Zhu T. Numerical Study on the Explosion Reaction Mechanism of Multicomponent Combustible Gas in Coal Mines. Fire. 2024; 7(10):368. https://doi.org/10.3390/fire7100368
Chicago/Turabian StyleMa, Dong, Leilin Zhang, Guangyuan Han, and Tingfeng Zhu. 2024. "Numerical Study on the Explosion Reaction Mechanism of Multicomponent Combustible Gas in Coal Mines" Fire 7, no. 10: 368. https://doi.org/10.3390/fire7100368
APA StyleMa, D., Zhang, L., Han, G., & Zhu, T. (2024). Numerical Study on the Explosion Reaction Mechanism of Multicomponent Combustible Gas in Coal Mines. Fire, 7(10), 368. https://doi.org/10.3390/fire7100368






