Explosive Characteristics Analysis of Gasoline–Air Mixtures within Horizontal Oil Tanks
Abstract
1. Introduction
2. Experimental Configuration
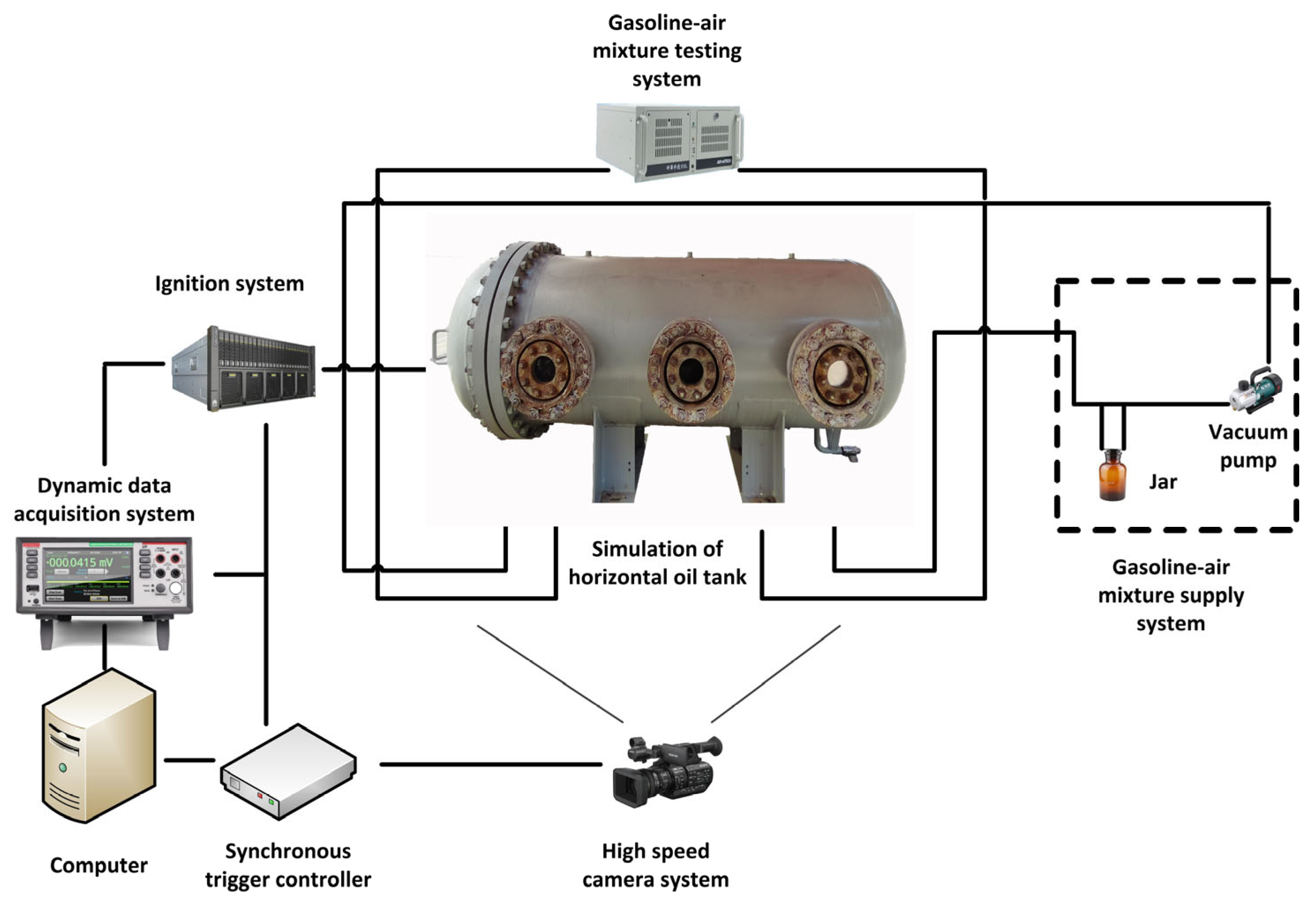
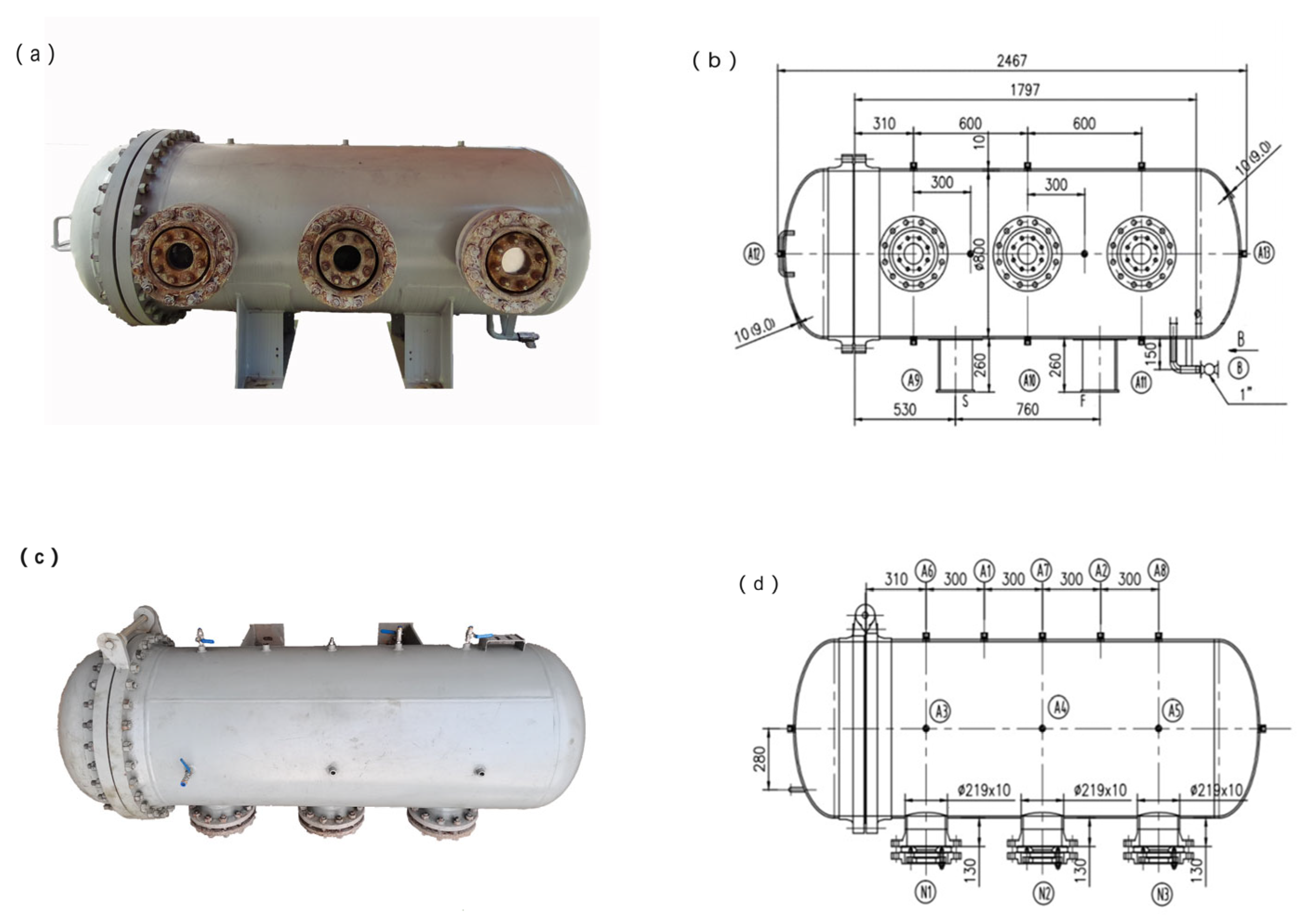
2.1. Experimental System
2.2. Experimental Conditions
3. Explosion Overpressure Analysis in a Horizontal Oil Tank When Ignition Occurs at the Top Position
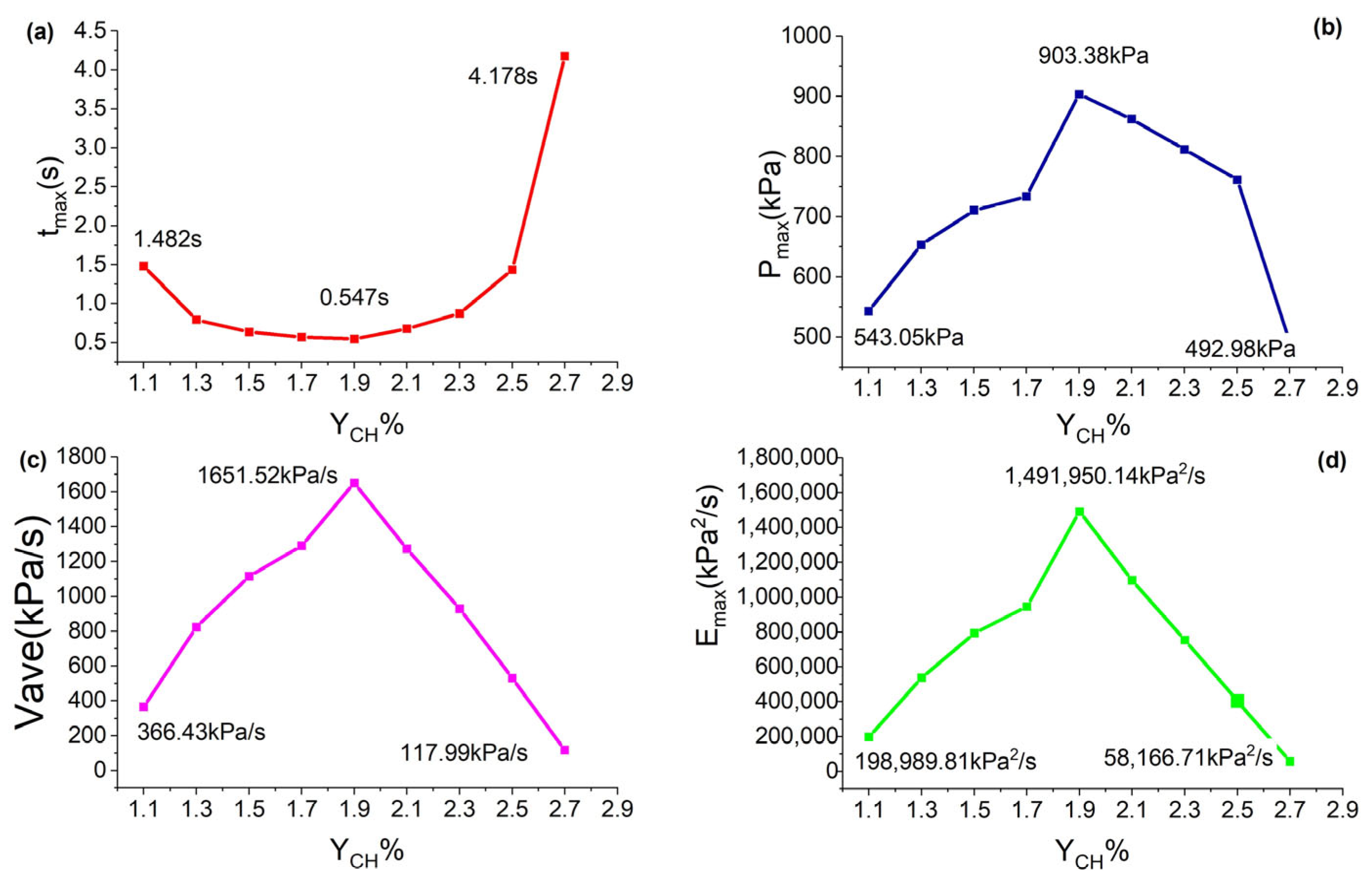
3.1. Effect of Different Initial Gasoline–Air Mixture Volume Fractions of Hydrocarbons on the Overpressure Characteristics of Explosions
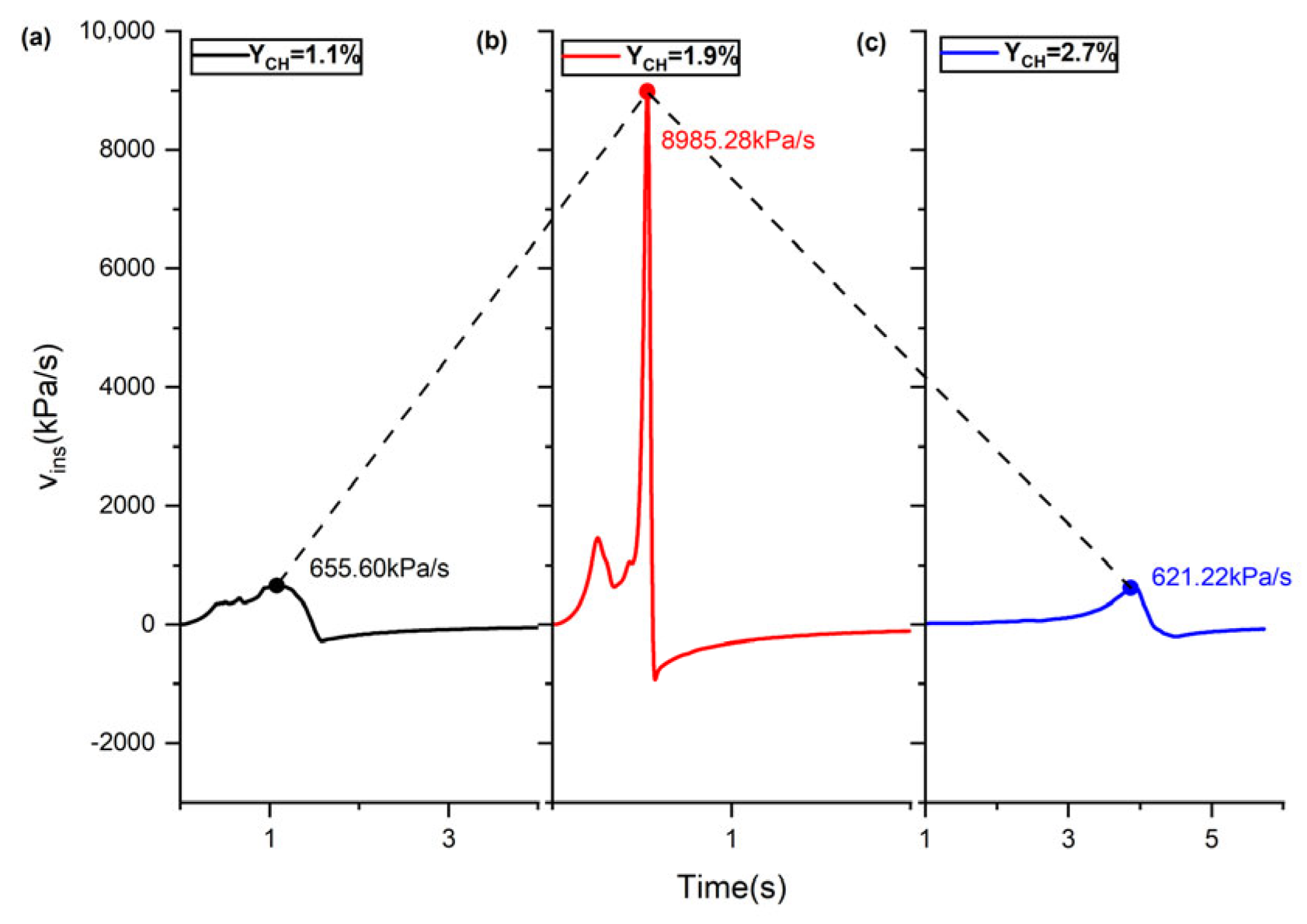
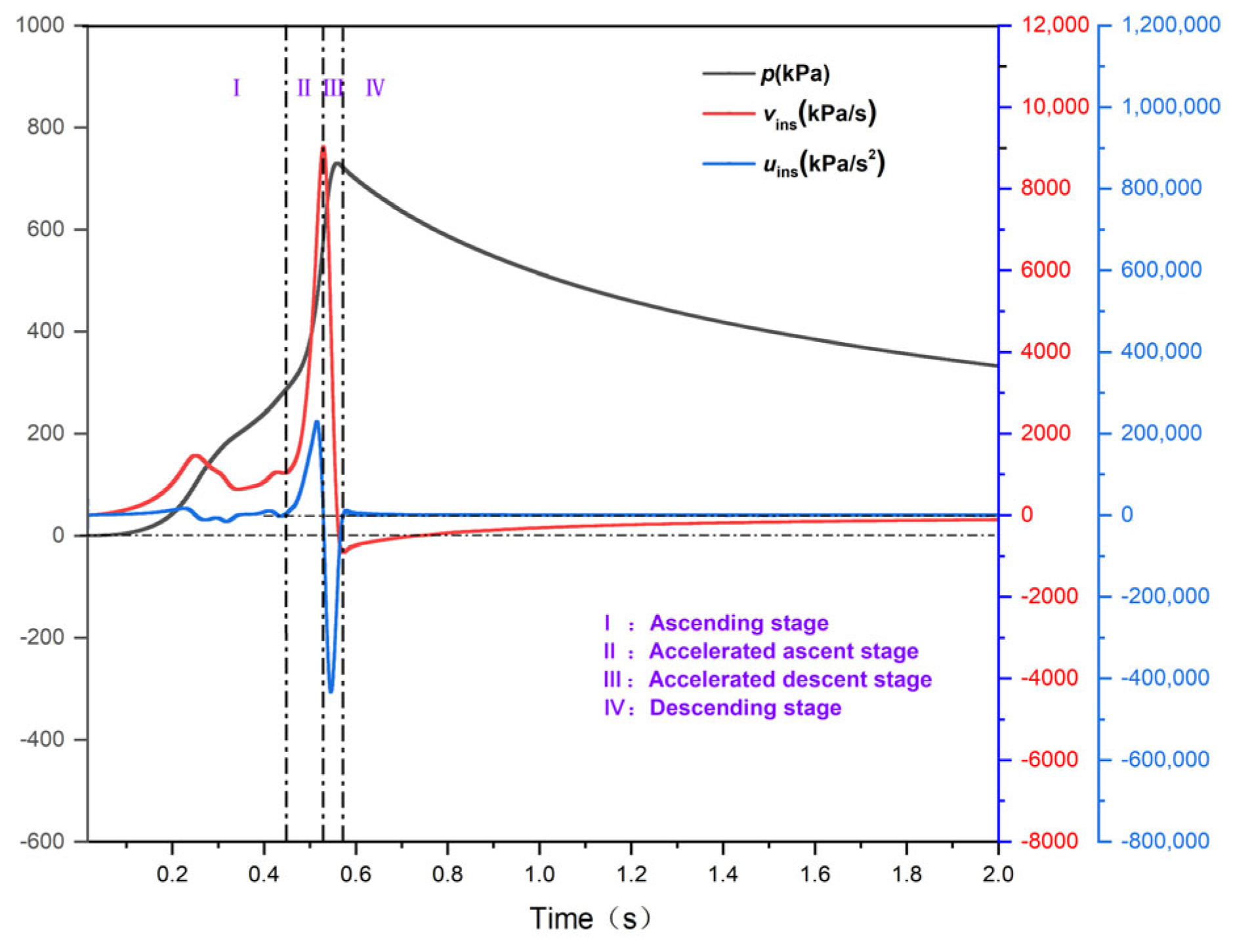
3.2. Analysis of the Explosion Overpressure Variation Process
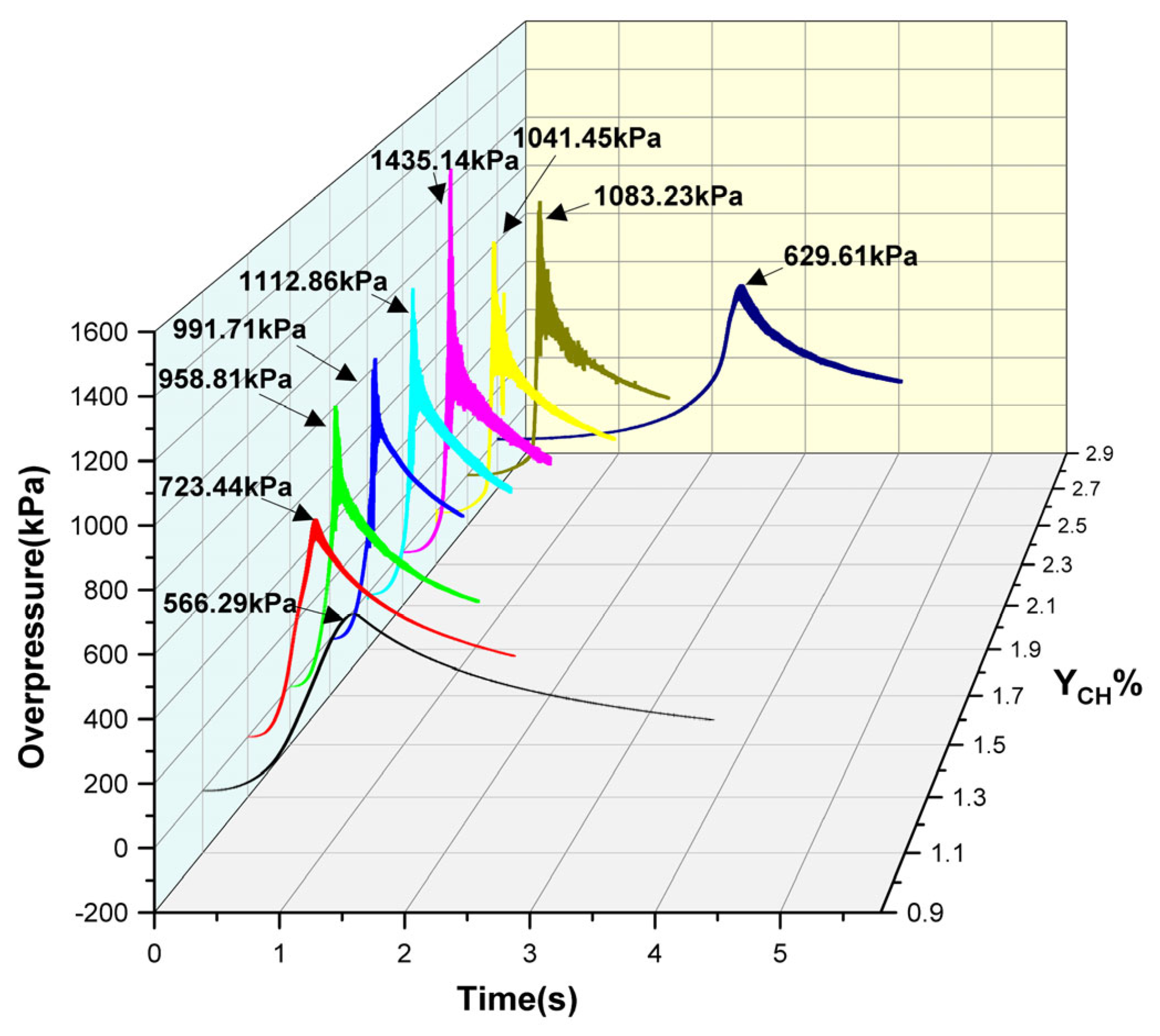
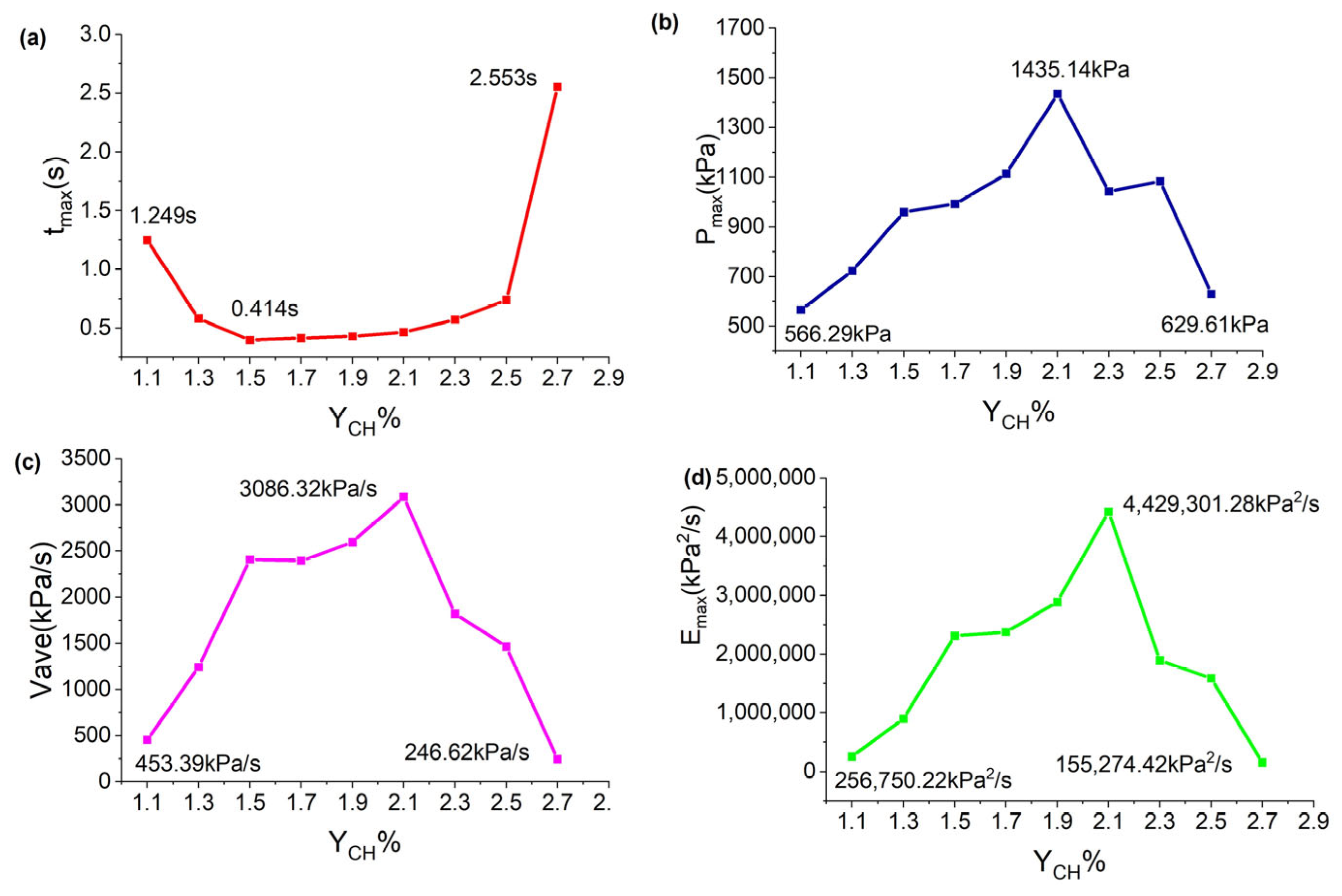
4. Explosion Overpressure Process and Characteristic Parameters for Different Initial Gasoline–Air Mixture Volume Fractions with Mid-Position Ignition
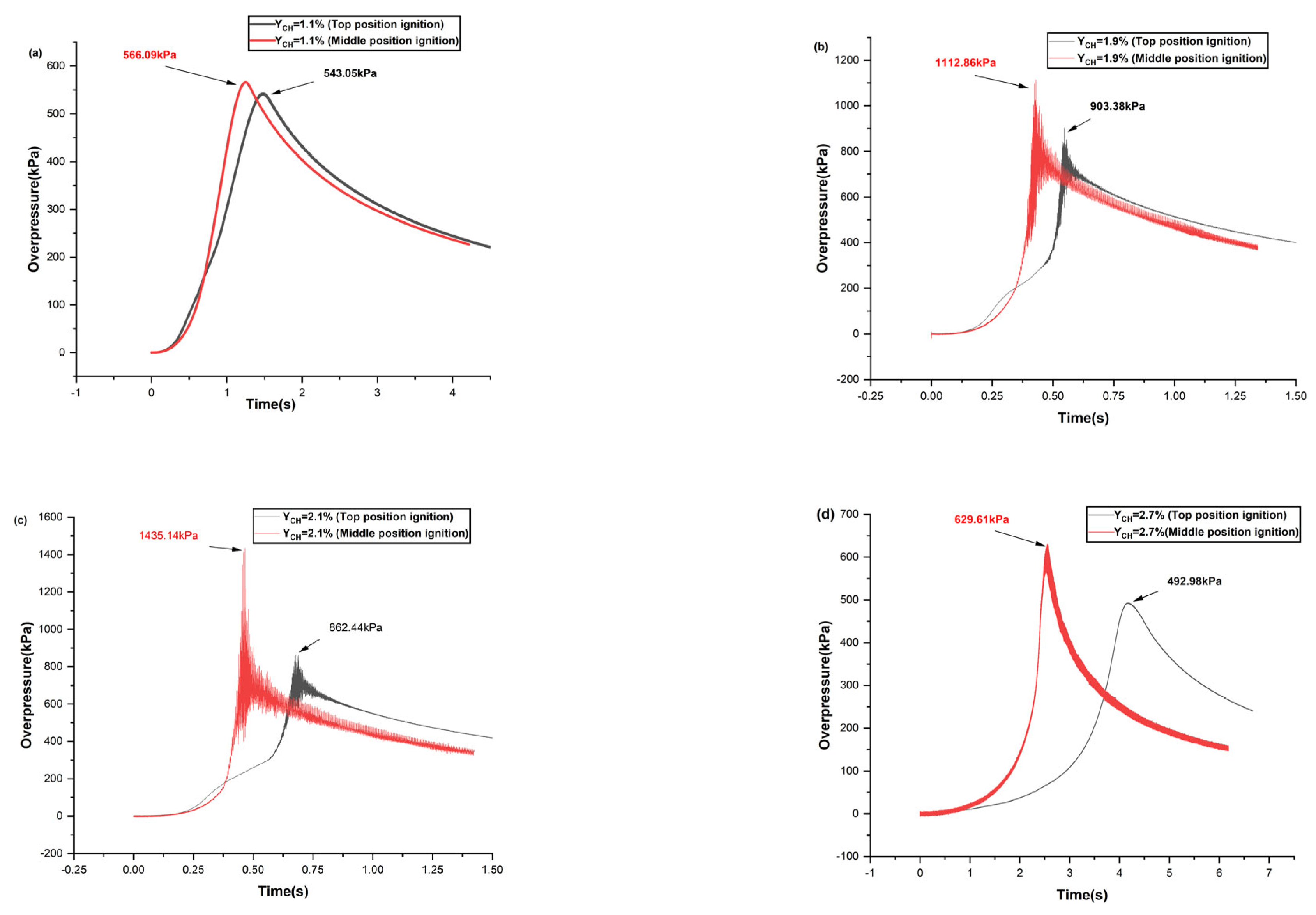
5. Comparison of Ignition-Induced Explosive Overpressure Characteristics between Top and Middle Positions
6. Analysis of Typical Flame Morphology
7. Conclusions
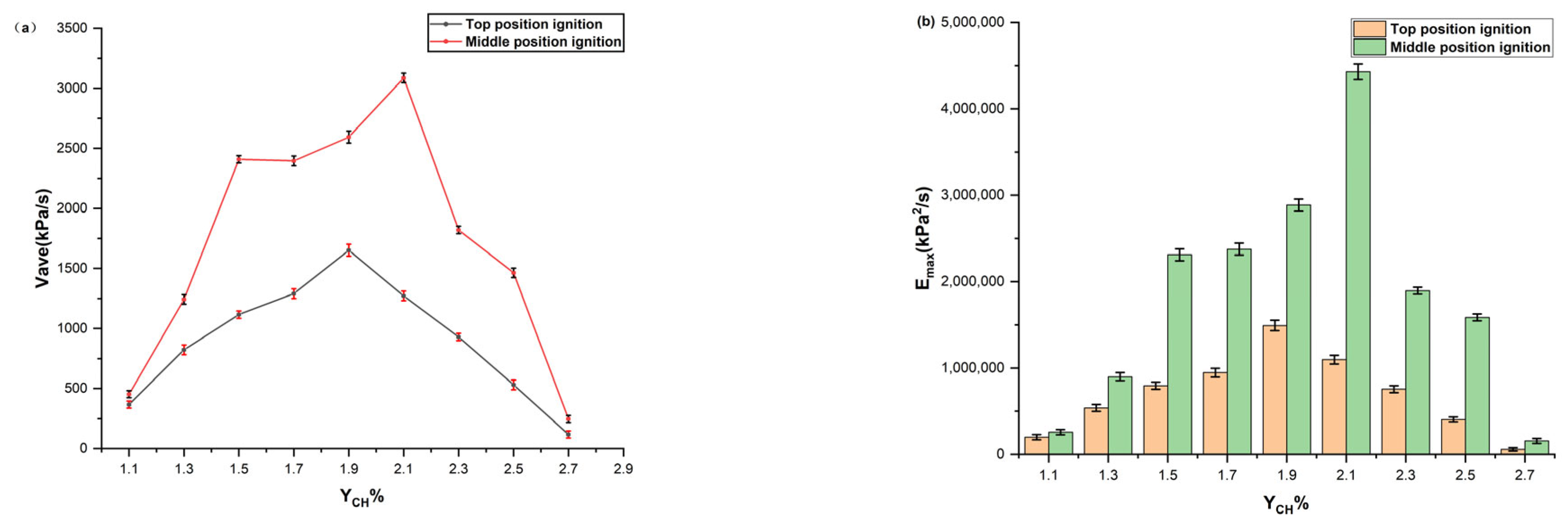
- With an increase in the initial gasoline–air mixture volume fraction, the peak overpressure increased and later decreased. The development of internal overpressure exhibits four stages: smooth rise, accelerated rise, fast transitions, and decreasing attenuation. When ignited at the top position, the most dangerous gasoline–air mixture volume fraction was 1.9%, with a maximum overpressure of 903.38 kPa. When the gasoline–air mixture ignited at the middle position, the most dangerous gasoline–air mixture volume fraction was 2.1%, with a maximum overpressure of 1435.14 kPa. In practical engineering, efforts must be made to avoid reaching gasoline–air mixture volume fractions near the aforementioned values.
- Different ignition positions exhibit similar trends in the variation in the explosion overpressure characteristic parameters. However, when YCH was equal, the values of , (dp/dt)max, and for ignition at the middle position were greater than those for ignition at the top position. Hence, ignition at the middle position results in a more powerful explosion, with greater destructive force at the top position.
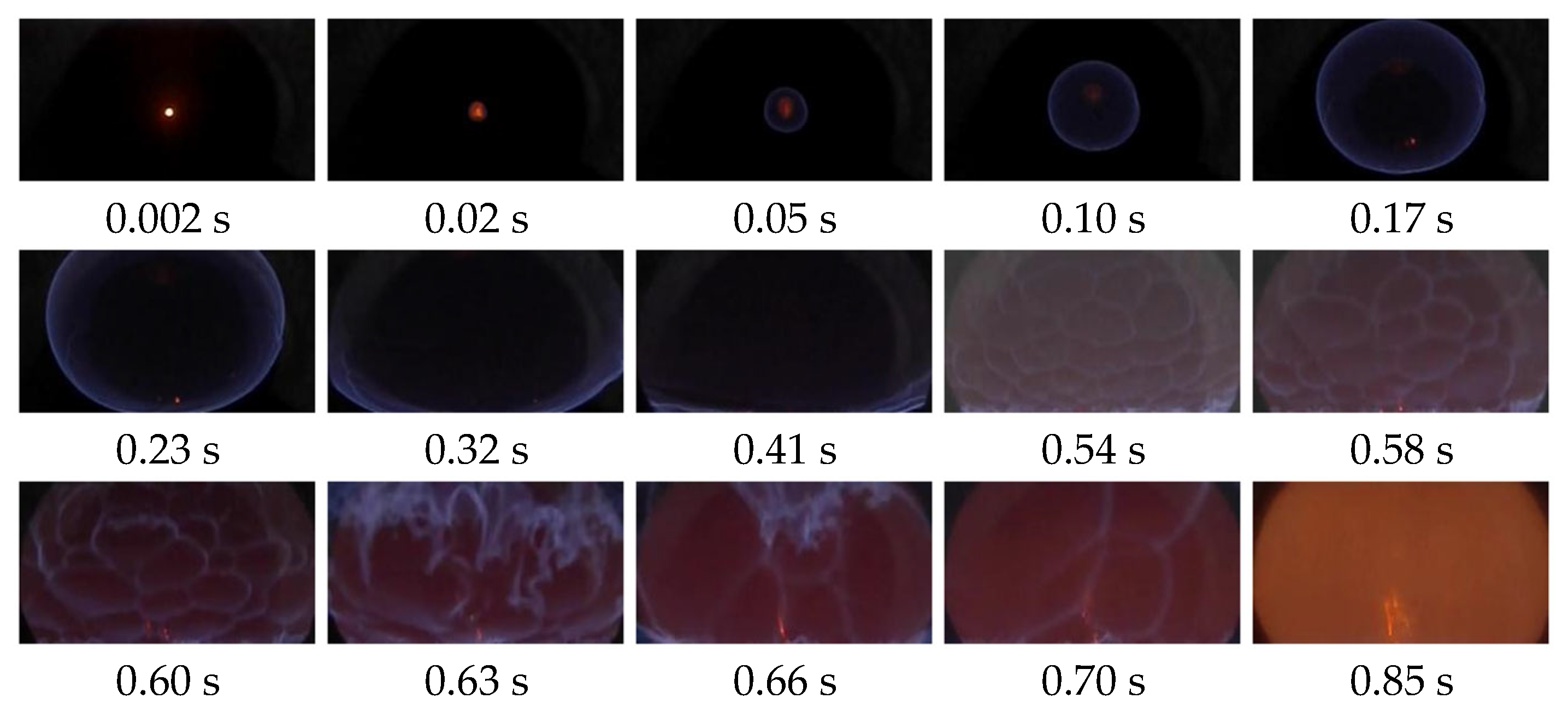
- Different initial gasoline–air mixture volume fractions and ignition positions result in distinct flame characteristics. When YCH = 1.9%, i.e., closer to the stoichiometric concentration, the laminar flame speed was faster than when YCH = 1.1%. The flame image exhibited more pronounced folds, thereby shortening the duration. At YCH = 2.7%, wherein the equivalent volume fraction is larger, the flame propagation is relatively slow owing to the rapid heat release, resulting in an irregular flame front.
- The experimental research in this paper was conducted within a horizontal oil tank. The results of the study not only enriched various experimental setups but also deepened our understanding of the explosive characteristics of gasoline–air mixtures. Due to various constraints in the laboratory, we did not conduct a study with a prototype horizontal oil tank and numerical simulation. In the future, when conditions permit, we will conduct experiments and numerical simulation studies on a prototype experimental test stand.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| YCH | Initial gasoline–air mixture volume fraction, |
| Maximum overpressure peak | |
| Time to reach maximum overpressure peak | |
| Average pressure boost rate | |
| Explosion power index | |
| Instantaneous pressure rise rate | |
| Maximum overpressure rise rates | |
| First-order derivative of the instantaneous pressure rise rate |
References
- Wang, S.M. Experimental Research and Numerical Simulation on the Evolution Characteristics of Oil and Gas Explosions in Confined Spaces with Weak Intensity Surface. Ph.D. Thesis, Army Logistics Academy, Chongqing, China, 2018. [Google Scholar]
- Guo, W.H.; Li, H. Prevention of Major Hazardous Fire and Explosion Accidents in Petrochemical Enterprises. Fire Sci. Technol. 2016, 35, 1335–1337. [Google Scholar]
- Yu, Z.B.; Lu, Y.L. Analysis of the Causes of Flash Explosion Accidents in Tanker Trucks and Countermeasures. Pet. Petrochem. Saf. Technol. 2005, 21, 12–13. [Google Scholar]
- Wei, S.H.; Du, Y.; Wang, S.M.; Li, M. Experimental Study on Oil and Gas Explosion Characteristics in Different Shaped Confined Spaces. China Saf. Sci. J. 2017, 13, 41–47. [Google Scholar]
- Zhang, P.L.; Wang, J.; Wang, D. Explosions of gasoline vapor/air mixture in closed vessels with different shapes and sizes. J. Loss Prev. Process Ind. 2018, 57, 327–334. [Google Scholar] [CrossRef]
- Cai, Y.X.; Jiang, X.S.; Wang, S.M.; Yu, B.B.; Wang, C.H.; Wang, Z.T.; Li, Y.X. Experimental Study on Oil and Gas Explosion in Simulated Vertical Domed Oil Tanks. Explos. Impact 2022, 42, 152–168. [Google Scholar]
- Huang, Y.; Xi, G.C.; Yang, J.; Xu, N.; Hao, Y.M. Research on the Flame Spread Law of Methane Pipeline Leakage and Explosion. Safety 2022, 43, 62–68. [Google Scholar] [CrossRef]
- Yu, J.L.; Kang, S.L.; Yan, X.Q.; Zhan, X.B. Study on Methane Explosion and Venting Characteristics in Pressurized Vessels. J. Saf. Environ. 2023, 1–9. [Google Scholar] [CrossRef]
- Hu, F.F.; Jia, Y.; Wang, W.T.; Jiang, B.Y.; Li, S.Z.; Cheng, Y.F. Study on the Combustion and Explosion Characteristics of Acetylene/Air Premixed Gas in Confined Spaces. Pyrotechnics 2022, 6, 50–55. [Google Scholar]
- Li, D.; Dai, S.J.; Zheng, H.W.; Cui, Z.Y. Research on the Maximum Explosion Pressure and Maximum Rate of Pressure Rise of Premixed Gas in Flameproof Enclosures. Coal Mine Saf. 2023, 54, 216–221. [Google Scholar] [CrossRef]
- Cao, W.G.; Huang, L.Y.; Liang, J.Y.; Miao, N.; Rao, G.N.; Pan, F. Study on the Explosion Characteristics of Coal Powder in Spherical Closed Containers. J. China Univ. Min. Technol. 2014, 43, 113–119. [Google Scholar] [CrossRef]
- Castellanos, D.; Carreto, V.; Skjold, T. Construction of a 36L dust explosion Apparatus and turbulence flow field comparison with a standard 20 L dust explosion vessel. J. Loss Prev. Process Ind. 2018, 55, 113–123. [Google Scholar] [CrossRef]
- Dong, B.Y.; Zha, Y.X.; Zou, Y.; Yin, P.P. Numerical Simulation and Experimental Study on Methane-Hydrogen-Air Explosion Process in Spherical Pressure Vessel. China Saf. Sci. J. 2023, 19, 157–163. [Google Scholar]
- Jiang, X.S.; Xie, W.; Zhao, Y.D.; Li, J.Y.; Li, J.; Yu, B.B. Experimental Study on Oil and Gas Explosion in Long and Narrow Pipes with Different Length-to-Diameter Ratios. Oil Gas Storage Transp. 2020, 39, 879–884. [Google Scholar]
- Ferrara, G.; Willacy, S.K.; Phylaktou, H.N.; Andrews, G.E.; Di Benedetto, A.; Salzano, E.; Russo, G. Venting of gas explosion through relief ducts: Interaction between internal and external explosions. J. Hazard. Mater. 2007, 155, 358–368. [Google Scholar] [CrossRef]
- Bauwens, C.R.; Chaffee, J.; Dorofeev, S. Effect of Ignition Location, Vent Size, and Obstacles on Vented Explosion Overpressures in Propane—Air Mixtures. Combust. Sci. Technol. 2010, 182, 1915–1932. [Google Scholar] [CrossRef]
- Cao, Y.; Guo, J.; Hu, K.; Xie, L.; Li, B. The effect of ignition location on explosion venting of hydrogen—air mixtures. Shock Waves 2017, 27, 691–697. [Google Scholar] [CrossRef]
- Guo, J.; Sun, X.X.; Rui, S.C.; Cao, Y.; Hu, K.L.; Wang, C.J. Effect of ignition position on vented hydrogen–air explosions. Int. J. Hydrog. Energy 2015, 40, 15780–15788. [Google Scholar] [CrossRef]
- Zhang, P.L.; Du, Y. Nitrogen Non-Premixed Inhibition Experiment for Oil and Gas Explosions. Explos. Impact 2016, 36, 347–348. [Google Scholar]
- Wang, S.M.; Yan, Z.H.; Li, X.D.; Li, G.Q.; Guo, H.; Wu, D.J. The venting explosion process of premixed fuel vapor and air in a half—open vessel: An analysis of the overpressure dynamic process and flame evolution behavior. Fuel 2020, 268, 117385. [Google Scholar] [CrossRef]
- Li, H.Z.; Xiao, H.H. Experimental study on the explosion characteristics of NH3/DME/air mixtures. Fuel 2023, 352, 129069. [Google Scholar] [CrossRef]
- Li, R.Z.; Si, R.J. Influence of Gas Concentration on Explosion Pressure and Pressure Rise Rate. J. Xi’an Univ. Sci. Technol. 2010, 30, 29–33. [Google Scholar] [CrossRef]
- Wang, X.F.; Shi, E.H.; Qi, C.; Yan, X.Q.; Zhang, Z.H.; Yu, J.L. Experimental study on aerosol explosion characteristics and flame propagation behavior of aluminum/ethanol nanofluid fuel. Fuel 2023, 352, 129022. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.B.; Cai, R. Analysis of Accidental Acetone Explosion Effects in a Closed Container and Safety Measures. Chem. Eng. Manag. 2022, 7, 88–91. [Google Scholar] [CrossRef]
- Li, G.Q.; Zheng, K.; Wang, S.M.; Chen, W.Z. Comparative study on explosion characteristics of hydrogen and gasoline vapor in a semi-confined pipe based on large eddy simulation. Fuel 2022, 328, 125334. [Google Scholar] [CrossRef]
- Wang, S.M.; Wu, D.J.; Guo, H.; Li, X.D.; Pu, X.Y.; Yan, Z.H.; Zhang, P.L. Effects of concentration, temperature, ignition energy and relative humidity on the overpressure transients of fuel-air explosion in a medium-scale fuel tank. Fuel 2020, 259, 116265. [Google Scholar] [CrossRef]
- Zhang, P.L.; Du, Y.; Zhou, Y.; Qi, S.; Wu, S.L.; Xu, J.F. Explosions of gasoline—air mixture in the tunnels containing branch configuration. J. Loss Prev. Process Ind. 2013, 26, 1279–1284. [Google Scholar] [CrossRef]
- Qi, S.; Du, Y.; Zhang, P.L.; Li, G.Q.; Zhou, Y.; Wang, B. Effects of concentration, temperature, humidity, and nitrogen inert dilution on the gasoline vapor explosion. J. Hazard. Mater. 2017, 323, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, Y.; Li, G.Q.; Yuan, G.Q.; Wang, S.M. Study on the explosion characteristics of oil and gas in long and narrow confined pipelines. Vib. Impact 2017, 36, 80–85+145. [Google Scholar] [CrossRef]
- Ge, Y.; Ma, H.H.; Wang, L.Q. On the smoothing explosion pressure curves using Savitzky—Golay method. J. Loss Prev. Process Ind. 2022, 80, 104929. [Google Scholar] [CrossRef]
- Jiang, X.S.; Chen, R.; Zhang, P.L.; Cai, Y.X.; Zhou, D.L.; He, D.H.; Qin, X.Z.; Zhu, S.J. Study on Gasoline—Air Mixture Explosion Overpressure Characteristics and Flame Propagation Behaviors in an Annular Cylindrical Confined Space with a Circular Arch. Energies 2023, 16, 6944. [Google Scholar] [CrossRef]
- Jiang, H.P.; Bi, M.S.; Gao, W. Effect of monoammonium phosphate particle size on flame propagation of aluminum dust cloud. J. Loss Prev. Process Ind. 2019, 60, 311–316. [Google Scholar] [CrossRef]
- Shang, S.; Bi, M.S.; Zhang, T.J.; Jiang, H.P.; Zhang, S.L.; Gao, W. Synthesis of green nanomaterial and discussion on its suppression performance and mechanism to aluminum dust explosion. Process Saf. Environ. Prot. 2021, 151, 355–364. [Google Scholar] [CrossRef]





| Initial Conditions | Value of Initial Parameters |
|---|---|
| Gas volume concentration | 1.1%, 1.3%, 1.5%, 1.7%, 1.9%, 2.1%, 2.3%, 2.5%, 2.7% |
| Ignition voltage | 1500 V |
| Ignition energy | 1.5 J |
| Ambient temperature | 294 k |
| Ambient pressure | 1 atm |
| Relative humidity | 65% |
| Experimental repetition times | 3 |
| YCH/% | /kPa | /s | /kPa·s−1 | /kPa2·s−1 |
|---|---|---|---|---|
| 1.1 | 543.05 | 1.482 | 366.43 | 198,989.81 |
| 1.3 | 653.23 | 0.794 | 822.71 | 537,418.85 |
| 1.5 | 710.69 | 0.637 | 1115.68 | 792,902.62 |
| 1.7 | 734.02 | 0.569 | 1290.02 | 946,900.48 |
| 1.9 | 903.38 | 0.547 | 1651.52 | 1,491,950.14 |
| 2.1 | 862.44 | 0.678 | 1272.04 | 1,097,058.18 |
| 2.3 | 811.67 | 0.873 | 929.75 | 754,650.18 |
| 2.5 | 761.47 | 1.435 | 530.64 | 404,066.44 |
| 2.7 | 492.98 | 4.178 | 117.99 | 58,166.71 |
| Volume/L | YCH/% | /kPa | /kPa·s−1 | |
|---|---|---|---|---|
| Present work | 1000 (tank) | 1.90 | 903.38 | 8985.28 |
| Wang et al. [26] | 907.50 (tank) | 1.71 | 844.00 | 23,041.00 |
| Zhang et al. [27] | 244 (straight tunnel) | 1.50 | 870.00 | 8190.00 |
| Zhang et al. [5] | 5190 (cylindrical) | 1.70 | 932.11 | 2540.00 |
| 1150 (cylindrical) | 1.70 | 498.12 | 3520.00 | |
| 280 (rectangular) | 1.70 | 398.75 | 3840.00 | |
| 20 (spherical vessel) | 1.70 | 626.52 | 19,790.00 | |
| Qi et al. [28] | 20 (spherical vessel) | 1.62 | 835.00 | 34,381.00 |
| YCH/% | /kPa | /s | /kPa·s−1 | /kPa2·s−1 |
|---|---|---|---|---|
| 1.1 | 566.29 | 1.249 | 453.39 | 256,750.22 |
| 1.3 | 723.44 | 0.582 | 1243.02 | 899,250.39 |
| 1.5 | 958.81 | 0.398 | 2409.07 | 2,309,840.41 |
| 1.7 | 991.71 | 0.414 | 2395.43 | 2,375,571.89 |
| 1.9 | 1112.86 | 0.429 | 2594.07 | 2,886,836.74 |
| 2.1 | 1435.14 | 0.465 | 3086.32 | 4,429,301.28 |
| 2.3 | 1041.45 | 0.572 | 1820.72 | 1,896,188.84 |
| 2.5 | 1083.23 | 0.740 | 1463.82 | 1,585,653.74 |
| 2.7 | 629.61 | 2.553 | 246.62 | 155,274.42 |
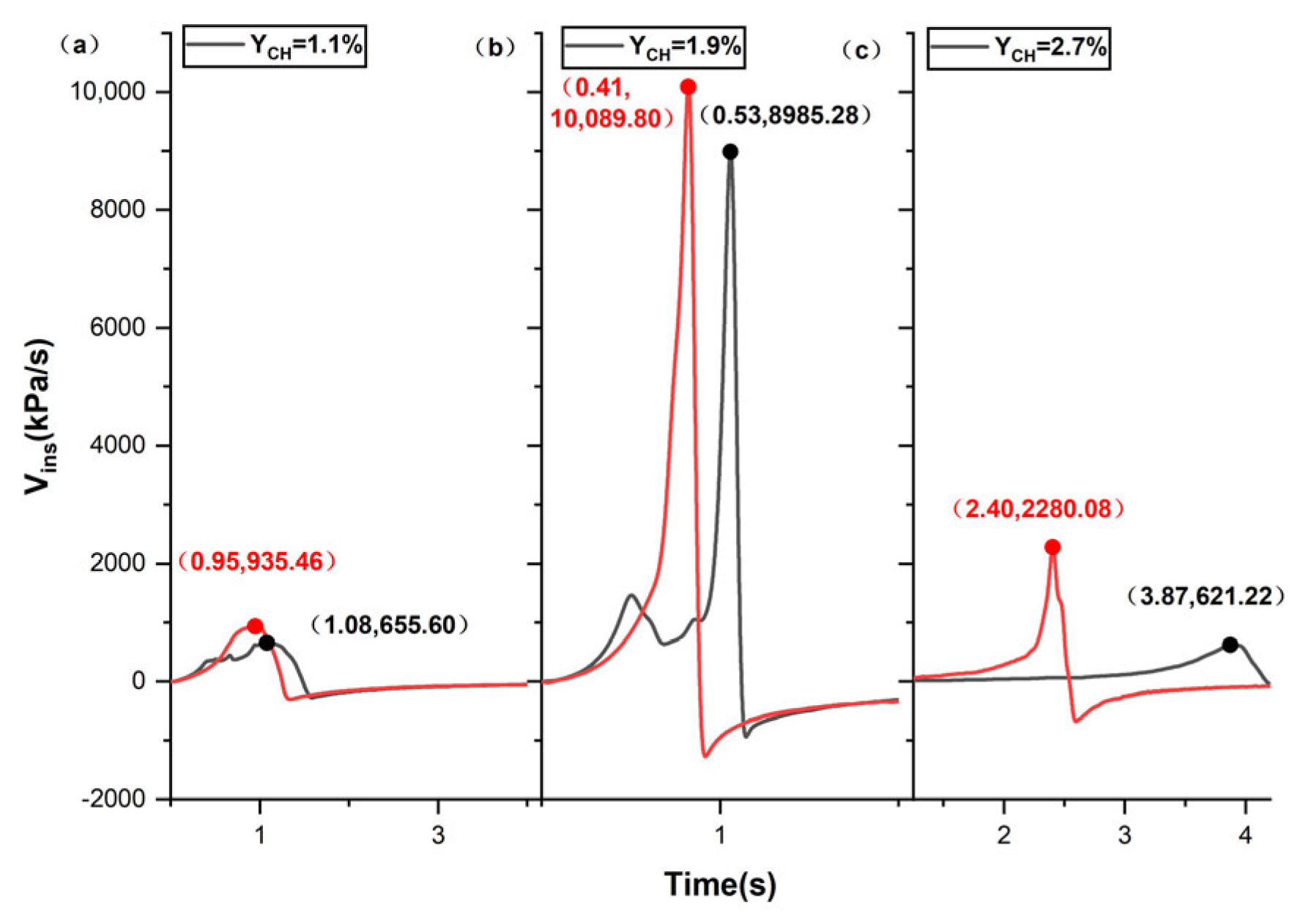
| Ignition Position | YCH/% | Maximum Overpressure Peaks (kPa) | Time to Reach Maximum Overpressure Peaks | Maximum Overpressure Rise Rates (kPa/ s) |
|---|---|---|---|---|
| Top–position ignition | (a) 1.1 | 543.05 | 1.482 | 655.60 |
| (b) 1.9 | 903.38 | 0.547 | 8985.28 | |
| (c) 2.1 | 862.44 | 0.678 | 5671.31 | |
| (d) 2.7 | 492.98 | 4.178 | 621.22 | |
| Middle–position ignition | (a) 1.1 | 566.29 | 1.249 | 935.46 |
| (b) 1.9 | 1112.86 | 0.429 | 10,089.80 | |
| (c) 2.1 | 1435.14 | 0.465 | 9324.29 | |
| (d) 2.7 | 629.61 | 2.553 | 2280.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zhou, D.; Zhang, P.; Cai, Y.; Chen, R.; He, D.; Qin, X.; Lin, K.; Wang, S. Explosive Characteristics Analysis of Gasoline–Air Mixtures within Horizontal Oil Tanks. Fire 2024, 7, 24. https://doi.org/10.3390/fire7010024
Jiang X, Zhou D, Zhang P, Cai Y, Chen R, He D, Qin X, Lin K, Wang S. Explosive Characteristics Analysis of Gasoline–Air Mixtures within Horizontal Oil Tanks. Fire. 2024; 7(1):24. https://doi.org/10.3390/fire7010024
Chicago/Turabian StyleJiang, Xinsheng, Dongliang Zhou, Peili Zhang, Yunxiong Cai, Ri Chen, Donghai He, Xizhuo Qin, Keyu Lin, and Sai Wang. 2024. "Explosive Characteristics Analysis of Gasoline–Air Mixtures within Horizontal Oil Tanks" Fire 7, no. 1: 24. https://doi.org/10.3390/fire7010024
APA StyleJiang, X., Zhou, D., Zhang, P., Cai, Y., Chen, R., He, D., Qin, X., Lin, K., & Wang, S. (2024). Explosive Characteristics Analysis of Gasoline–Air Mixtures within Horizontal Oil Tanks. Fire, 7(1), 24. https://doi.org/10.3390/fire7010024





