Abstract
CO, H2, and other combustible gases will be produced during coal oxidation in coal mines, which will increase the risk of explosion when mixed with methane. Therefore, it is very important to understand the production characteristics of combustible gas during coal oxidation. In this paper, a programmed temperature gas test system is built to study the impact of lignite on the production of gases at different particle sizes and temperatures, and the release characteristics of gases are also analyzed. The result shows that the production of combustible gas is influenced by the coal particle size significantly when the temperature is above 200 °C, and it decreases as the particle size increases. CO is the main gas during the early stage of coal spontaneous combustion, and the release of CH4 and H2 increases after 300 °C. The fitted equations of gas generation and temperature are consistent with the experimental results. The research results are helpful in understanding the hazards of coal spontaneous combustion and have a certain guiding significance for coal mine monitoring and prevention of coal spontaneous combustion.
1. Introduction
During the oxidation process of coal, oxygen is absorbed through physical and chemical adsorption, simultaneously releasing gaseous products. The primary gaseous products during coal self-ignition include carbon oxides and hydrocarbons [,]. The mine fires and gas explosions caused by coal spontaneous combustion are major safety hazards in coal mines, resulting in serious casualties and property losses. In recent years, there have been multiple accidents caused by coal spontaneous combustion in major coal-producing countries around the world. In 2021, a coal spontaneous combustion occurred at Shenmu Coal Mine in Shaanxi, China, causing a fire and gas explosion, resulting in the death of 5 workers. In 2022, a coal mine in the Indian state of Jharkhand caught fire due to spontaneous combustion, causing the mine to shut down for several weeks. Based on the frequent occurrence of coal spontaneous combustion accidents, a large number of scholars have conducted research on the process of coal spontaneous combustion.
In the low-temperature oxidation stage of coal, the quantity of gaseous products increases with the rise in coal oxidation temperature, and the concentration of gas products exponentially grows [,,]. Furthermore, since some coals inherently adsorb gases like CO2, CH4, and C2H6, these gases gradually desorb as the coal’s temperature rises, resulting in relatively high emissions at the early stages of coal oxidation [,,]. The concentration of gaseous products produced during coal oxidation correlates with changes in coal temperature, allowing for predictions of coal self-ignition states based on the release patterns of gaseous products [,]. Yuan et al. [] found that the CO/CO2 ratio tends to a constant value of 0.2 in different coal samples, regardless of the properties of the coal. The rate of CO production is highly correlated with coal temperature. When the initial temperature of the coal body is 90 °C, the concentration of CO increases rapidly and can reach a maximum value of 1300 ppm. Green et al. [,] studied the generation characteristics of CO and CO2 during the coal self-ignition process and further analyzed the pathways of their gaseous products’ formation. They found that the thermal decomposition of oxygen-containing functional groups to produce CO does not depend on the coal grade, and the production of CO2 depends on the coal grade; the decomposition of air oxygen by chemical adsorption presents a completely opposite situation to the former. Li Zenghua et al. [] measured the production patterns of H2 during coal oxidation, finding that H2 is detected in the temperature range of 200–250 °C, and its concentration subsequently increases exponentially with temperature. To study the gaseous product generation patterns at high temperatures, Xi’an University of Science and Technology independently developed a testing system for the self-ignition characteristics of coal under high-temperature conditions and examined the generation patterns of gaseous products during high-temperature oxidation stages of coal self-ignition [,,,]. Dong et al. [] used programmed temperature experiments to study the gas production patterns of different coal samples during heating and oxidation processes. They discovered that the order of gas production was CO, C2H6, C2H4, and C3H8, and that the amount of gas produced was approximately exponentially related to the temperature. Ren and colleagues [] studied the relationship between temperature and exothermic intensity with the amount of gas produced under different oxygen concentrations. They found that CO, CH4, C2H4, and C2H6 increased with rising temperature. The exothermic intensity had the greatest correlation with CO2, and under low oxygen conditions, the exothermic intensity had a stronger correlation with CO and CH4. Sun et al. [] studied the CO gas production characteristics of low metamorphic coals, CYM, RNM, and QM, through programmed temperature experiments. They found that CYM had the fastest CO concentration and gas production rate, reaching a CO concentration of 1000 × 10−11 mol cm−3 s−1 at 170 °C. Yan et al. [] studied the relationship between the indicator gases and their ratios during the coal oxidation process with coal temperature using grey correlation analysis. They concluded that between 90 °C and 150 °C, the grey relational degrees of C3H8/CH4, C2H6/CH4, and C3H8/C2H6 were 0.9701, 0.9562, and 0.9240, respectively.
The particle size of coal also affects the release patterns of gaseous products during the self-ignition process. It was found that the critical particle size of coal is in the range of 38–74 μm (D50 = 50.56 μm), and below this particle size, the oxidation rate does not change significantly. CO2/CO was proposed as the main indicator for quantitative coal spontaneous combustion under the influence of coal particle size [,,]. Singh et al. [] tested the characteristics of gaseous products produced by coal below 200 °C, finding that the particle size of coal significantly impacts the production of CO while having a lesser effect on H2 production. Song et al. [] studied the characteristics of gas production from weathered coal of different particle sizes under conditions of high temperature and hypoxia. They found that during low-temperature oxidation (<250 °C), the concentration of indicator gases increased with decreasing particle size. During high-temperature oxidation (>250 °C), weathered coal of different particle sizes exhibited their own characteristics, with a 3 mm particle size being the critical size for high-temperature oxidation.
These studies illustrate the characteristics of the primary gaseous products in coal self-ignition under various environmental conditions and can provide guidance for predicting and preventing coal self-ignition at coal mine sites. However, regarding the underground fire areas in coal mines, it is crucial to understand the generation pattern of combustible gas products, which may be mixed with methane to form multi-component gases and increase the risk of gas explosion. At present, both domestically and internationally, the relationship between coal temperature and gas-phase products is mainly focused on low-temperature oxidation (<200 °C), and most studies focus on the relationship between single CO or H2 and coal temperature. There is a lack of research on the relationship between multi-component products and coal temperature changes at high temperatures (>200 °C). The systematic research on the generation law of combustible gases generated by coal spontaneous combustion is not sufficient, so it is impossible to grasp the explosive characteristics of mixed gases in the coal spontaneous combustion environment. This article systematically analyzes the generation patterns of multiphase products at different coal temperatures (<500 °C), and explores the effects of particle size differences to further reveal the dynamic changes of products during coal spontaneous combustion. Unlike conventional experiments, this study simulated an actual environment closer to the spontaneous combustion of coal in the fire zone and examined the synergistic effect of temperature and particle size, two key factors. The research results can provide important references for early warning of coal spontaneous combustion in fire zones, and also propose new ideas for fire prevention and extinguishing strategies in actual coal mines.
In this work, lignite from Datong Ermuwan Coal Mine is taken as a study object, and the coal spontaneous combustion programmed temperature gas test system is used to study the production of gas with different coal particle sizes and temperatures. The amount of combustible gas is fitted by segment, and the generation characteristic is analyzed. The results can predict the degree of coal spontaneous combustion, and further provide guidance on avoiding coal fires.
2. Materials and Methods
2.1. Coal Samples
In the experiment, lignite from Datong Ermuwan Coal Mine in Shanxi Province, China (referred to as EMW), was chosen as the coal sample. Fresh coal samples collected from underground were sealed in multi-layer plastic bags and transported to the laboratory. The oxidized layer of the coal was removed, and the samples were crushed under vacuum conditions. Coal samples of three different particle sizes—0.096~0.18 mm, 0.18~0.38 mm, and 0.38~0.83 mm—were sieved and set aside. Prior to experimentation, the coal samples were placed in a vacuum drying oven at 40 °C for 48 h to remove extraneous moisture. An automatic industrial analyzer, model 5E-MAG6600, was used to conduct the industrial analysis of five coal samples. The results of the analysis are presented in Table 1.

Table 1.
Industrial analysis of coal samples.
2.2. Experimental Equipment
The coal spontaneous combustion programmed temperature gas test system is illustrated in Figure 1. The system comprises a quartz reactor, a programmable heating system, a gas distribution system, a data acquisition system, and a gas chromatograph. The quartz reactor has an inner diameter of 100 mm and a length of 680 mm. The programmable heating system includes a heating wire and a temperature control module, which allows for the adjustment of the heating rate, as well as a real-time temperature display. The gas distribution system consists of two gas flow meters with a range of 0 to 10 L/min and two gas cylinders of oxygen and nitrogen, enabling intelligent control of the gas composition and flow rate during coal spontaneous combustion. The data acquisition system primarily includes temperature sensors, a multi-channel data collector, and a PLC main control screen, facilitating real-time recording and storage of the coal temperature during the combustion process. Gases produced during the coal combustion are analyzed using a GC-4000A gas chromatograph.
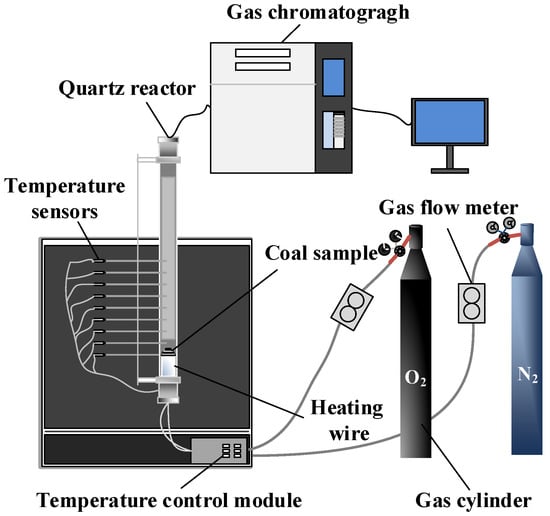
Figure 1.
Coal spontaneous combustion programmed temperature gas test system.
2.3. Experimental Procedure
Prior to the commencement of the experiment, nitrogen gas was introduced into a quartz reaction tube at a flow rate of 5 L/min for 10 min to expel any atmospheric air present, thereby mitigating potential interference with the experimental outcomes. Subsequently, both the electric heating system and the automatic gas delivery system were activated. The target temperature for the electric heating program’s control module was set at 300 °C, employing a linear temperature increase approach. Concurrently, the intake of oxygen and nitrogen was adjusted to mimic the proportions found in air, with a total inflow rate of 500 mL/min. Once the temperature of the molybdenum heating element reached 300 °C, the temperature control system was deactivated, and heating was discontinued to allow the coal to continue oxidizing and burning in a ventilated environment. When the coal temperature dropped below 200 °C, an increase of 10 °C prompted gas analysis via gas chromatography. For temperatures exceeding 200 °C, gas analysis was conducted for every 20 °C increment. Real-time data on the temperatures of both the molybdenum wire and the coal were recorded by the data acquisition system.
3. Results and Discussion
3.1. Influence of Coal Particle Size on the Generation of Combustible Gas
The concentration of combustible gas produced during the heating process with different particle sizes is illustrated in Figure 2. Figure 2a reveals that larger coal particles produce lower concentrations of CO. This phenomenon is attributed to the larger particles having a smaller specific surface area and lower porosity, which results in fewer active sites for coal surface interaction with oxygen, thus reducing the CO concentration during the oxidation process. Furthermore, it is observed that the peak temperature of CO concentration significantly shifts towards higher values with increasing coal particle size. For example, for coal particles sized between 0.096 and 0.18 mm, the peak CO concentration occurs at 400 °C, whereas for particles sized between 0.38 and 0.83 mm, it occurs at 440 °C.
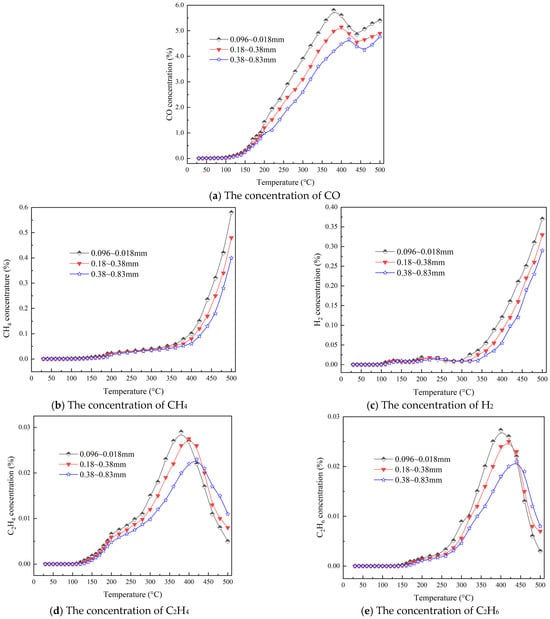
Figure 2.
The concentration of gas with temperature during the heating process.
Figure 2b illustrates the variation in CH4 under different particle size conditions as a function of temperature. The graph indicates that prior to reaching 300 °C, the methane concentrations generated from coal samples of varying particle sizes are relatively low. However, once the temperature of the coal exceeds 300 °C, the methane concentrations significantly increase due to the oxidative cleavage of aromatic rings and cycloalkanes within the coal molecules. The methane production shows exponential growth with increasing temperature until the end of the experiment. A comparison among coal samples of different particle sizes reveals that the methane concentration produced during the coal oxidation process increases as the particle size decreases. For instance, at a coal temperature of 500 °C, coal samples with a particle size range from 0.096 to 0.18 mm generate a methane concentration of 0.58%, while those with a particle size range from 0.38 to 0.83 mm produce a methane concentration of 0.41%.
Figure 2c presents H2 under varying particle sizes. The graph illustrates a similar pattern across different coal particle sizes. Prior to reaching a temperature of 300 °C, the concentration of H2 produced by coal samples of various sizes exhibits certain fluctuations and remains relatively low, suggesting a minimal impact of particle size within this temperature range. Additionally, the graph indicates a downward trend in H2 concentration as the coal particle size increases. For instance, coal samples with particle sizes ranging from 0.096 to 0.18 mm produced an H2 concentration of 0.37% at 500 °C, whereas those between 0.38 and 0.83 mm produced a CH4 concentration of 0.28%. Figure 2d,e display the production volumes of C2H4 and C2H6 under different particle sizes, respectively. During the oxidation phase from 30 °C to 200 °C, the coal particle size exhibits little impact on the production of C2H4 and C2H6, with gas concentrations remaining low during this stage. Peak concentrations of gases produced by spontaneous coal combustion vary with particle size; for C2H4, the peak concentrations for particle sizes of 0.096~0.18 mm, 0.18~0.38 mm, and 0.38~0.83 mm are 0.029%, 0.027%, and 0.023%, respectively. Similarly, for C2H6, the peak concentrations are 0.027%, 0.025%, and 0.021%, respectively, demonstrating a gradual decrease in peak concentrations of both gases as coal particle size increases. Moreover, the peak temperatures for C2H4 and C2H6 significantly shift towards higher values with increasing coal particle sizes. For instance, for coal samples of 0.096~0.18 mm, the peak concentrations of both C2H4 and C2H6 occur at 380 °C, whereas for samples of 0.38~0.83 mm, the corresponding peak temperatures rise to 420 °C.
The total quantity of combustible gases produced during the coal oxidation process under various particle size conditions and the proportion of the main combustible gases is illustrated in Figure 3. As shown in Figure 3a, the total amount of combustible gases generated by coal of three different particle sizes begins to rise significantly after reaching 100 °C and continues to increase as the temperature rises. Comparing the production of combustible gases among different particle sizes, it is evident that as the particle size of the coal sample increases, the total volume of combustible gases gradually decreases. It also illustrates that different types of coal exhibit peak values between 350 °C and 450 °C, primarily due to a significant reduction in CO concentration during this phase. Comparing the production of combustible gases at particle sizes of 0.096~0.18 mm, 0.18~0.38 mm, and 0.38~0.83 mm, the peak concentrations are 6.02%, 5.37%, and 4.91%, respectively, indicating a gradual decrease in the peak concentration of combustible gases as coal particle size increases. Figure 3b–d display the percentages of the three primary combustible gases—CO, CH4, and H2—relative to the total combustible gas volume under different particle size conditions. It is observed that the proportional concentration of CO, CH4, and H2 follows a similar trend across varying particle sizes, accounting for over 99% of the combustible gases in the temperature range of 30 to 500 °C. A comparison of the production patterns of these three gases across different particle sizes reveals that particle size has a minimal impact on their proportion within the total volume of combustible gases.
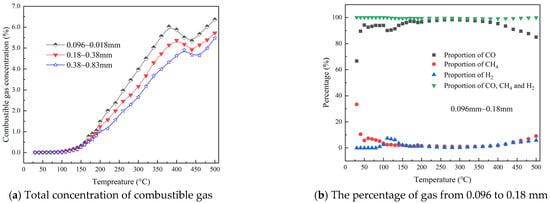
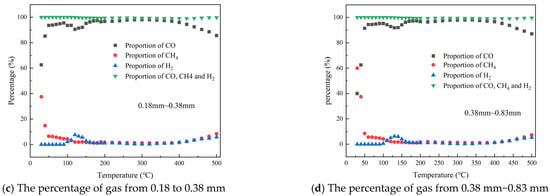
Figure 3.
The percentage of combustible gas during the heating process with different particle sizes.
This section examines the impact of coal particle size on the production of combustible gases—CO, CH4, H2, C2H4, and C2H6—during the heating process. It shows that larger coal particles generate lower concentrations of CO, CH4, H2, C2H4, and C2H6 due to smaller specific surface areas and lower porosity, reducing their interaction with oxygen. The peak concentrations of these gases shift to higher temperatures as particle size increases. Smaller particles produce higher concentrations of combustible gases, especially CO, CH4, and H2, with the most pronounced gas production occurring between 350 °C and 450 °C. Although the total volume of combustible gases decreases with increasing particle size, CO, CH4, and H2 make up over 99% of the total gas volume produced, regardless of particle size. The research conclusions of Zhang et al. [,,] on the influence of coal particle size and coal temperature on combustible gases, such as CO, are similar to those in this paper.
3.2. Segmental Analysis of Combustible Gas during the Coal Combustion Process
It is evident that the combustible gases produced during the coal self-ignition process primarily include CO, CH4, and H2. In the initial stages of coal self-ignition, the production of combustible gases is predominantly CO, and with the temperature of the coal rising to 300 °C, the release of CH4 and H2 significantly increases, with these stages primarily characterized by CH4, CO, and H2 as the main combustible gases. It indicates that the generation of combustible gases during coal self-ignition exhibits distinct segmental characteristics. The concentration and composition of these gases vary according to the different temperature stages of coal self-ignition. The concentration of combustible gases is segmented and fitted, as shown in Figure 4.
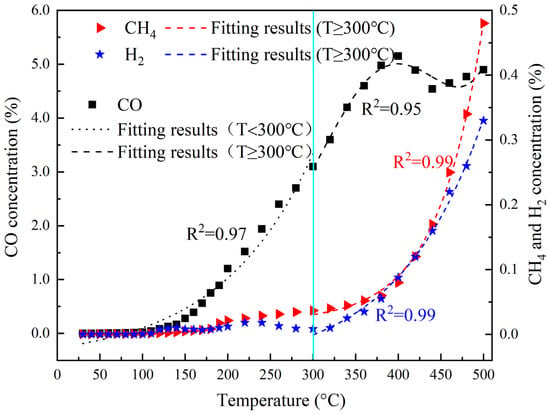
Figure 4.
Fitting results of combustible gas production in the oxidation process.
At temperatures below 300 °C, CO concentration rapidly increases as an exponential function; subsequently, due to a decrease in the content of carbonyl and aliphatic hydrocarbon functional groups in the coal, CO gas concentration exhibits fluctuating changes within the temperature range of 300 °C to 500 °C. For CH4 and H2, which are also combustible gases produced during the coal self-ignition process, their main production occurs at the high-temperature stages above 300 °C, with both gases increasing in an approximately exponential manner.
When the coal temperature is lower than 300 °C, the release law of CO gas produced by spontaneous combustion of coal with the coal temperature can be fitted as follows:
In the formula, and are exponential function fitting coefficients, and represents an exponential function fitting constant.
When the coal temperature is over 300 °C, the CO, CH4, and H2 gases generated by spontaneous combustion of coal can be fitted with the following formula, respectively:
where is a polynomial function fitting constant, while , , … are the fitting coefficients of the polynomial function, respectively. , , and are exponential function fitting coefficients, while and are exponential function fitting constants.
The fitting results obtained for the generation of combustible gases during the coal oxidation process, as a function of temperature, are presented in Table 2. The results indicate that the correlation coefficients for the generated quantities of combustible gases and the temperature fitting equations are both above 0.95. Using these fitting equations, calculations were conducted on the generation of combustible gases during the oxidation process for different types of coals, and these were compared with experimental results, as shown in Figure 5. The established fitting equation has a high degree of fit with experimental data in different temperature ranges, indicating the reliability of the fitting equation and its ability to better capture the changes in the generation of combustible gases during coal spontaneous combustion.

Table 2.
Fitting results of combustible gas generation concentration and temperature.
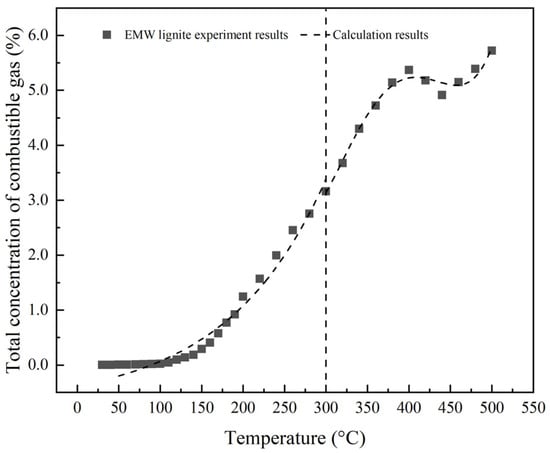
Figure 5.
Comparison of experimental data and calculated results of the total concentration of combustible gas generated from EMW lignite.
According to Table 3, it can be concluded that the error between experimental data and calculated results gradually decreases with increasing temperature. When the temperature is below 100 °C, there is a significant difference between the fitted value and the experimental value. For example, at 50 °C, the experimental value is 0.0062, while the calculated value is −0.19842. This may be due to the complexity of oxidation reactions at low temperatures, as well as uncertainties such as instrument measurement errors and experimental environments. However, when the temperature reaches above 300 °C, the error between the calculated results and the experimental results gradually decreases, indicating that the fitting equation performs more accurately in the high-temperature stage.

Table 3.
Comparison of experimental data and calculated results of the total concentration of combustible gas generated from EMW lignite.
Although there is a large error in the low-temperature stage, in practical applications, the amount of gas generated in the low-temperature stage is relatively low, so the impact of this error on predicting the risk of coal spontaneous combustion is limited. Moreover, the overall trend of the fitted equation is highly consistent with the experimental results, verifying the feasibility of using this fitted equation for gas generation prediction.
This section analyzes the generation patterns of combustible gases (CO, CH4, and H2) during coal self-ignition and highlights distinct temperature-dependent phases. At temperatures below 300 °C, CO is the primary gas, increasing exponentially. As the temperature exceeds 300 °C, the production of CH4 and H2 rises exponentially, making them the dominant gases at higher temperatures. Fitting equations were used to simulate the release of these gases at different temperature stages, showing a strong correlation (R2 > 0.95) between predicted and experimental results. The obvious segmented characteristics of combustible gases during coal spontaneous combustion can predict the danger level of coal mine spontaneous combustion based on changes in gas concentration, which is consistent with the research results of Guo et al. [,]. These equations provide a reliable method for predicting the concentration of combustible gases during coal oxidation, aiding in the prevention of coal combustion-related hazards.
4. Conclusions
This paper analyzes the primary sources and components of combustible gases in goaf areas where coal spontaneous combustion occurs. It focuses on the relationship between the concentration of combustible gases and temperature during coal oxidation, using a specially constructed high-temperature programmed heating experimental system for coal spontaneous combustion. This study clarifies the composition of combustible gases under different conditions, and the main conclusions are as follows:
- To master the production patterns of combustible gases during coal oxidation, a high-temperature programmed heating test system for coal spontaneous combustion was established. It mainly consists of a quartz reactor, a programmed heating system, an automatic gas distribution system, a data acquisition system, and a gas chromatograph, which enables the detection of gaseous products during the high-temperature stage of coal spontaneous combustion;
- The influence of coal particle size on the production of CH4, H2, C2H4, and C2H6 is not significant at low temperatures but becomes considerably significant above 200 °C. As the coal particle size increases, the total amount of combustible gases gradually decreases; however, the impact of particle size on the percentage of CO, CH4, and H2 among the total combustible gases is minimal, with their concentration ratios reaching over 99% within the temperature range of 30 °C to 500 °C;
- The generation of combustible gases during coal spontaneous combustion exhibits distinct phased characteristics. Initially, CO is the predominant combustible gas produced, but as the temperature rises above 300 °C, the release of CH4 and H2 significantly increases. During this phase, the primary combustible gases in the coal spontaneous combustion environment are CH4, CO, and H2. Based on these observations, functional equations relating the generation of combustible gases at different temperature stages to temperature were fitted and compared with experimental results, indicating a good agreement between the calculated results using the fitted equations and the experimental findings.
Author Contributions
D.M.: methodology, software, investigation, validation, visualization, data curation, and writing—original draft. L.Z.: supervision, conceptualization, resources, and writing—review and editing. T.Z.: supervision and resources. P.Y.: software, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China grant number (52204250) and Independent Research Project of State Key Laboratory for Fine Exploration and Intelligent Development of Coal Resources, CUMT grant number (SKLCRSM23X007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available due to commercial confidentiality, as they contain information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baris, K.; Kizgut, S.; Didari, V. Low-temperature oxidation of some Turkish coals. Fuel 2012, 93, 423–432. [Google Scholar] [CrossRef]
- Wang, H.; Dlugogorski, B.Z.; Kennedy, E.M. Coal oxidation at low temperatures: Oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Prog. Energy Combust. Sci. 2003, 29, 487–513. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, S.; Gu, J.; Gao, J. Differences in physical properties and CO2 gasification reactivity between coal char and petroleum coke. Process Saf. Environ. Prot. 2009, 87, 323–330. [Google Scholar] [CrossRef]
- Zhang, D.; Cen, X.; Wang, W.; Deng, J.; Wen, H.; Xiao, Y.; Shu, C.M. The graded warning method of coal spontaneous combustion in Tangjiahui Mine. Fuel 2021, 288, 119635. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol. 2020, 30, 303–311. [Google Scholar] [CrossRef]
- Li, Q.W.; Xiao, Y.; Zhong, K.Q.; Shu, C.M.; Lü, H.F.; Deng, J.; Wu, S. Overview of commonly used materials for coal spontaneous combustion prevention. Fuel 2020, 275, 117981. [Google Scholar] [CrossRef]
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. Res. 2017, 24, 23453–23470. [Google Scholar] [CrossRef]
- Adamus, A.; Šancer, J.; Guřanová, P.; Zubíček, V. An investigation of the factors associated with interpretation of mine atmosphere for spontaneous combustion in coal mines. Fuel Process. Technol. 2011, 92, 663–670. [Google Scholar] [CrossRef]
- Szurgacz, D.; Tutak, M.; Brodny, J.; Sobik, L.; Zhironkina, O. The method of combating coal spontaneous combustion hazard in goafs—A case study. Energies 2020, 13, 4538. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Wang, L.K.; Zhang, Z.; Zhang, D.; Li, C. Experimental study and thermodynamic analysis of coal spontaneous combustion characteristics. Combust. Theory Model. 2023, 27, 118–137. [Google Scholar] [CrossRef]
- Yuan, L.; Smith, A. Experimental study on CO and CO2 emissions from spontaneous heating of coals at varying temperatures and O2 concentrations. J. Loss Prev. Process Ind. 2013, 26, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Green, U.; Aizenshtat, Z.; Hower, J.C.; Stark, S.; Weidner, C.; Cohen, H. Modes of formation of carbon oxides (COx (x = 1, 2)) from coals during atmospheric storage: Part 1: Effect of coal rank. Energy Fuels 2010, 24, 6366–6374. [Google Scholar] [CrossRef]
- Green, U.; Aizenshtat, Z.; Hower, J.; Hatch, R.; Cohen, H. Modes of formation of carbon oxides (COx (x = 1, 2)) from coals during atmospheric storage: Part 2: Effect of coal rank on the kinetics. Energy Fuels 2011, 25, 5625–5631. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Tang, Y.; Liu, Z.; Ji, H. Fine coal covering for preventing spontaneous combustion of coal pile. Nat. Hazards 2014, 74, 603–622. [Google Scholar] [CrossRef]
- Wen, H.; Yu, Z.; Deng, J.; Zhai, X. Spontaneous ignition characteristics of coal in a large-scale furnace: An experimental and numerical investigation. Appl. Therm. Eng. 2017, 114, 583–592. [Google Scholar] [CrossRef]
- Wang, K.; Du, Y.; Sun, W.; Deng, J.; Zhang, Y. Coal Secondary Oxidation Thermodynamic Behaviors Affected by Pre-Heating at Different Oxygen Concentrations. Combust. Sci. Technol. 2023, 1–13. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.; Deng, J.; Wen, H.; Xiao, Y.; Jia, H. Research on coal spontaneous combustion period based on pure oxygen adiabatic oxidation experiment. Fuel 2021, 288, 119651. [Google Scholar] [CrossRef]
- Wen, H.; Wang, H.; Liu, W.; Cheng, X. Comparative study of experimental testing methods for characterization parameters of coal spontaneous combustion. Fuel 2020, 275, 117880. [Google Scholar] [CrossRef]
- Dong, X.; Wen, Z.; Wang, F.; Meng, Y. Law of gas production during coal heating oxidation. Int. J. Min. Sci. Technol. 2019, 29, 617–620. [Google Scholar] [CrossRef]
- Ren, L.; Yu, X.; Li, Q.; Tao, F.; Weng, T.F.; Zhai, X.W.; Ma, T. Thermodynamic characteristics of weakly caking coal oxidation and variation law of gaseous products in low oxygen concentration environment. Case Stud. Therm. Eng. 2024, 62, 10171. [Google Scholar] [CrossRef]
- Sun, L.; Han, H.; Cheng, W.; Wang, H.; Shi, Q.; Qi, G.; Guo, Z. Exploring the factors influencing the low temperature CO gas production characteristics of low-metamorphic coal. Fuel 2024, 359, 13027. [Google Scholar] [CrossRef]
- Yan, H.; Nie, B.; Liu, P.; Chen, Z.; Yin, F.; Gong, J.; Lin, S.; Wang, X.; Kong, F.; Hou, Y. Experimental assessment of multi-parameter index gas correlation and prediction system for coal spontaneous combustion. Combust. Flame 2023, 247, 112485. [Google Scholar] [CrossRef]
- Mishra, D.P. Effects of intrinsic properties, particle size and specific surface area on WOP and spontaneous combustion susceptibility of coal. Adv. Powder Technol. 2022, 33, 103454. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Zhang, Y.; Deng, J. An approach for evaluation of grading forecasting index of coal spontaneous combustion by temperature-programmed analysis. Environ. Sci. Pollut. Res. 2023, 30, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Küçük, A.; Kadıoğlu, Y.; Gülaboğlu, M.Ş. A study of spontaneous combustion characteristics of a Turkish lignite: Particle size, moisture of coal, humidity of air. Combust. Flame 2003, 133, 255–261. [Google Scholar] [CrossRef]
- Singh, A.; Singh, R.; Singh, M.; Chandra, H.; Shukla, N.K. Mine fire gas indices and their application to Indian underground coal mine fires. Int. J. Coal Geol. 2007, 69, 192–204. [Google Scholar] [CrossRef]
- Song, J.; Deng, J.; Zhao, J.; Zhang, Y.; Wang, C.; Shu, C.M. Critical particle size analysis of gas emission under high-temperature oxidation of weathered coal. Energy 2021, 214, 118995. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Deng, J.; Zhao, J.Y.; Li, H.T.; Yang, H. Influence of granularity on thermal behaviour in the process of lignite spontaneous combustion. J. Therm. Anal. Calorim. 2019, 135, 2247–2255. [Google Scholar] [CrossRef]
- Ren, L.; Li, Q.; Xiao, Y.; Hao, J.C.; Yi, X.; Zou, L.; Li, Z.B. Critical parameters and risk evaluation index for spontaneous combustion of coal powder in high-temperature environment. Case Stud. Therm. Eng. 2022, 38, 102331. [Google Scholar] [CrossRef]
- Yan, H.; Nie, B.; Kong, F.; Liu, Y.; Liu, P.; Wang, Y.; Chen, Z.; Yin, F.; Gong, J.; Lin, S.; et al. Experimental investigation of coal particle size on the kinetic properties of coal oxidation and spontaneous combustion limit parameters. Energy 2023, 270, 126890. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, W.; Lu, W. Risk evaluation of coal spontaneous combustion from the statistical characteristics of index gases. Thermochim. Acta 2022, 715, 179287. [Google Scholar]
- Chen, X.; Bi, R.; Huang, J.; Shan, W.; Xiao, J.; Wang, D. Experimental study on early prediction index gas for spontaneous combustion. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 46, 7003–7017. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).