Abstract
Prescribed fire is a management tool that is frequently used to foster biodiversity. Simultaneously, insects that provide essential ecosystem services are globally declining. Within the pyroentomology literature, there are mixed reports of positive and negative effects that prescribed fires have on insect communities. This is likely due to not accounting for fire heterogeneity created by fire severity. To better understand prescribed fire severity effects on insect communities, we used multispectral reflectance data collected by Sentinel-2 to methodically quantify prescribed fire severity and compared ground beetle (Coleoptera: Carabidae) taxonomic and functional community composition responses between an unburned site and two burned sites with contrasting fire impacts. We found 23 ground beetle species and used 30 morphological, physiological, phenological, and ecological functional traits for each species. We found that our moderate fire severity site had different taxonomic and functional community compositions from both our unburned and high-severity sites. Surprisingly, we did not find a strong difference in taxonomic or functional ground beetle composition between our unburned and high-severity sites. Our results encourage future pyroentomology studies to account for fire severity, which will help guide conservation managers to make more accurate decisions and predictions about prescribed fire effects on insect biodiversity.
1. Introduction
Biodiversity loss is one of the leading threats to ecosystem function and human society, as both are dependent on the unique, complex, and irreplaceable interactions of individual species and their abundances to their surrounding environment [1,2,3,4]. Historically, biodiversity was fostered by natural wildfire regimes creating early successional habitats and spatio-temporal heterogeneity (i.e., patchiness), particularly in fire-adapted ecosystems [5]. Currently, land managers are attempting to mimic historic fire regimes via prescribed fire to conserve the natural resources that they are responsible for [6,7,8]. Even though there is evidence that reintroducing prescribed fires back into our landscapes can benefit biodiversity for certain animal and plant taxa, there can still be detrimental effects on richness, abundance, and/or community composition [5,9,10,11]. Thus, it is vital to study post-prescription fire effects on biodiversity to continually help guide and strengthen future prescribed fire implementation and management plans for ecological and conservation objectives.
Insects are the most biodiverse animal group in the world and provide essential ecosystem services such as pollination, decomposition, nutrient cycling, and pest control [12,13,14,15]. However, there are conflicting reports of positive and negative results regarding their interaction with wildland fire in both the taxonomic and functional pyroentomology literature [10,16]. Taxonomically, some prescribed fires have been shown to increase insect biodiversity by creating new, open habitats that increase bare ground for nesting insects, decreasing competition for early successional species (e.g., resource partitioning), and attracting obligatory and facultative pyrophilic and saproxylic species that rely on dead and decaying wood [17,18,19,20,21]. In contrast, other prescribed fires have been shown to cause declines in insect biodiversity through direct mortality, elimination of leaf litter, floral resources, and host plants, and by reducing the availability of soil moisture, which many species rely on [16,22,23,24,25,26,27]. Functionally, larger-sized insects were found to be indicative of the post-fire environment [28,29]. However, Glasier et al. 2015 [30] and Lazarina et al. 2016 [31] found that larger-bodied insects declined after fires. Despite evidence indicating that body size is a successful and useful morphological effect-and-response trait indicative of niche filtering, temperature relationships, and mobility across and within insect taxa [32,33], the conflicting pyroentomology results suggest that more detail is missing within these studies.
The conflicting results may be partially attributed to ignoring the variable effects of fire severity [10]; fire severity is the degree of loss of organic matter from before and after the fire [34]. Out of 100 pyroentomology studies on bees (Apoidea), butterflies (Rhopalocera), and ground beetles (Carabidae), only 7% of the studies methodically quantified fire severity [10]. Fires are not all the same due to their heterogeneity and complexity [5]; thus, sampling different fire severity sites within the same fire can provide different results for any fire study. This has been demonstrated with various insect taxa, including true flies (Diptera), beetles (Coleoptera), wasps (Aculeata), sawflies (Symphyta), bees, and soil microarthropods (e.g., Collembola) [35,36,37,38,39,40,41]. Hence, if there can be different results within the same “fire” due to differences in fire severity, it would be expected that comparing results across pyroentomology studies that do not account for fire severity would not be conclusive either.
Specifically for ground beetles (Coleoptera: Carabidae), one of the most species-rich (~40,000 globally) and functionally diverse beetle families in the world [32,42], there are also conflicting taxonomic and functional trait pyroentomology studies. A meta-analysis completed by Mason et al. 2021 [10] summarized most of the taxonomic ground beetle pyroentomology studies (n = 106) but did not look at any functional traits in their analyses. The few studies that quantitatively investigate ground beetle functional traits from fires again reported conflicting results, particularly with wing morphology and diet traits. Samu et al. 2010 [43] showed that macropterous and granivorous species were significantly higher in their wildfire plots than unburned plots. Barber et al. 2017 [44] also supported this by showing that their young burned sites from prescribed fires were typified by macropterous and phytophagous species. In contrast, though, Bargmann et al. 2016 [45] investigated prescribed fires and found that wing morphology had no significant effect and the diet trait that was tolerant of fire was collembola specialists (predators) and not species that fed on “plant matter.” However, none of these studies accounted for fire severity. As far as we know, there are only three ground beetle pyroentomology studies that considered fire severity in their taxonomic approach by examining ground beetle richness, abundance, and/or species composition [36,46,47], and only one study that included a functional trait approach via guild analysis [48], which lumped all ground beetles as predators, despite many being granivores and omnivores [49]. Each of these studies measured the loss of organic matter visually in the field [46,47,48] or visually using aerial photos [36]. Also, each study focused on wildfires. Even though Koivula et al. 2006 [46] did also include prescribed fire, that study concentrated on two ground beetle species instead of an entire ground beetle community.
We build on the four aforementioned severity studies by objectively quantifying fire severity based on satellite imagery for the first time in a ground beetle pyroentomology study. Additionally, our study is novel, not only as the first to focus on prescribed fire severity effects on both ground beetle taxonomic and functional trait community composition but also as the first study to examine fire effects on ground beetle communities in the northern part of the Atlantic Coastal Plain, and the first in the New Jersey Pinelands National Reserve [10].
We predicted that there would be dissimilarities in (1) ground beetle taxonomic community composition and (2) functional trait community composition between our different prescribed fire severity sites and our unburned control site. If our predictions are supported, it further demonstrates the importance and need to methodically quantify fire severity in pyroentomology studies. We also investigated specific functional traits that could explain any differences in our taxonomic and functional community compositions. Lastly, we explored the mechanisms of what could cause any differences between taxonomic and functional community composition between our fire severity and unburned sites by specifically looking at the fire severity itself, year of sampling, and seasonal phenology of the ground beetles. Thus, this exploratory research will allow scientists and conservation managers to better understand the processes that are involved with the complex impact that prescribed fires can have on insect communities and, in this case, on one of the most biodiverse and functionally important beetle families in the world.
2. Materials and Methods
2.1. Study Site and Treatment
This study was conducted at the New Jersey Conservation Foundation’s 4500 hectare Franklin Parker Preserve (FPP) (N39.81496,W-74.54796) located in Woodland Township, Burlington County, NJ, USA. FPP is located within the New Jersey Pinelands National Reserve (PNR), which has an average spring/summer temperature of 18.06 °C, precipitation of 123 mm [50], and is known for its sandy, acidic, nutrient-poor sedimentary soils that are typical of New Jersey’s Atlantic Coastal Plain physiographic province [51]. The upland forests are mostly pine-dominated (Pinus rigida, Miller, 1768 and P. echinata, Miller, 1768) with an understory of shrub oaks (Quercus ilicifolia, Wangenheim, 1787 and Q. marilandica, Munchhausen, 1770) and ericaceous plants (e.g., Gaylussacia baccata (Wangenheim, 1872) and Vaccinium angustifolium Aiton, 1789). These fire-adapted plant communities have been shaped by a historic, frequent wildfire regime with estimated return intervals of 10–30 years before pre-European settlement [52,53,54]. Most importantly though, FPP and the rest of the PNR is the northernmost portion of the North American Coastal Plain, which is considered to be the 36th Global Biodiversity Hotspot due to the high amount of endemic vascular plant species (>1500) and has lost more than 70% of historic vegetation cover [55,56,57]. Additionally, the PNR is a United Nations Educational, Scientific and Cultural Organization (UNESCO) International Biosphere Reserve and World Heritage Site [58]. Unfortunately, little is known about the insect biodiversity in this fire-adapted region [59], particularly insect responses after prescribed fires. The only pyroentomology study published from the PNR was by Buffington 1967 [60], which focused on soil arthropods and whose conclusions were driven by ant (Hymenoptera: Formicidae) responses.
Three prescribed fires were conducted at FPP in upland pine forests by the New Jersey Forest Fire Service (NJFFS) to reduce hazardous fuel loads and lower the wildfire risk in the wildland–urban interface. The site we designated as Eagle Burned (EB) was approximately 342 hectares and was burned on 20 February 2017, and the Main Burned (MB) site was approximately 293 hectares and was burned on 6 March 2017. The third, smaller burn (PPN) was a portion of MB and was the focus of additional fire effect surveys to calibrate a satellite-based fire effects index for the entirety of all the burned areas (Figure 1) [61,62,63]. All prescribed burns were conducted under similar dormant season conditions and are described in Supplementary Data S1. These prescribed burn conditions in the PNR generally occur during relatively cool air temperatures, low—moderate wind speeds, and low relative humidity conditions (20–39%) [64].
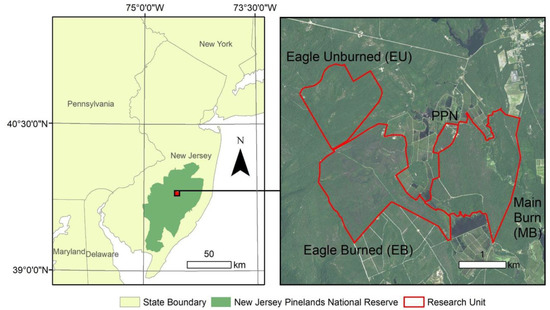
Figure 1.
Map showing the border of the New Jersey Pinelands National Reserve, along with our insect study sites that are outlined in red: Eagled Unburned (control), Eagled Burned, and Main Burned. The PPN was a portion of Main Burned to calibrate a satellite-based fire severity effects index for the entirety of all the burned areas.
Beyond reducing fuels, increasing structural and compositional heterogeneity of the treated land was an important secondary objective of these burns, with the intended response of increasing native biodiversity. To accomplish this goal, a mix of heading, flanking, and backing fire ignitions were used to achieve a range of fire intensities that involved the tree canopy to produce a mix of low, moderate, and high-severity impacts. Lastly, a fire-excluded control site, Eagle Unburned (EU), was maintained immediately north of EB and was approximately 166 hectares in area (Figure 1). All our study sites were not managed with fire or other means following a stand replacing wildfire in the spring of 1954 [61,62].
2.2. Fire Severity Metrics
We used remote sensing to estimate fire severity across our study area to objectively identify areas of high fire impacts for sampling. Near-infrared (NIR) and shortwave infrared (SWIR) reflectance data of forests collected from satellite platforms are frequently used to estimate burn severity in terms of an index known as the differenced normalized burn ratio (dNB) [65,66]. This is possible because NIR and SWIR are highly sensitive to changes in chlorophyll content, leaf area, moisture conditions, and the presence of char in an environment [66]. This approach is commonly used around the world for monitoring fire effects and has proven useful in the PNR when comparing field observations with NIR and SWIR data from Landsat 5/7 [67] and Worldview-3 [66]. Due to data availability issues with the previously used sensors, we estimated burn severity for the study at hand using NIR and SWIR data from Sentinel-2, which we calibrated using the Composite Burn Index (CBI) field method, which is a standard method of collecting burn severity observational data in the field. This method involves visually observing and ranking the severity of fire effects within specific forest strata based on a common rubric. Following standard procedures for this method, CBI was observed within 2 weeks of burning. Observations were collected in a grid of 50 plots at PPS and a grid of 44 plots at PPN (Supplementary Data S2). Plots had a radius of 10 m and were 24–40 m apart at their edges. Sentinel-2 NIR and SWIR data were obtained from the Copernicus database and reflected the period of similar phenology before burns and within 2 weeks after burns to provide pre- and post-burn data required to calculate dNBR (Supplementary Data S2). dNBR was calibrated to the CBI data using a regression model, and that calibration was used to classify and provide severity areas based on CBI thresholds within the prescribed fire (Figure 2).
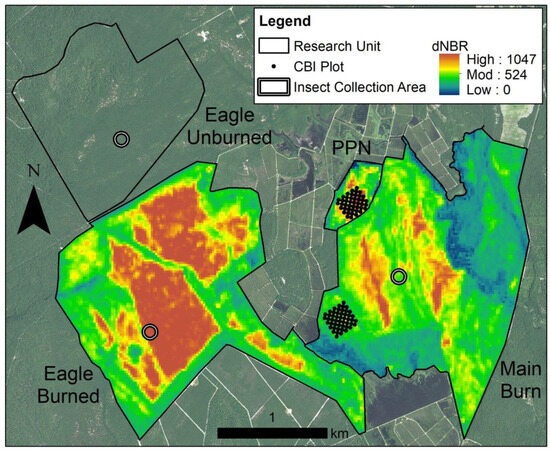
Figure 2.
Prescribed fire severity map in 2017 at Franklin Parker Preserve, Chatsworth, New Jersey. Green indicates lower-severity sites and red indicates higher-severity sites. White circles are pitfall trapping sites. Therefore, Eagle Unburned was our control site, Eagle Burn was our high fire severity site, and Main Burned was our moderate fire severity site.
2.3. Ground Beetle Sampling
Pitfall traps are an effective way to collect surface-active ground beetles and have been successfully used in other pyroentomology studies [36,68,69,70,71]. Therefore, we monitored pitfall traps at each site from April to September in 2017 and 2018. Traps were installed at the center of each site before the prescribed fires to avoid potential unburned edge effects. At each collection site, traps were set up in a diamond design consisting of twelve 532 mL (18 oz) individual red plastic “solo cups” that were 10 m apart from each other. Each cup had an opening diameter of approximately 9 cm and was 12 cm deep. Traps were installed in the ground such that the rim was at surface level and filled halfway with Splash RV & Marine Environmentally Friendly Antifreeze as a safe and effective insect preservative agent that would not readily evaporate [72]. To prevent rain and other debris from getting into the traps, 19 cm diameter plastic plates were propped approximately 15 cm above the traps by three wooden sticks. All pitfall traps were collected every other week and combined at the site level for later sorting and identification in the lab.
Laboratory processing consisted of multiple steps to sort, preserve, and identify the captured specimens. First, samples were strained of the RV antifreeze with a 0.5 mm mesh—a size that retains even the smallest ground beetle specimens. The strained samples were then combined with 70% ethanol for preservation and, later, ground beetle specimens were eventually sorted out, pinned, and curated. We used Ball and Bousquet 2001 [73] to determine each specimen’s genus. Additionally, to determine each specimen’s species, we used Gidaspow 1959 [74] (Calosoma spp.), Van Dyke 1945 [75] (Carabus spp.), Pearson et al. 2006 [76] (Cicindela spp.) Lindroth 1961–1969 [77] (Amara spp., Apenes spp., Cymindis spp., Notiophilus spp., Polyderis spp., Stenolophus spp., Syntomus spp.), Lindroth 1956 [78] (Synuchus spp.), Ball 1959 [79] (Dicaelus spp.), Ball and Nimmo 1983 [80] (Galerita spp.), Bousquet 1996 [81] (Oodes spp.), Liebherr and Will 1996 [82] (Platynus spp.), Messer and Raber 2021 [83] (Selenophorus spp.), and Purrington and Drake 2005 [84] (Pasimachus spp.) Lastly, we used regional taxonomic keys to additionally help with determinations [85,86]. All ground beetle specimens are now housed at the Academy of Natural Sciences in Philadelphia, with representative species in the personal research collection of author Evan S. Waite [87].
2.4. Ground Beetle Traits
We followed [32] Fountain-Jones et al. 2015′s framework, the “M-P-P-E” trait approach, for using functional traits on terrestrial beetles. This includes morphological, physiological, phenological, and ecological trait groups that impact species reproduction, growth, and survival, and that link to ecosystem function [32,88]. This trait approach has been successfully used in recent pyroentomology studies involving ground beetles [44,89]. In total, there were ten specific trait groups we used: wing morphology (brachypterous, macropterous, dimorphic), relative size (small, medium, large) according to their mean body length, diet (predator, omnivore), speed (slow, moderate, fast, based on leg characteristics), breeding season (spring, summer, fall, a combination, unknown), activity time (nocturnal, diurnal, or cathemeral), if species were gregarious (yes, no, a combination, unknown), attracted to light (yes, no, unknown), locomotory behavior (burrower, climber, swimmer, a combination, unknown), and if they were favored by human activities (yes or no) (Supplementary Data S3). We specifically chose these traits because they (1) contribute to ground beetle dispersal, colonization, habitat use, and emergence time [32,45,90,91], which likely would be affected by prescribed fires, and (2) they were traits that have already been documented for ground beetles [49].
2.5. Data Analyses
To examine the effects between our moderate- and high-severity and control sites, we conducted a Permutational Multivariate Analysis of Variance (PERMANOVA), also known as a non-parametric MANOVA [92,93]. We used this analysis because it allows us to determine if fire and, consequently, its severity, had an effect on the overall taxonomic and functional composition of post-fire ground beetle communities. It additionally allows us to explore other effects that can contribute to any potential ground beetle community composition change. This analysis has successfully been used in recent fire studies to analyze differences between communities [94,95,96]. It is important to note that our data were pseudoreplicated due to combining all the individual pitfall traps at the site level. We still proceeded with the above analyses because we felt that it would be pertinent to explore the similarity across the sites with the full recognition that other, non-fire characteristics may have also influenced site-level diversity across the sampling period (e.g., annual phenology). Considering this, we encourage careful interpretation of our results and advocate for further investigation of the effects of fire on ground beetle communities.
To start, we transformed our pitfall trap data into a trap by species matrix (Supplementary Data S4). We then calculated the relative abundance across the matrix by dividing each species’ raw abundance by the total abundance of the trap. We used these relative abundances to standardize our metric of community composition across all traps regardless of the total number of ground beetles captured. For functional trait composition, we calculated a functional composition matrix from our matrix of relative abundances and species-associated trait data using the package “FD” [97,98]. From there, we analyzed the trap data with non-metric multidimensional scaling (NMDS) to create a distance matrix using Bray–Curtis dissimilarity as our distance metric. NMDS has commonly been used to ordinate community composition data to lower-dimensional spaces represented by dissimilarities [93]. This method has also been successfully used in pyroentomology studies to map differences in communities [35,99,100]. Bray–Curtis also prioritizes differences in taxonomic/functional compositions over abundances [101].
After generating a resulting distance matrix, we employed PERMANOVA using the function “adonis” in the package “vegan” with fire severity, year, and trap week as covariates (i.e., predictors), following the form: Taxonomic/Functional Composition Dissimilarity ~ Treatment + Year + Trap Week). From there, we conducted pairwise PERMANOVA tests using our fire severity categories to investigate which trap communities differed. Sites would be considered different if the distance matrix centroids of each treatment were non-overlapping (“Pseudo F-statistic”) [93]. Since we performed multiple pairwise comparisons, which can increase the probability of obtaining falsely significant results [102], we used a Benjamini–Hochberg procedure to adjust our p-values. We used 10,000 permutations across all our analysis scenarios.
Lastly, to further examine how seasonal phenology impacted different ground beetle taxonomic and functional compositions, we utilized the “envfit” function in the package “vegan” to fit an environmental vector to our NMDS space for the trap week at each of our sites. In this case, the trap week was used as the environmental information for the “Envfit” analysis. Additionally, “Envfit” has been successfully used to map the correlation of continuous predictors in relation to site dissimilarity in former fire ecology studies [96,103].
All analyses described above were performed in R v. 4.2.0 [104] using the packages “FD” [97,98], “vegan” [105], and were visualized using the package “ggordiplots” [106]. Code and data are available in the Supplementary Data S5 and as a static repository on FigShare via https://doi.org/10.6084/m9.figshare.21587862.v2 (accessed on 2 August 2023).
3. Results
3.1. Summary Statistics
In total, 447 ground beetle specimens were collected, representing 23 species in 18 genera during 2017 and 2018 across our control and two burned sites (Table 1). For 2017, there were nine collection dates, resulting in 201 specimens total (Control: 112, Mid Severity: 75, and High Severity: 14) and 18 species total (Control: 13, Mid Severity: 8, and High Severity: 6). For 2018, there were 10 collection dates, resulting in 246 specimens total (Control: 122, Mid Severity: 54, and High Severity: 70) and 16 species total (Control: 9, Mid Severity: 9, and High Severity: 10). Pasimachus depressus (Fabricius, 1787) was by far the most common species collected across sites and years, with 254 individuals, which account for 57% of the total ground beetles collected. Polyderis laeva (Say, 1823) and Galerita janus (Fabricius, 1792) were the next most common species, with 67 and 58 specimens, respectively. These three species represent approximately 85% of all the ground beetles that we collected. Interestingly, two of our most common species, Pasimachus depressus and Galerita janus, seemed to have preferences for certain sites. We collected 71% of our P. depressus at the control site, while 86% of our G. janus were collected in the mid-severity site. We collected seven unique species that only occurred at the unburned control site, each with fewer than five individuals recorded. We had similar patterns of unique species at each severity site occurring in low numbers, with three species only at the mid-severity site and four only at the high-severity site (Table 1).

Table 1.
Number of ground beetles (Carabidae) of each species trapped by site and their presence by year.
The 23 species we collected were represented by 10 functional trait groups that included 30 unique traits that have been documented by Larochelle and Lariviere 2003 [49] (Supplementary Data S3). The four trait groups that included “unknown” traits were breeding season, being gregarious, locomotory behavior, and being attracted to light. For wing morphology, 15 species were macropterous, 5 were brachypterous, and 3 were considered dimorphic. The relative size (length) of the species we collected was divided between small (1.5–8.95 mm), medium (9.95–13.25 mm), and large (16–27.5 mm), with seven small, eight medium, and eight large species. All the traits we examined occurred in at least two sites, except for breeding in summer/fall, swimmer, and swimmer/climber, which occurred only at the high, moderate, and control sites, respectively. Overall, the most common traits represented for the ground beetles we collected were predators (19 species), being attracted to light (16 species), and having macropterous wings (15 species) (Supplementary Data S3).
3.2. Taxonomic Community Composition
The best fit for our NMDS was a two-dimensional solution with a stress value of 0.075 (Non-metric R2 = 0.994, Linear R2 = 0.984; Supplementary Data S6). There were significant differences according to our PERMANOVA across fire severity treatments (R2 = 0.146, p < 0.001) (Table 2). Among pairwise comparisons of ground beetle communities, the taxonomic composition was influenced differently between the control and moderate-severity fire treatments (p = 0.001) and the moderate- and high-severity sites (p = 0.009). For specific species, the moderate-severity community was distinctly characterized by higher relative abundances of Galerita janus, Platynus tenuicollis (LeConte, 1846), and Carabus vinctus. (Figure 3a). The pairwise comparison revealed no difference between the control and high-severity taxonomic ground beetle community composition (p = 0.075) (Table 2). However, distinctive species for our high-severity site were Dicaelus elongatus, Cicindela patruela, Cicindela punctulata, and Amara aenea, and distinctive species for our control site were Pasimachus depressus, Scaphinotus sp., Cicindela sexguttata, and Apenes sinuata. (Figure 3a).

Table 2.
Results of an PERMANOVA analysis on the role of treatment, year, and trap-week on both taxonomic and functional community composition across traps. Pairwise comparisons are denoted with their Benjamini–Hochberg corrected p-values. A significant result is indicated by the asterisks by an alpha level of 0.05.
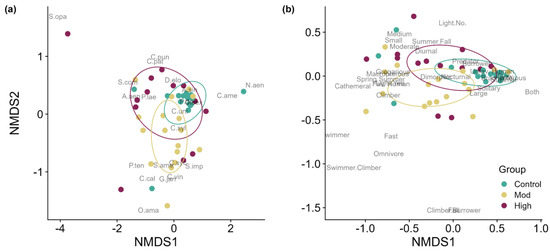
Figure 3.
Non-metric multidimensional scaling output for (a) taxonomic and (b) functional community composition for ground beetle community compositions in our trap data (note that the NMDS axes are not shared across panels). Trap treatments are denoted by color. According to our PERMANOVA analysis (Table 1), significant differences in taxonomy across traps were found between control and mid-severity fire as well as mid-severity and high-severity fire (corrected-p < 0.05). The year of sampling was also a significant delimiter for variance in taxonomic composition (Supplementary Data S8). Differences in functional community composition were found only between control and mid-severity fire (corrected-p < 0.05).
Additionally, our NMDS highlighted taxonomic differences in community composition by sampling year (2017 vs. 2018). This distinction was found to be significant based on the results of our PERMANOVA (R2 = 0.062, p = 0.0011) (Table 2). The distinctive ground beetle species found in 2017 were Galerita janus, Platynus tenuicollis, Syntomus americanus, Synuchus impunctatus, Calosoma sycophanta, and Carabus vinctus. The ground beetle species that were distinctive for 2018 were Polyderis laeva, Dicaelus elongatus, Cincindela patruela, Cicindela punctulata, and Amara aenea (Supplementary Data S8a). As for seasonal phenology (i.e., trap week), there were differences in taxonomic community composition (PERMANOVA R2 = 0.324; p < 0.001) (Table 2). According to our trap week vector fit, these differences appear to be aligned largely along NMDS axis 1 in ordination space (p < 0.001) (Supplementary Data S8c, Table 3).

Table 3.
Results of the fitting of an environmental vector to NMDS space for taxonomic and functional ground beetle community composition. For both taxonomic and functional composition, the trap week vector correlates most strongly with the NMDS1 axis. A significant result is indicated by the asterisks by an alpha level of 0.05.
3.3. Functional Community Composition
The best fit for our NMDS was a two-dimensional solution with a stress value of 0.081 (non-metric R2 = 0.993, linear R2 = 0.976; Supplementary Data S6). There were significant differences according to our PERMANOVA across fire severity treatments (R2 = 0.169, p < 0.001) (Table 2). Among pairwise comparisons of ground beetle communities, the functional trait composition was influenced differently between the control and moderate-severity fire treatments (p = 0.001) (Table 2). Visually, this can be seen since the control community ellipse narrowly overlaps with the moderate-severity community ellipse (Figure 3b). There was also a difference between the moderate- and high-severity community (p = 0.041), with both these community ellipses approximately overlapping by 25%. The pairwise community comparison revealed no difference between the control and high-severity sites (p = 0.07), which can visually be seen with the control community ellipse nearly being encompassed by the high-severity community ellipse (Figure 3b). Additionally, our NMDS did not show any functional differences in community composition by sampling year (2017 vs. 2018) (PERMANOVA R2 = 0.014; p = 0.2489) (Table 2). As for seasonal phenology, there were differences in taxonomic community composition (PERMANOVA R2 = 0.379; p = 0.001). According to our trap week vector fit, these differences appear to be aligned largely along NMDS axis 1 in ordination space (p = 0.001) (Supplementary Data S8d, Table 3).
As for traits that distinctly characterized our field sites, our unburned control community had higher relative abundances of slow and brachypterous ground beetle species that breed during summer and were not favored by human activity (Figure 3b). In contrast, general “fire” traits that appeared between both our fire site ellipses were macropterous, gregarious species that breed in spring and summer and are favored by human activity. Traits that seem to be pulling the high-severity fires ellipse upward and left are diurnal, small, and medium-sized species, suggesting that they are traits associated with only high-severity fires and not moderate severity. The clear trait indicative of moderate-severity fires is being a climber (Figure 3b).
4. Discussion
Our goal was to demonstrate that by methodically quantifying fire severity via satellite imagery and stratifying our sampling across this classification, we would show differences for surface-active ground beetle communities, particularly in the NJ Pinelands National Reserve, the northern portion of the Atlantic Coastal Plain. We predicted that ground beetle taxonomic and functional trait community compositions would be different when comparing control, moderate, and high fire severity sites, and these hypotheses have been supported. Our data show that there were pairwise differences in both ground beetle taxonomic and functional community composition, specifically for our control and moderate-severity fire and moderate- and high-severity fire sites. Even with some taxonomic and functional community composition changes, our results indirectly support the “habitat heterogeneity hypothesis” by showing that different fire severity sites lead to more/higher diversity of ground beetle community compositions [107,108,109]. Additionally, we report on representative species for each of our control and fire severity taxonomic communities along with unique species that did not occur at the other sites (e.g., Syntomus americanus at control; Calosoma calidum at moderate severity; Cicindela patruela at high severity) and functional traits that characterized our study sites (e.g., macropterous and gregarious species that favored human activity characterizing both our fire sites; climbing species at moderate severity; small and medium-sized species at high severity).
4.1. Fire Severity Sites
Our moderate-severity site showed taxonomic and functional differences with both the unburned and the high-severity site. This is not surprising since the direct biotic association between the study species and their functional traits can often explain similar responses when studying both taxonomic and functional approaches [33,110,111]. We mostly attribute the moderate-severity ground beetle community composition to the unique post-fire forest structure. Afterall, the differenced normalized burn ratio (dNBR) accounts for changes in vegetation cover following a disturbance event [112,113]. Unburned pine forests, particularly in the PNR, that have not been burned over a long period of time can create a near-climax and stable community that is homogeneous with established, competitively successful plant and animal species [51,114,115]. Similarly, high-severity fires can completely consume all the forest’s above-ground biomass (i.e., canopy, understory, leaf litter) and expose the mineral soil [35,64,116], leaving another homogeneous community (“structural simplification”) [117] without much niche diversity.
However, moderate-severity fires have the ability to create a unique vegetation community with characteristics similar to both low- and high-severity fires, e.g., consumed litter layer and understory (i.e., high severity) and a canopy that is still mostly intact (i.e., low severity) [34,40,118]. In other words, sites with moderate-severity fires include micro-habitats where species from unburned and high-severity sites can co-exist. Ponisio et al. 2016 [119] found that early and late colonizer species were characteristic of their moderate-severity site, which included a diversity of niches from the soil and canopy structure. Specifically for ground beetles, there are opportunistic species that prefer open (burned) areas, while unburned forests will contain forest specialist species that prefer thicker vegetation and higher humidity [36,120,121,122]. We believe that moderate-severity fire has enough niches for both kinds of ground beetles to coexist, which result in a unique taxonomic and functional community composition, even if it is potentially for the short term. As for our unburned and high fire severity sites that were nearly dissimilar (taxonomically: p = 0.075), we still believe that there would have been differences between them. This is mostly because they are essentially two extremes on opposite ends of a fire severity gradient: a site that has not been burned in approximately 70 years with an established forest canopy, understory, and decades of accumulated leaf litter, and a site that lost all of its vegetation cover and biomass (Supplementary Data S7). Thus, we agree with the “dynamic vegetation hypothesis” in which species’ post-fire responses are driven by vegetation structure [123], which is mostly affected by fire severity.
Distance is another possible contribution to the unique moderate fire severity community. Distance (i.e., scale) is both the solution and issue in ecological studies, especially when considering spatial autocorrelation [124,125]. The analyses we used, NMDS and PERMANOVA, do not inherently account for spatial relationships. The moderate-severity fire is approximately three kilometers away from our control site, while the high-severity site is approximately one-and-a-half kilometers away from the control site (i.e., closer to each other). Even though a one-and-a-half kilometer distance might not be a relatively long distance, there is very little information about how far ground beetles can disperse via flying and/or running [126,127,128]. It is possible that the ground beetles from the unburned site could rapidly recolonize into the high-severity burn site much easier and faster than the moderate-fire severity site. This high resilience is likely reflected in their evolutionary history through functional traits, especially if they occur in fire-adapted ecosystems like the PNR [5,16,33]. Additionally, the ground beetle community at our moderate-severity site might have been different from the start, as suggested by a much higher abundance of Galerita janus. Ideally, having more fire severity and control sites would have helped to give more clarity to the actual fire severity effects on ground beetle communities. Unfortunately, being able to obtain an adequate replication of study sites, especially for landscape-scale events such as fires, is difficult to achieve; hence, treatment effects are often tested at the trap level vs. overall treatment [129,130].
4.2. Dispersal Traits
Traits related to dispersal capabilities were strong indicators of the species that occurred at our study sites. These types of traits can limit a species’ ability to escape fires, resulting in direct or indirect mortality, and can affect the rate at which habitats are recolonized [32,131]. For example, macropterous species were associated at both our burn sites and brachypterous species were found at our unburned site. Our results support Samu et al. 2010 [43] and Barber et al. 2017 [44], who also found that macropterous species were linked to their fire sites. However, our results were likely dependent on the brachypterous Pasimachus depressus since this species represented 57% of the total ground beetles collected, and 71% of Pasimachus depressus specimens were collected at our unburned site. Also, our moderate-severity site was dominated by the macropterous Galerita janus, and our high-severity site had an influx of the also macropterous Polyderis laeva in 2018, but with no specimens collected in 2017. Thus, our data are in agreement with many studies suggesting that the high-dispersal capabilities of macropterous species are early colonizers to the newly burned and disturbed landscapes [16,132,133,134]; Polyderis laeva is a great example of that. Additionally, our data support former studies that suggest that brachypterous species are more frequent in undisturbed habitats and rare in early successional habitats [44,45,132,134]. It is important to note though that the majority of ground beetles we collected were strictly macropterous anyway and only five species were strictly brachypterous, three of which were collected more at our burned sites (i.e., Dicaelus elongatus, Carabus sylvosus, Carabus vinctus). Due to this, our interpretation is that the functional community composition turnover from brachypterous to macropterous traits might not just be an influx of macropterous species, but also the loss of brachypterous species that directly and indirectly perish from fires and cannot rapidly colonize the new burned habitat.
Being attracted to light, body length, and being a climber are other dispersal traits that could be useful predictors for fire sites. Even though being attracted to light represented the moderate-severity site, we suspect this trait could also be a general fire trait and be included with high-severity fires. Insects that are attracted to lights are known to be able to disperse relatively farther distances than species that are not attracted to light [135,136]. Thus, with a more open forest structure at moderate (and high) fire severity sites, light can travel farther distances and essentially attract insects that are attracted to light. It is not surprising that 12 of the 16 species we collected that were known to be attracted to light were also macropterous, reflecting how the two traits are connected and potentially have co-evolved together. Body length has been shown to be a functionally important indicator of a higher dispersal ability for beetles [32,134,137]. For this study, small- and medium-sized individuals were indicative of our high fire severity site. High fire severity sites will have a more open canopy, allowing smaller dispersers to take advantage of the new habitat. Additionally, the absence of larger species of ground beetles can be due to the absence of suitable prey for them, such as lepidopterous caterpillars [132]. Lastly, for moderate-severity fires, the indicative trait was being a climber. For the species that were strictly climbers, eight out of the nine were found at our burned sites. This result was likely influenced by the climber Galerita janus, the only gregarious species that we collected with enough individuals that can support that this species was actually gregarious. Climbing as a functional trait has been understudied in pyroentomology, but there is some evidence of “vertical dispersal” up trees to escape fires [138].
4.3. Non-Dispersal Indicator Traits
Ground beetles that were favored by human activity was another strong indicator of our study sites. Since humans constantly alter natural landscapes via urbanization [139], we felt this trait would be a reliable indicator for general disturbance preference. Our results show that species with preference for human activities are indicative of both our disturbed (burned) sites, while species without this preference were indicative of our undisturbed (unburned) site. The changes that fire disturbances create generally provide new and/or additional resources and preferred habitat preferences to disturbance specialists [16,133]. These resources can be more dead wood that provide shelter, food, and breeding microhabitats, along with a lower humidity and increased temperature that specialist and generalist beetles can readily take advantage of [70,140,141]. In contrast though, non-disturbance species might benefit from a late successional community due to more leaf litter and a closed canopy [142,143,144]. Regardless, it seems that our species pool of ground beetles that are favored by human activity also benefit from fires, suggesting that this functional trait could be used to infer species responses to other types of disturbance (e.g., grazing, thinning). It should be noted though that the ten species we collected that were favored by humans are also all macropterous species. Therefore, it is important to consider and test if disturbance specialist ground beetles are actually using post-fire environment resources or are simply dispersing through.
Our analysis showed two other surface-active ground beetle functional traits that can be indicators of overall “fire” since they occurred in both moderate- and high-severity burn sites. The first trait is being gregarious, which can be beneficial or detrimental from “fires” for ground beetles. As a benefit, this trait could mean quick re-establishment after fires by hiding out in large numbers together in refugia from fires [16,24,145]. As a detriment though, it could mean that populations get locally extirpated if they are all relatively close together and vulnerable to a fire. However, we suspect that this trait might be a byproduct of other traits that directly contribute to our fire sites. As two examples, the gregarious species Galerita janus and Polyderis laeva were two of the three most dominant species we collected throughout 2017 and 2018 and likely influenced our analysis. Specifically, for Galerita janus, 40 individuals were caught at our moderate-severity site in 2017 and only 10 were found at the site in 2018, suggesting that many individuals survived the fire, but cannot survive the post-fire environment. Thus, this species did not re-establish itself in 2018, despite being gregarious. Furthermore, for Polyderis laeva, we collected 0 specimens at our high-severity site and 2 specimens at our moderate-severity site in 2017, but, in 2018, we collected 28 at our high-severity site and 16 at our moderate-severity site. This suggests that Polyderis laeva were more common at our burned sites in 2018, likely because they flew in (being macropterous) and also just so happen to be gregarious.
Spring and summer breeding species was the second trait indicative of our fire sites. In contrast, only summer breeding species and not spring was a trait indicative of our unburned site. However, these traits should be explored further, mostly because 11 out of the 23 species we collected were considered unknown for the breeding season (Supplementary Data S3). Learning what these unknowns are would give us a much better sense of how spring and summer breeders are affected by fires and strengthen or weaken our initial result. Nevertheless, the breeding season pattern is a functional trait that still represents the ground beetle activity period, which fires can greatly influence [132]. With more support from future pyroentomology studies, the timing of prescribed fires could be better guided, particularly when this trait can be associated with uncommon/rare species (e.g., Cicindela patruela, Calosoma calidum for NJ).
For activity periods in beetles, diurnal and nocturnal traits are often linked to sensory mechanisms that are related to eye size and structure [32]. Larger eyes (i.e., more ommatidia and larger surface area) usually indicate diurnal species of more open habitats, while nocturnal species have smaller eyes and are associated with more complex habitats [91,146,147]. Because of these traits, we expected that diurnal species would be indicative of our more open fire sites and nocturnal for the more closed canopies of our unburned sites. Our results do show some signals that this might be supported. As for diet, we could not either support or reject Barber et al. 2017 [44], Bargmann et al. 2016 [45], and Samu et al. 2010 [43] because we had no species that were phytophagous and granivorous. However, predators seem to make up the center of our burned functional compositions, while also being associated with our unburned community, while omnivores could potentially be influencing the moderate-severity composition. In general though, Koltz et al. 2018 [133] note that species with general feeding habits are the most likely to benefit from post-fire resources.
4.4. Predictors (Covariates)
The seasonal phenology of ground beetles plays an important role in contributing to ground beetle community composition. This is often correlated with the activity period of local climate, looking for mates and shelter, and foraging for food [49,117,121]. Thus, it is not a surprise that seasonal phenology explained approximately a third of the variation in our study as a predictor of our ground beetle community composition (taxonomically: 0.324; functionally: 0.379). Even though a third of the variation can be accounted for by phenology, most of the variation remains unaccounted for, and we suspect that the fire severity explains this variation. Due to the pooling of samples, pinpointing fire severity as the cause of this additional variation was difficult to establish, despite us still providing some evidence that fire severity was the main driver. Under a replicated sampling design with independent prescribed fires of varying severity, we anticipate that the fire severity predictor would have a larger effect on our ground beetle taxonomic and functional community composition. Post-fire effects from different fire severities not only affect vegetation structure but also ambient and ground temperatures and soil nitrogen and pH [34,148], which can directly and indirectly affect insect biodiversity and behavior [10,16].
As for surface active ground beetle community composition by year, we found it interesting that the taxonomic composition was different between 2017 and 2018, but the functional composition was not dissimilar. As previously mentioned, functional traits directly associated to specific species are often tied together and similar patterns can emerge when comparing both taxonomic and functional approaches. However, this was not the case when we investigated year as a predictor for ground beetle communities. This result is likely an artifact of our analyses since we combined different ground beetle community compositions together from sites. Regardless, we have indirectly supported that ground beetle functional community composition was not driven by taxonomic community composition when considering temporal variation. We suspect that, in the following years, the taxonomic community composition will get back to being more stable as indicated by the 2018 ellipse being smaller than the 2017 ellipse. The additional residual variation could be explained by a combination of other variables that are not directly associated with fire severity, such as interspecific competition and behavior, phenotypic plasticity, and/or stochastic noise [149,150,151].
5. Conclusions
Wild and prescribed fires often result in heterogeneous mixtures of severity, and subsequent habitats, across the landscape [5,52,152]. In this study, we provide exploratory evidence that shows that ground beetle taxonomic and functional community compositions at moderate fire severities are different from both unburned and high-severity fire sites. Therefore, pyroentomology studies that generalize “fire” could be misleading since their results might be more dependent on the specific fire severity site and/or mix of fire severity sites they sampled. We encourage future pyroentomology studies to account for fire severity, particularly when accounting for ground beetles and other insect taxa. We also report on ground beetle species that respond to different prescribed fire severities and unburned sites along with their potentially universal functional traits that can be used to make better predictions about insect responses in the post-fire environment (e.g., dispersal traits, breeding season, disturbance specialists). This will ultimately help conservation managers to make more informed decisions and accurate predictions about prescribed fire effects on biodiversity in our native landscapes. Based on this research, we see no detrimental effects of high prescribed fire severity in the PNR if the management goal is to increase ground beetle biodiversity in the form of taxonomic and functional community composition.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fire6090366/s1, Supplementary Data S1: Prescribed Burning Conditions Data; Supplementary Data S2: Composite Burn Index Data; Supplementary Data S3: Functional Trait Data; Supplementary Data S4: Trap by Species Matrix; Supplementary Data S5: R analyses; Supplementary Data S6: Stress Plots; Supplementary Data S7: Site Images; Supplementary Data S8: Year and Phenology NMDS.
Author Contributions
Conceptualization, S.C.M.J.; methodology, S.C.M.J., M.R.G., V.S. and E.S.W.; formal analysis, V.S., M.R.G. and S.C.M.J.; investigation, S.C.M.J., V.S., E.S.W., M.R.G. and N.S.S.; resources, S.C.M.J., M.R.G. and N.S.S.; data curation, S.C.M.J., V.S., E.S.W., M.R.G. and N.S.S.; specimen determination: E.S.W. and S.C.M.J.; writing—original draft preparation, S.C.M.J.; writing—review and editing, S.C.M.J., V.S., E.S.W., M.R.G. and N.S.S.; visualization, V.S. and M.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are thankful for funding for this work that was provided by the Joint Fire Science Program (15-1-04-55), the Strategic Environmental Research and Development Program (RC-2641), the Chesapeake (and Delaware) Bay Watershed Research Fund (390105-9557), the New Jersey Department of Environmental Protection Conserve Wildlife Matching Grant (240986-9557), and the Claudio Elia Memorial Fellowship in Environmental Science or Environmental Engineering award in 2017. We graciously thank two donors that contributed to this research who would like to remain anonymous.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Code and data are available in the Supplementary Data S5 and as a static repository on FigShare via https://doi.org/10.6084/m9.figshare.21587862.v2 (accessed on 2 August 2023).
Acknowledgments
We thank New Jersey Forest Fire Service, and specifically fire wardens Tom Gerber, Samuel Moore III, and Shawn Judy, for conducting the prescribed burns and providing expert wisdom that were critical to the development of this study. We also thank Emile DeVito and Russell Juelg of the New Jersey Conservation Foundation for facilitating our use of the Franklin Parker Preserve for our research. We thank Jon Gelhaus, Sean O’Donnell, Marina Potapova, Alain Maasri, and Richard Horwitz for providing direction for this research. We thank Jacquelyne Ng, Madeline Sabo, Marina Jackson, Cameron Chung, and Nathan D. Hunt for helping with the field work and/or sorting pitfall trap samples. We thank Academy of Natural Sciences’ librarians Alexandria Capone and Kelsey Manahan-Phelan for helping to gather the literature, as well as Isabelle Betancourt, Greg Cowper, and Jason Weintraub for helping to manage and curate the pitfall trap collection at the Academy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ Warning to Humanity on Insect Extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- van der Plas, F. Biodiversity and Ecosystem Functioning in Naturally Assembled Communities. Biol. Rev. 2019, 94, 1220–1245. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent Responses to Climate Change Reveal the Drivers of Species Extinction and Survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R. How Does Biodiversity Relate to Ecosystem Functioning in Natural Ecosystems. In Unsolved Problems in Ecology; Princeton University Press: Princeton, NJ, USA, 2020; p. 393. [Google Scholar]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a Key Driver of Earth’s Biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef]
- Arkle, R.S.; Pilliod, D.S. Prescribed Fires as Ecological Surrogates for Wildfires: A Stream and Riparian Perspective. For. Ecol. Manag. 2010, 259, 893–903. [Google Scholar] [CrossRef]
- Knapp, E.E.; Estes, B.L.; Skinner, C.N. Ecological Effects of Prescribed Fire Season: A Literature Review and Synthesis for Managers; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2009; p. PSW-GTR-224.
- Sample, M.; Thode, A.E.; Peterson, C.; Gallagher, M.R.; Flatley, W.; Friggens, M.; Evans, A.; Loehman, R.; Hedwall, S.; Brandt, L.; et al. Adaptation Strategies and Approaches for Managing Fire in a Changing Climate. Climate 2022, 10, 58. [Google Scholar] [CrossRef]
- Eales, J.; Haddaway, N.R.; Bernes, C.; Cooke, S.J.; Jonsson, B.G.; Kouki, J.; Petrokofsky, G. What Is the Effect of Prescribed Burning in Temperate and Boreal Forest on Biodiversity, beyond Tree Regeneration, Pyrophilous and Saproxylic Species? A Systematic Review Protocol. Environ. Evid. 2016, 5, 24. [Google Scholar] [CrossRef]
- Mason, S.C.; Shirey, V.; Ponisio, L.C.; Gelhaus, J.K. Responses from Bees, Butterflies, and Ground Beetles to Different Fire and Site Characteristics: A Global Meta-Analysis. Biol. Conserv. 2021, 261, 109265. [Google Scholar] [CrossRef]
- Valkó, O.; Deák, B.; Magura, T.; Török, P.; Kelemen, A.; Tóth, K.; Horváth, R.; Nagy, D.D.; Debnár, Z.; Zsigrai, G.; et al. Supporting Biodiversity by Prescribed Burning in Grasslands—A Multi-Taxa Approach. Sci. Total Environ. 2016, 572, 1377–1384. [Google Scholar] [CrossRef]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. Ecological Functions and Ecosystem Services Provided by Scarabaeinae Dung Beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Schowalter, T.D. Decomposition and Pedogenesis. In Insect Ecology: An Ecosystem Approach, 4th Edition; Academic Press: Cambridge, MA, USA, 2016; pp. 477–510. [Google Scholar]
- Stewart, A.J.A.; New, T.R.; Lewis, O.T. Insect Conservation Biology; CAB International: Wallingford, UK, 2007; ISBN 978-1-84593-254-1. [Google Scholar]
- Waite, E.S.; Houseman, G.R.; Jensen, W.E.; Reichenborn, M.M.; Jameson, M.L. Ground Beetle (Coleoptera: Carabidae) Responses to Cattle Grazing, Grassland Restoration, and Habitat across a Precipitation Gradient. Insects 2022, 13, 696. [Google Scholar] [CrossRef] [PubMed]
- New, T. Insects, Fire, and Conservation; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Bargmann, T.; Hatteland, B.A.; Grytnes, J.-A. Effects of Prescribed Burning on Carabid Beetle Diversity in Coastal Anthropogenic Heathlands. Biodivers. Conserv. 2015, 24, 2565–2581. [Google Scholar] [CrossRef]
- Campbell, J.W.; Hanula, J.L.; Waldrop, T.A. Effects of Prescribed Fire and Fire Surrogates on Floral Visiting Insects of the Blue Ridge Province in North Carolina. Biol. Conserv. 2007, 134, 393–404. [Google Scholar] [CrossRef]
- Gongalsky, K.; Midtgaard, F.; Overgaard, H. Effects of Prescribed Forest Burning on Carabid Beetles (Coleoptera: Carabidae): A Case Study in South-Eastern Norway. Entomol. Fenn. 2006, 17, 325–333. [Google Scholar] [CrossRef]
- Hyvärinen, E.; Kouki, J.; Martikainen, P. Prescribed Fires and Retention Trees Help to Conserve Beetle Diversity in Managed Boreal Forests despite Their Transient Negative Effects on Some Beetle Groups. Insect Conserv. Divers. 2009, 2, 93–105. [Google Scholar] [CrossRef]
- Moylett, H.; Youngsteadt, E.; Sorenson, C. The Impact of Prescribed Burning on Native Bee Communities (Hymenoptera: Apoidea: Anthophila) in Longleaf Pine Savannas in the North Carolina Sandhills. Environ. Entomol. 2019, 49, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Bohls, P.A.; Nelson, M.T.; Cooper, K.N.; Clark, J.M. Short-Term Effects of a Prescribed Burn on Butterfly Abundance and Diversity in a Restored Northeastern Ohio Prairie. Ohio Biol. Surv. Notes 2016, 6, 7–13. [Google Scholar]
- Brand, R.H. The Effect of Prescribed Burning on Epigeic Springtails (Insecta: Collembola) of Woodland Litter. Am. Midl. Nat. 2002, 148, 383. [Google Scholar] [CrossRef]
- Iglay, R.B.; Miller, D.A.; Leopold, B.D.; Wang, G. Carabid Beetle Response to Prescribed Fire and Herbicide in Intensively Managed, Mid-Rotation Pine Stands in Mississippi. For. Ecol. Manag. 2012, 281, 41–47. [Google Scholar] [CrossRef]
- Tooker, J.F.; Hanks, L.M. Impact of Prescribed Burning on Endophytic Insect Communities of Prairie Perennials (Asteraceae: Silphium Spp.). Biodivers. Conserv. 2004, 13, 1875–1888. [Google Scholar] [CrossRef]
- Underwood, E.C.; Quinn, J.F. Response of Ants and Spiders to Prescribed Fire in Oak Woodlands of California. J. Insect Conserv. 2010, 14, 359–366. [Google Scholar] [CrossRef][Green Version]
- Verble, R.M.; Yanoviak, S.P. Short-Term Effects of Prescribed Burning on Ant (Hymenoptera: Formicidae) Assemblages in Ozark Forests. Ann. Entomol. Soc. Am. 2013, 106, 198–203. [Google Scholar] [CrossRef]
- Arnan, X.; Cerdá, X.; Rodrigo, A.; Retana, J. Response of Ant Functional Composition to Fire. Ecography 2013, 36, 1182–1192. [Google Scholar] [CrossRef]
- Moretti, M.; Legg, C. Combining Plant and Animal Traits to Assess Community Functional Responses to Disturbance. Ecography 2009, 32, 299–309. [Google Scholar] [CrossRef]
- Glasier, J.R.N.; Nielsen, S.E.; Acorn, J.H. The Real “Fire Ants”: Colony Size and Body Size of Workers Influence the Fate of Boreal Sand Hill Ants (Hymenoptera: Formicidae) after Wildfires in Alberta, Canada. Can. Entomol. 2015, 147, 396–404. [Google Scholar] [CrossRef]
- Lazarina, M.; Sgardelis, S.P.; Tscheulin, T.; Kallimanis, A.S.; Devalez, J.; Petanidou, T. Bee Response to Fire Regimes in Mediterranean Pine Forests: The Role of Nesting Preference, Trophic Specialization, and Body Size. Basic Appl. Ecol. 2016, 17, 308–320. [Google Scholar] [CrossRef]
- Fountain-Jones, N.M.; Baker, S.C.; Jordan, G.J. Moving beyond the Guild Concept: Developing a Practical Functional Trait Framework for Terrestrial Beetles. Ecol. Entomol. 2015, 40, 1–13. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Guénard, B.; Lewis, O.T. Trait-based Ecology of Terrestrial Arthropods. Biol. Rev. 2019, 94, 999–1022. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire Intensity, Fire Severity and Burn Severity: A Brief Review and Suggested Usage. Int. J. Wildland Fire 2009, 18, 116. [Google Scholar] [CrossRef]
- Galbraith, S.M.; Cane, J.H.; Moldenke, A.R.; Rivers, J.W. Wild Bee Diversity Increases with Local Fire Severity in a Fire-prone Landscape. Ecosphere 2019, 10, e02668. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Wikars, L.-O.; Persson, T.; Ãîíãàëüñêèé, Ê.Á. Ground Beetle (Coleoptera: Carabidae) Responses to a Forest Wildfire in Northern Europe. Russ. Entomol. J. 2008, 17, 273–282. [Google Scholar]
- Jung, J.-K.; Kim, M.; Nam, Y.; Koh, S.-H. Changes in Spatial and Temporal Distributions of Monochamus Beetles along the Fire Severity in Burned Pinus Densiflora Forests. J. Asia-Pac. Entomol. 2020, 23, 404–410. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, C. Abundance of Soil Microarthropods Associated with Forest Fire Severity in Samcheok, Korea. J. Asia-Pac. Entomol. 2008, 11, 77–81. [Google Scholar] [CrossRef]
- Lazarina, M.; Devalez, J.; Neokosmidis, L.; Sgardelis, S.P.; Kallimanis, A.S.; Tscheulin, T.; Tsalkatis, P.; Kourtidou, M.; Mizerakis, V.; Nakas, G.; et al. Moderate Fire Severity Is Best for the Diversity of Most of the Pollinator Guilds in Mediterranean Pine Forests. Ecology 2019, 100, e02615. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A. The Importance of Measuring Fire Severity—Evidence from Microarthropod Studies. For. Ecol. Manag. 2010, 260, 62–70. [Google Scholar] [CrossRef]
- Simanonok, M.P.; Burkle, L.A. High-Severity Wildfire Limits Available Floral Pollen Quality and Bumble Bee Nutrition Compared to Mixed-Severity Burns. Oecologia 2020, 192, 489–499. [Google Scholar] [CrossRef]
- Johnson, N.; Triplehorn, C.A. Borror and DeLong’s Introduction to the Study of Insects, 7th ed.; Brooks/Cole: Pacific Grove, CA, USA, 2004. [Google Scholar]
- Samu, F.; Kádár, F.; Ónodi, G.; Kertész, M.; Szirányi, A.; Szita, É.; Fetykó, K.; Neidert, D.; Botos, E.; Altbäcker, V. Differential Ecological Responses of Two Generalist Arthropod Groups, Spiders and Carabid Beetles (Araneae, Carabidae), to the Effects of Wildfire. Community Ecol. 2010, 11, 129–139. [Google Scholar] [CrossRef]
- Barber, N.A.; Lamagdeleine-Dent, K.A.; Willand, J.E.; Jones, H.P.; McCravy, K.W. Species and Functional Trait Re-Assembly of Ground Beetle Communities in Restored Grasslands. Biodivers. Conserv. 2017, 26, 3481–3498. [Google Scholar] [CrossRef]
- Bargmann, T.; Heegaard, E.; Hatteland, B.A.; Chipperfield, J.D.; Grytnes, J.-A. Species Trait Selection along a Prescribed Fire Chronosequence. Insect Conserv. Divers. 2016, 9, 446–455. [Google Scholar] [CrossRef]
- Koivula, M.; Cobb, T.; Déchêne, A.; Jacobs, J.; Spence, J. Responses of Two Sericoda Kirby, 1837 (Coleoptera: Carabidae) Species to Forest Harvesting, Wildfire, and Burn Severity. Entomol. Fenn. 2006, 17, 315–324. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Alekseev, S.K.; Ecological Club «Stenus»; Khapugin, A.A. Joint Directorate of the Mordovia State Nature Reserve and National Park "Smolny"; Tyumen State University. Post-Fire Fauna of Carabid Beetles (Coleoptera, Carabidae) in Forests of the Mordovia State Nature Reserve (Russia). Nat. Conserv. Res. 2019, 4, 11–20. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Park, Y.K.; Lim, J.-H.; Ryou, S.H.; Lee, C.M. Change of Arthropod Abundance in Burned Forests: Different Patterns According to Functional Guilds. J. Asia-Pac. Entomol. 2013, 16, 321–328. [Google Scholar] [CrossRef]
- Larochelle, A.; Lariviere, M.C. A Natural History of the Ground-Beetles (Coleoptera: Carabidae) of America North of Mexico; Pensoft: Newport News, VA, USA, 2003. [Google Scholar]
- Office of the New Jersey State Climatologist. Available online: https://climate.rutgers.edu/stateclim/ (accessed on 1 June 2021).
- Forman, R.T.T. Pine Barrens Ecosystem and Landscape; Revised; Rutgers University Press: New Brunswick, NJ, USA, 1998. [Google Scholar]
- Forman, R.T.T.; Boerner, R.E. Fire Frequency and the Pine Barrens of New Jersey. Bull. Torrey Bot. Club 1981, 108, 34. [Google Scholar] [CrossRef]
- Givnish, T.J. Serotiny, Geography, and Fire in the Pine Barrens of New Jersey. Evolution 1981, 35, 101. [Google Scholar] [CrossRef]
- Little, S. Fire and Plant Succession in the New Jersey Pine Barrens. In Pine Barrens: Ecosystem and Landscape; Academic Press: New York, NY, USA, 1979; pp. 297–314. ISBN 0-12-263450-0. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Noss, R.F.; Platt, W.J.; Sorrie, B.A.; Weakley, A.S.; Means, D.B.; Costanza, J.; Peet, R.K. How Global Biodiversity Hotspots May Go Unrecognized: Lessons from the North American Coastal Plain. Divers. Distrib. 2015, 21, 236–244. [Google Scholar] [CrossRef]
- Critical Ecosystem Partnership Fund. Available online: https://www.cepf.net/node/1996 (accessed on 8 November 2022).
- Moscovici, D.; Clarke, C. Planning, Conservation, and Education in the Pinelands National Reserve. Case Stud. Environ. 2019, 3, 1–10. [Google Scholar] [CrossRef]
- Mason, S.C., Jr. Butterflies & Skippers (Lepidoptera: Papilionoidea, Hesperioidea) of the Franklin Parker Preserve, Burlington County, New Jersey. Trans. Am. Entomol. Soc. 2015, 141, 351–368. [Google Scholar] [CrossRef]
- Buffington, J.D. Soil Arthropod Populations of the New Jersey Pine Barrens as Affected by Fire1. Ann. Entomol. Soc. Am. 1967, 60, 530–535. [Google Scholar] [CrossRef]
- Belcher, C.M.; New, S.L.; Gallagher, M.R.; Grosvenor, M.J.; Clark, K.; Skowronski, N.S. Bark Charcoal Reflectance May Have the Potential to Estimate the Heat Delivered to Tree Boles by Wildland Fires. Int. J. Wildland Fire 2021, 30, 391. [Google Scholar] [CrossRef]
- Zen, S.; Thomas, J.C.; Mueller, E.V.; Dhurandher, B.; Gallagher, M.; Skowronski, N.; Hadden, R.M. Development of a Field Deployable Firebrand Flux and Condition Measurement System. Fire Technol. 2021, 57, 1401–1424. [Google Scholar] [CrossRef]
- Thomas, J.C.; Mueller, E.V.; Gallagher, M.R.; Clark, K.L.; Skowronski, N.; Simeoni, A.; Hadden, R.M. Coupled Assessment of Fire Behavior and Firebrand Dynamics. Front. Mech. Eng. 2021, 7, 650580. [Google Scholar] [CrossRef]
- Clark, K.L.; Heilman, W.E.; Skowronski, N.S.; Gallagher, M.R.; Mueller, E.; Hadden, R.M.; Simeoni, A. Fire Behavior, Fuel Consumption, and Turbulence and Energy Exchange during Prescribed Fires in Pitch Pine Forests. Atmosphere 2020, 11, 242. [Google Scholar] [CrossRef]
- van Gerrevink, M.J.; Veraverbeke, S. Evaluating the Near and Mid Infrared Bi-Spectral Space for Assessing Fire Severity and Comparison with the Differenced Normalized Burn Ratio. Remote Sens. 2021, 13, 695. [Google Scholar] [CrossRef]
- Warner, T.A.; Skowronski, N.S.; Gallagher, M.R. High Spatial Resolution Burn Severity Mapping of the New Jersey Pine Barrens with WorldView-3 near-Infrared and Shortwave Infrared Imagery. Int. J. Remote Sens. 2017, 38, 598–616. [Google Scholar] [CrossRef]
- Gallagher, M.R.; Skowronski, N.S.; Lathrop, R.G.; McWilliams, T.; Green, E.J. An Improved Approach for Selecting and Validating Burn Severity Indices in Forested Landscapes. Can. J. Remote Sens. 2020, 46, 100–111. [Google Scholar] [CrossRef]
- Campbell, J.W.; Grodsky, S.M.; Keller, O.; Vigueira, C.C.; Vigueira, P.A.; Waite, E.S.; Greenberg, C.H. Response of Beetles (Coleoptera) to Repeated Applications of Prescribed Fire and Other Fuel Reduction Techniques in the Southern Appalachian Mountains. For. Ecol. Manag. 2018, 429, 294–299. [Google Scholar] [CrossRef]
- Cook, W.M.; Holt, R.D. Fire Frequency and Mosaic Burning Effects on a Tallgrass Prairie Ground Beetle Assemblage. Biodivers. Conserv. 2006, 15, 2301–2323. [Google Scholar] [CrossRef]
- Hammond, H.E.J.; Hoffman, P.G.K.; Pinno, B.D.; Pinzon, J.; Klimaszewski, J.; Hartley, D.J. Response of Ground and Rove Beetles (Coleoptera: Carabidae, Staphylinidae) to Operational Oil Sands Mine Reclamation in Northeastern Alberta, a Case Study. J. Insect Conserv. 2018, 22, 687–706. [Google Scholar] [CrossRef]
- Parmenter, R.R.; Kreutzian, M.; Moore, D.I.; Lightfoot, D.C. Short-Term Effects of a Summer Wildfire on a Desert Grassland Arthropod Community in New Mexico. Environ. Entomol. 2011, 40, 1051–1066. [Google Scholar] [CrossRef]
- Thomas, D.B. Nontoxic Antifreeze for Insect Traps. Entomol. News 2008, 119, 361–365. [Google Scholar] [CrossRef]
- Ball, G.E.; Bousquet, Y. Carabidae Latreille, 1810. In American beetles. Volume 1. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia; CRC Press: Boca Raton, FL, USA, 2001; pp. 32–132. [Google Scholar]
- Gidaspow, T. North American Caterpillar Hunters of the Genera Calosoma and Callisthenes (Coleoptera, Carabidae). Bull. Am. Mus. Nat. Hist. 1959, 116, 225–344. [Google Scholar]
- van Dyke, E.C. A Review of the North American Species of the Genus Carabus Linnaeus. Entomol. Am. 1945, 24, 87–137. [Google Scholar]
- Pearson, D.L.; Knisley, C.B.; Kazilek, C.J. A Field Guide to the Tiger Beetles of the United States and Canada: Identification, Natural History, and Distribution of the Cicindelidae; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Lindroth, C.H. The Ground-Beetles (Carabidae, Excl. Cicindelinae) of Canada and Alaska; Opuscula Entomologica: Stockholm, Sweden, 1961. [Google Scholar]
- Lindroth, C.H. A Revision of the Genus Synuchus Gyllenhal (Coleoptera: Carabidae) in the Widest Sense, with Notes on Pristosia Motschulsky (Eucalathus Bates) and Calathus Bonelli. Trans. R. Entomol. Soc. Lond. 1956, 108, 485–585. [Google Scholar] [CrossRef]
- Ball, G.E. A Taxonomic Study of the North American Licinini with Notes on the Old World Species of the Genus Diplochelia Brulle (Coleoptera). Mem. Am. Entomol. Soc. 1959, 16, 1–258. [Google Scholar]
- Ball, G.E.; Nimmo, A.P. Synopsis of the Species of Subgenus Progaleritina Jeannel, Including Reconstructed Phylogeny and Geographical History (Coleoptera: Carabidae: Galerita Fabricius). Trans. Am. Entomol. Soc. 1983, 109, 295–356. [Google Scholar]
- Bousquet, Y. Taxonomic Revision of Neartic, Mexican, and West Indian Oodini (Coleoptera: Carabidae). Can. Entomol. 1996, 128, 443–537. [Google Scholar] [CrossRef]
- Liebherr, J.K.; Will, K.W. New North American Platynus Bonelli (Coleoptera: Carabidae), a Key to Species North of Mexico, and Notes on Species from the Southwestern United States. Coleopt. Bull. 1996, 50, 21. [Google Scholar]
- Messer, P.W.; Raber, B.T. A Review of Nearctic Selenophorus Dejean (Coleoptera: Carabidae: Harpalini) North of Mexico with New Species, New Synonyms, Range Extensions, and a Key. Coleopt. Bull. 2021, 75, 9–55. [Google Scholar] [CrossRef]
- Purrington, F.F.; Drake, C.J. A Key to Adult Nearctic Pasimachus (Pasimachus) Bonelli (Coleoptera: Carabidae: Scaritini), with Comments on Their Functional Mouthpart Morphology. Entomol. News 2005, 116, 253–262. [Google Scholar]
- Bousquet, Y. Illustrated Identification Guide to Adults and Larvae of Northeastern North American Ground Beetles (Coleoptera: Carabidae); Pensoft: Newport News, VA, USA, 2010. [Google Scholar]
- Ciegler, J.C. Ground Beetles and Wrinkled Bark Beetles of South Carolina (Coleoptera: Geadephaga: Carabidae and Rhysodidae); Biota of South Carolina; Clemson University: Clemson, SC, USA, 2000; Volume 1. [Google Scholar]
- Waite, E.S. Arizona State University Biocollections. Available online: https://doi.org/10.15468/5d9kk2 (accessed on 5 July 2021).
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Nelson, M.; Hosler, S.C.; Boetzl, F.A.; Jones, H.P.; Barber, N.A. Reintroduced Grazers and Prescribed Fire Effects on Beetle Assemblage Structure and Function in Restored Grasslands. Ecol. Appl. 2021, 31, e02217. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Forsythe, T. A Comparison of Adaptations to Running, Pushing and Burrowing in Some Adult Coleoptera, Specially Carabidae. J. Zool. 1984, 202, 513–534. [Google Scholar] [CrossRef]
- Talarico, F.; Brandmayr, P.; Giglio, A.; Massolo, A.; Zetto Brandmayr, T. Morphometry of Eyes, Antennae and Wings in Three Species of Siagona (Coleoptera, Carabidae). ZooKeys 2011, 100, 203–214. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–15. ISBN 978-1-118-44511-2. [Google Scholar]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar]
- de Arruda, F.V.; Teresa, F.B.; Layme, V.M.G.; Vicente, R.E.; Camarota, F.; Izzo, T.J. Fire and Flood: How the Pantanal Ant Communities Respond to Multiple Disturbances? Perspect. Ecol. Conserv. 2022, 20, 197–204. [Google Scholar] [CrossRef]
- Hanula, J.L.; Horn, S.; O’Brien, J.J. Have Changing Forests Conditions Contributed to Pollinator Decline in the Southeastern United States? For. Ecol. Manag. 2015, 348, 142–152. [Google Scholar] [CrossRef]
- Vázquez-Veloso, A.; Dejene, T.; Oria-de-Rueda, J.A.; Guijarro, M.; Hernando, C.; Espinosa, J.; Madrigal, J.; Martín-Pinto, P. Prescribed Burning in Spring or Autumn Did Not Affect the Soil Fungal Community in Mediterranean Pinus Nigra Natural Forests. For. Ecol. Manag. 2022, 512, 120161. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology, R Package Version 1.0-12; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Hjältén, J.; Hägglund, R.; Löfroth, T.; Roberge, J.-M.; Dynesius, M.; Olsson, J. Forest Restoration by Burning and Gap Cutting of Voluntary Set-Asides Yield Distinct Immediate Effects on Saproxylic Beetles. Biodivers. Conserv. 2017, 26, 1623–1640. [Google Scholar] [CrossRef]
- Tello, F.; González, M.E.; Valdivia, N.; Torres, F.; Lara, A.; García-López, A. Diversity Loss and Changes in Saproxylic Beetle Assemblages Following a High-Severity Fire in Araucaria–Nothofagus Forests. J. Insect Conserv. 2020, 24, 585–601. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Gibson, K.S.; Johnson, N.C.; Laturno, C.; Parmenter, R.R.; Antoninka, A. Abundance of Mites, but Not of Collembolans or Nematodes, Is Reduced by Restoration of a Pinus Ponderosa Forest with Thinning, Mastication, and Prescribed Fire. Trees For. People 2022, 7, 100190. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, R Package Version 2.2-0; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Quensen, J. Ggordiplots: The Comprehensive R Archive Network: Make Ggplot Versions of Vegans Ordiplots. 2018. Available online: https://cran.r-project.org/web/packages/ggordiplots/index.html (accessed on 2 August 2023).
- Beal-Neves, M.; Chiarani, E.; Abreu Ferreira, P.M.; Fontana, C. The Role of Fire Disturbance on Habitat Structure and Bird Communities in South Brazilian Highland Grasslands. Sci. Rep. 2020, 10, 19708. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.J.; Willig, M.R. Habitat Heterogeneity, Habitat Associations, and Rodent Species Diversity in a Sand-Shinnery-Oak Landscape. J. Mammal. 2002, 83, 743–753. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Gagic, V.; Bartomeus, I.; Jonsson, T.; Taylor, A.; Winqvist, C.; Fischer, C.; Slade, E.M.; Steffan-Dewenter, I.; Emmerson, M.; Potts, S.G.; et al. Functional Identity and Diversity of Animals Predict Ecosystem Functioning Better than Species-Based Indices. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142620. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Stockan, J.A. Drivers of Carabid Functional Diversity: Abiotic Environment, Plant Functional Traits, or Plant Functional Diversity? Ecology 2014, 95, 1213–1224. [Google Scholar] [CrossRef]
- Miller, J.D.; Thode, A.E. Quantifying Burn Severity in a Heterogeneous Landscape with a Relative Version of the Delta Normalized Burn Ratio (DNBR). Remote Sens. Environ. 2007, 109, 66–80. [Google Scholar] [CrossRef]
- Miller, J.D.; Knapp, E.E.; Key, C.H.; Skinner, C.N.; Isbell, C.J.; Creasy, R.M.; Sherlock, J.W. Calibration and Validation of the Relative Differenced Normalized Burn Ratio (RdNBR) to Three Measures of Fire Severity in the Sierra Nevada and Klamath Mountains, California, USA. Remote Sens. Environ. 2009, 113, 645–656. [Google Scholar] [CrossRef]
- Ammer, C. Unraveling the Importance of Inter- and Intraspecific Competition for the Adaptation of Forests to Climate Change. In Progress in Botany; Springer: Cham, Germany, 2016; Volume 78, pp. 345–367. [Google Scholar]
- Molles, M.C., Jr. Ecology Concepts and Applications, 6th ed.; McGraw-Hill Companies: New York, NY, USA, 2013. [Google Scholar]
- Skowronski, N.S.; Gallagher, M.R.; Warner, T.A. Decomposing the Interactions between Fire Severity and Canopy Fuel Structure Using Multi-Temporal, Active, and Passive Remote Sensing Approaches. Fire 2020, 3, 7. [Google Scholar] [CrossRef]
- Radea, C.; Arianoutsou, M. Soil Arthropod Communities and Population Dynamics Following Wildfires in Pine Forests of the Mediterranean Basin: A Review. Isr. J. Ecol. Evol. 2012, 58, 137–149. [Google Scholar]
- Ryan, K. Dynamic Interactions between Forest Structure and Fire Behavior in Boreal Ecosystems. Silva Fenn. 2002, 36, 13–39. [Google Scholar] [CrossRef]
- Ponisio, L.C.; Wilkin, K.; M’Gonigle, L.K.; Kulhanek, K.; Cook, L.; Thorp, R.; Griswold, T.; Kremen, C. Pyrodiversity Begets Plant-Pollinator Community Diversity. Glob. Chang. Biol. 2016, 22, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Gongalsky, K.B.; Wikars, L.-O.; Persson, T. Dynamics of Pyrophilous Carabids in a Burned Pine Forest in Central Sweden. Balt. J. Coleopterol. 2003, 6, 107–111. [Google Scholar]
- Fernández Fernández, M.M.; Salgado Costas, J.M. Recolonization of a Burnt Pine Forest (Pinus Pinaster) by Carabidae (Coleoptera). Eur. J. Soil Biol. 2004, 40, 47–53. [Google Scholar] [CrossRef]
- Zdzioch, P. Effect of Fire of Various Intensities on Assemblages of Ground Beetles (Coleoptera: Carabidae) Inhabiting Pine-Stands at Different Ages. Balt. J. Coleopterol. 2003, 3, 101–105. [Google Scholar]
- Nimmo, D.G.; Kelly, L.T.; Farnsworth, L.M.; Watson, S.J.; Bennett, A.F. Why Do Some Species Have Geographically Varying Responses to Fire History? Ecography 2014, 37, 805–813. [Google Scholar] [CrossRef]
- Legendre, P. Spatial Autocorrelation: Trouble or New Paradigm? Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Negret, P.J.; Marco, M.D.; Sonter, L.J.; Rhodes, J.; Possingham, H.P.; Maron, M. Effects of Spatial Autocorrelation and Sampling Design on Estimates of Protected Area Effectiveness. Conserv. Biol. 2020, 34, 1452–1462. [Google Scholar] [CrossRef]
- Den Boer, P.J. On the Significance of Dispersal Power for Populations of Carabid-Beetles (Coleoptera, Carabidae). Oecologia 1970, 4, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Den Boer, P.J. The Survival Value of Dispersal in Terrestrial Arthropods. Biol. Conserv. 1990, 54, 175–192. [Google Scholar] [CrossRef]
- Frampton, G.K.; Çilgi, T.; Fry, G.L.A.; Wratten, S.D. Effects of Grassy Banks on the Dispersal of Some Carabid Beetles (Coleoptera: Carabidae) on Farmland. Biol. Conserv. 1995, 71, 347–355. [Google Scholar] [CrossRef]
- Davies, G.M.; Gray, A. Don’t Let Spurious Accusations of Pseudoreplication Limit Our Ability to Learn from Natural Experiments (and Other Messy Kinds of Ecological Monitoring). Ecol. Evol. 2015, 5, 5295–5304. [Google Scholar] [CrossRef]
- van Mantgem, P.; Schwartz, M.; Keifer, M. Monitoring Fire Effects for Managed Burns and Wildfires: Coming to Terms with Pseudoreplication. Nat. Areas J. 2001, 21, 266–273. [Google Scholar]
- Barton, P.S.; Gibb, H.; Manning, A.D.; Lindenmayer, D.B.; Cunningham, S.A. Morphological Traits as Predictors of Diet and Microhabitat Use in a Diverse Beetle Assemblage: Morphological traits of beetles. Biol. J. Linn. Soc. 2011, 102, 301–310. [Google Scholar] [CrossRef]
- Holliday, N.J. Species Responses of Carabid Beetles (Coleoptera: Carabidae) during Post-Fire Regeneration of Boreal Forest. Can. Entomol. 1991, 123, 1369–1389. [Google Scholar] [CrossRef]
- Koltz, A.M.; Burkle, L.A.; Pressler, Y.; Dell, J.E.; Vidal, M.C.; Richards, L.A.; Murphy, S.M. Global Change and the Importance of Fire for the Ecology and Evolution of Insects. Curr. Opin. Insect Sci. 2018, 29, 110–116. [Google Scholar] [CrossRef]
- Ribera, I.; Doledec, S.; Downie, I.S.; Foster, G.N. Effect of Land Disturbance and Stress on Species Traits of Ground Beetle Assemblages. Ecology 2001, 82, 19. [Google Scholar] [CrossRef]
- Firebaugh, A.; Haynes, K.J. Experimental Tests of Light-Pollution Impacts on Nocturnal Insect Courtship and Dispersal. Oecologia 2016, 182, 1203–1211. [Google Scholar] [CrossRef]
- Perkin, E.K.; Hölker, F.; Tockner, K. The Effects of Artificial Lighting on Adult Aquatic and Terrestrial Insects. Freshw. Biol. 2014, 59, 368–377. [Google Scholar] [CrossRef]
- Koivula, M. Useful Model Organisms, Indicators, or Both? Ground Beetles (Coleoptera, Carabidae) Reflecting Environmental Conditions. ZooKeys 2011, 100, 287–317. [Google Scholar] [CrossRef] [PubMed]
- Dell, J.; O’Brien, J.; Doan, L.; Richards, L.; Dyer, L. An Arthropod Survival Strategy in a Frequently Burned Forest. Ecology 2017, 98, 2972–2974. [Google Scholar] [CrossRef] [PubMed]
- Varet, M.; Burel, F.; Lafage, D.; Pétillon, J. Age-Dependent Colonization of Urban Habitats: A Diachronic Approach Using Carabid Beetles and Spiders. Anim. Biol. 2013, 63, 257–269. [Google Scholar] [CrossRef]
- Gandhi, K.J.K.; Gilmore, D.W.; Katovich, S.A.; Mattson, W.J.; Zasada, J.C.; Seybold, S.J. Catastrophic Windstorm and Fuel-Reduction Treatments Alter Ground Beetle (Coleoptera: Carabidae) Assemblages in a North American Sub-Boreal Forest. For. Ecol. Manag. 2008, 256, 1104–1123. [Google Scholar] [CrossRef]
- Wikars, L.-O. Dependence on Fire in Wood-Living Insects: An Experiment with Burned and Unburned Spruce and Birch Logs. J. Insect Conserv. 2002, 6, 1–12. [Google Scholar] [CrossRef]
- Cobb, T.P.; Langor, D.W.; Spence, J.R. Biodiversity and Multiple Disturbances: Boreal Forest Ground Beetle (Coleoptera: Carabidae) Responses to Wildfire, Harvesting, and Herbicide. Can. J. For. Res. 2007, 37, 1310–1323. [Google Scholar] [CrossRef]
- Niwa, C.G.; Peck, R.W. Influence of Prescribed Fire on Carabid Beetle (Carabidae) and Spider (Araneae) Assemblages in Forest Litter in Southwestern Oregon. Environ. Entomol. 2002, 31, 785–796. [Google Scholar] [CrossRef]
- Oliver, I.; Mac Nally, R.; York, A. Identifying Performance Indicators of the Effects of Forest Management on Ground-Active Arthropod Biodiversity Using Hierarchical Partitioning and Partial Canonical Correspondence Analysis. For. Ecol. Manag. 2000, 139, 21–40. [Google Scholar] [CrossRef]
- Brennan, K.E.C.; Moir, M.L.; Wittkuhn, R.S. Fire Refugia: The Mechanism Governing Animal Survivorship within a Highly Flammable Plant: Plants as Fire Refugia for Animals. Austral Ecol. 2011, 36, 131–141. [Google Scholar] [CrossRef]
- Stanger-Hall, K.F.; Sander Lower, S.E.; Lindberg, L.; Hopkins, A.; Pallansch, J.; Hall, D.W. The Evolution of Sexual Signal Modes and Associated Sensor Morphology in Fireflies (Lampyridae, Coleoptera). Proc. R. Soc. B Biol. Sci. 2018, 285, 20172384. [Google Scholar] [CrossRef]
- Tocco, C.; Dacke, M.; Byrne, M. Eye and Wing Structure Closely Reflects the Visual Ecology of Dung Beetles. J. Comp. Physiol. A 2019, 205, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, T.S.; Rütting, T.; Nilsson, M.-C.; Wardle, D.A.; Gundale, M.J. Mid-Term Effects of Wildfire and Salvage Logging on Gross and Net Soil Nitrogen Transformation Rates in a Swedish Boreal Forest. For. Ecol. Manag. 2022, 517, 120240. [Google Scholar] [CrossRef]
- Mitchell, D.J.; Beckmann, C.; Biro, P.A. Understanding the Unexplained: The Magnitude and Correlates of Individual Differences in Residual Variance. Ecol. Evol. 2021, 11, 7201–7210. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, J.J.C.; Visser, M.E.; Gienapp, P. Quantifying Individual Variation in Reaction Norms: Mind the Residual. J. Evol. Biol. 2020, 33, 352–366. [Google Scholar] [CrossRef]
- Shoemaker, L.G.; Sullivan, L.L.; Donohue, I.; Cabral, J.S.; Williams, R.J.; Mayfield, M.M.; Chase, J.M.; Chu, C.; Harpole, W.S.; Huth, A.; et al. Integrating the Underlying Structure of Stochasticity into Community Ecology. Ecology 2020, 101, e02922. [Google Scholar] [CrossRef]
- Hiers, J.K.; O’Brien, J.J.; Varner, J.M.; Butler, B.W.; Dickinson, M.; Furman, J.; Gallagher, M.; Godwin, D.; Goodrick, S.L.; Hood, S.M.; et al. Prescribed Fire Science: The Case for a Refined Research Agenda. Fire Ecol. 2020, 16, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).