Fertile Island Soils Promote the Restoration of Shrub Patches in Burned Areas in Arid Saline Land
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
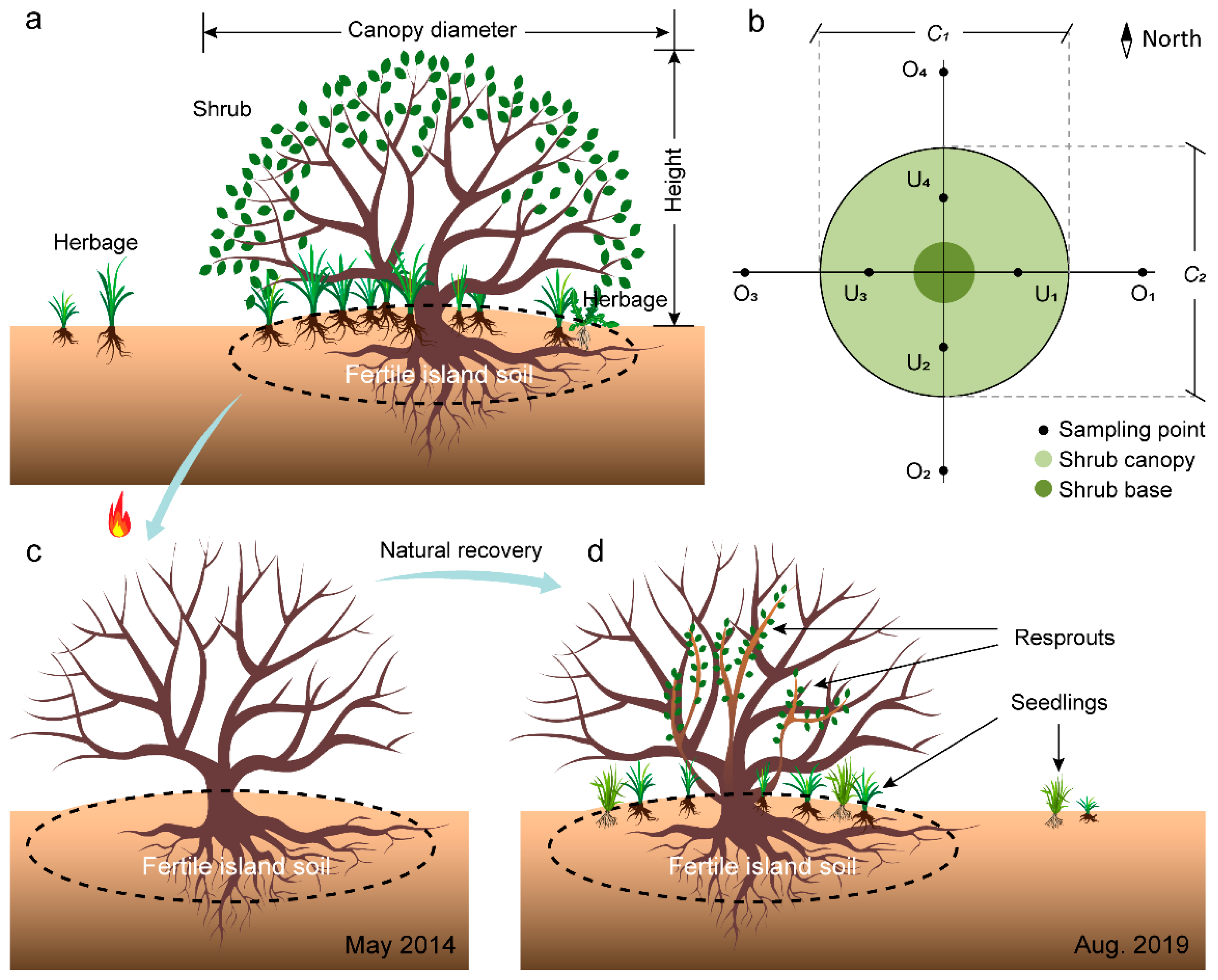
2.2. Vegetation Survey and Soil Sampling of Shrub Patches
2.3. Restoration of Shrub Patches in Burned Areas
2.4. Laboratory Analysis
2.5. Quantification of Shrub Volume and Plant Diversity
2.6. Statistical Analysis
3. Results
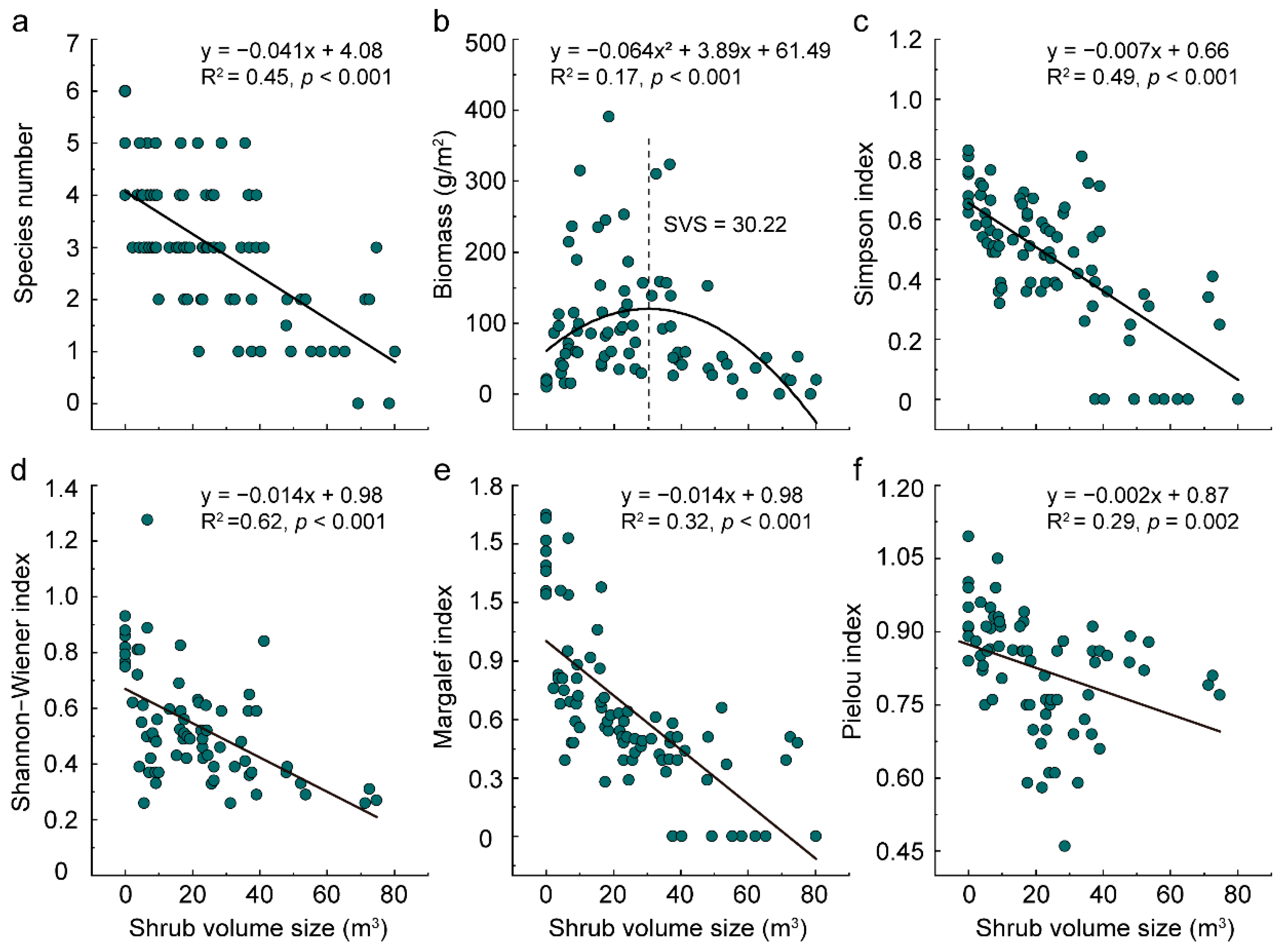
3.1. Features of Herbage Beneath Shrubs in Unburned Area
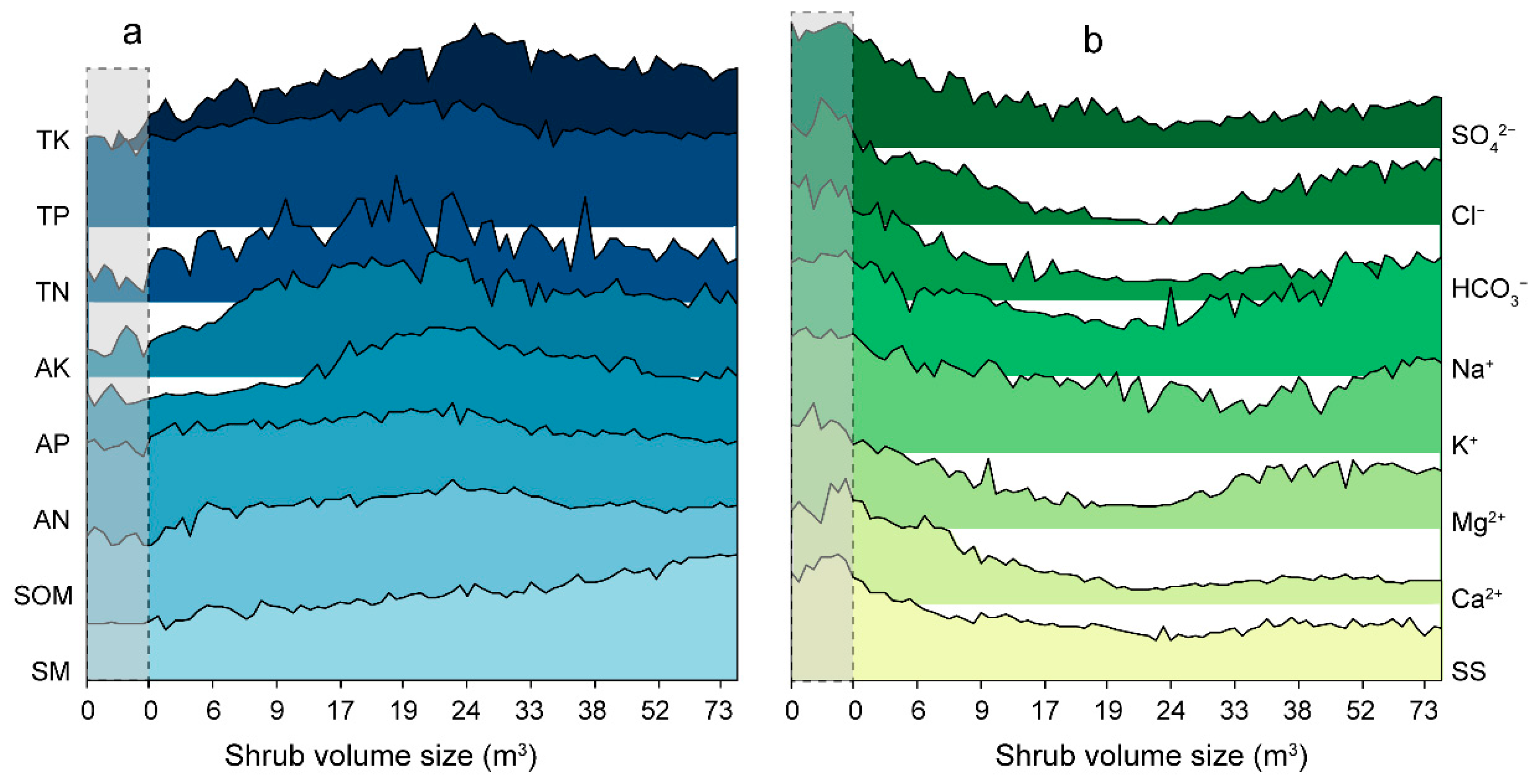
3.2. Soil Properties Beneath the Shrub Canopy in Unburned Area
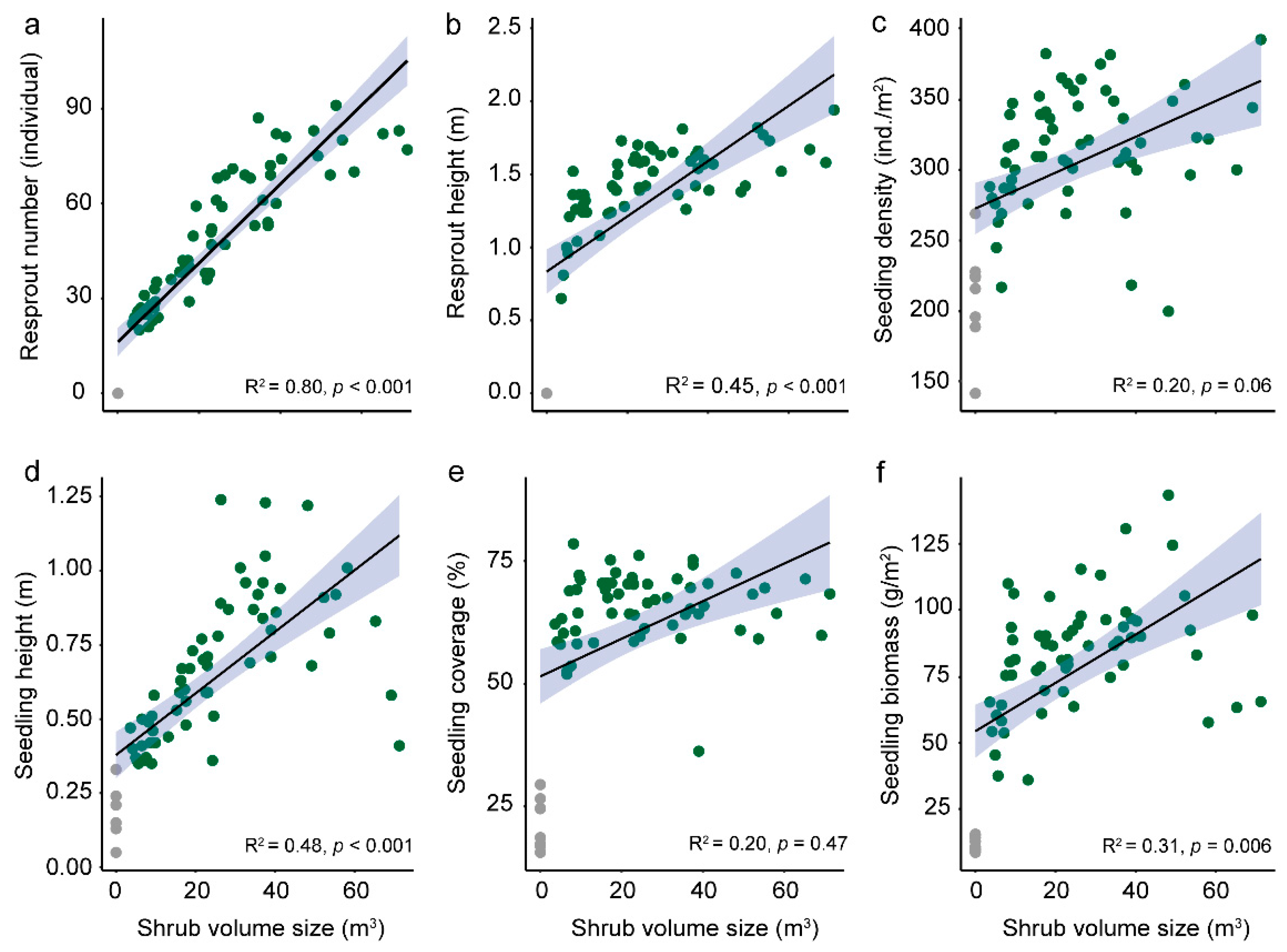
3.3. Vegetation Recovery of Shrubland in Burnrd Area
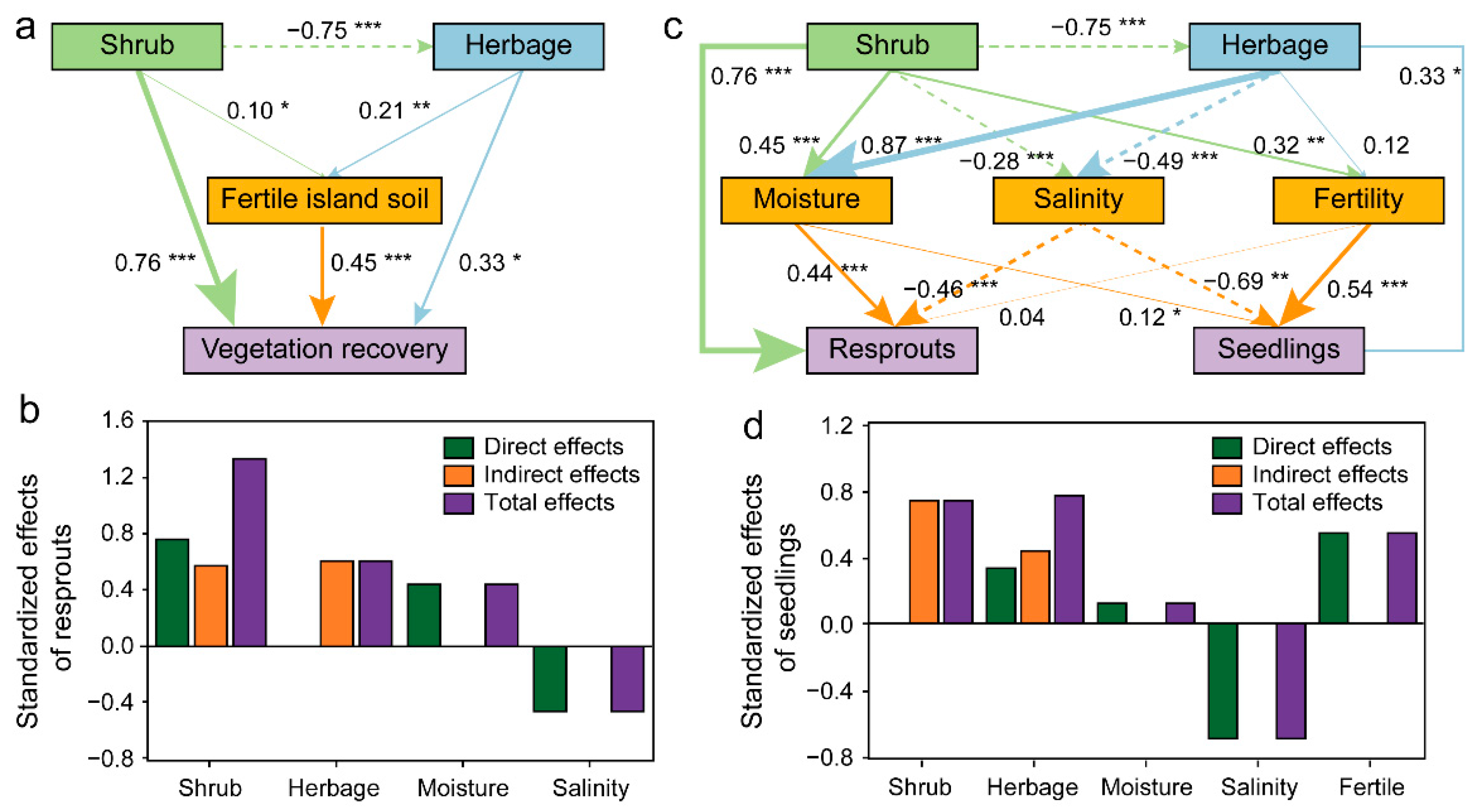
3.4. SEMs of Shrub Patch Restoration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mclaren, J.R.; Buckeridge, K.M.; van de Weg, M.J.; Shaver, G.R.; Schimel, J.P.; Gough, L. Shrub encroachment in Arctic tundra: Betula nana effects on above- and belowground litter decomposition. Ecology 2017, 98, 1361–1376. [Google Scholar] [CrossRef]
- Dahl, R.; Dalgaard, T.; Bork, E.W. Shrub encroachment following wetland creation in mixedgrass prairie alters grassland vegetation and soil. Environ. Manag. 2020, 66, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.L.; Wang, P.C.; Zhang, W.; Zhang, Y.; Li, S.G.; Wei, X.; Chen, X.; Zhang, Y.J.; Yang, F.L. Shrub encroachment shapes soil nutrient concentration, stoichiometry and carbon storage in an abandoned subalpine grassland. Sustainability 2019, 11, 1732. [Google Scholar] [CrossRef]
- Sepp, S.K.; Davison, J.; Moora, M.; Neuenkamp, L.; Oja, J.; Roslin, T.; Vasar, M.; Opik, M.; Zobel, M. Woody encroachment in grassland elicits complex changes in the functional structure of above- and belowground biota. Ecosphere 2021, 12, e03512. [Google Scholar] [CrossRef]
- Vivoni, E.R.; Perez-Ruiz, E.R.; Keller, Z.T.; Escoto, E.A.; Templeton, R.C.; Templeton, N.P.; Anderson, C.A.; Schreiner-McGraw, A.P.; Mendez-Barroso, L.A.; Robles-Morua, A.; et al. Long-term research catchments to investigate shrub encroachment in the Sonoran and Chihuahuan deserts: Santa Rita and Jornada experimental ranges. Hydrol. Process. 2021, 35, e14031. [Google Scholar] [CrossRef]
- Terzi, M.; Fontaneto, D.; Casella, F. Effects of Ailanthus altissima invasion and removal on high-biodiversity mediterranean grasslands. Environ. Manag. 2021, 68, 914–927. [Google Scholar] [CrossRef]
- Manjoro, M.; Kakembo, V.; Rowntree, K.M. Trends in soil erosion and woody shrub encroachment in Ngqushwa District, Eastern Cape Province, South Africa. Environ. Manag. 2012, 49, 570–579. [Google Scholar] [CrossRef]
- Hopkinson, P.; Hammond, M.; Bartolome, J.W.; Macaulay, L. Using consecutive prescribed fires to reduce shrub encroachment in grassland by increasing shrub mortality. Restor. Ecol. 2020, 28, 850–858. [Google Scholar] [CrossRef]
- Liu, Y.S.; Shi, Z.J.; Gong, L.Y.; Cong, R.C.; Yang, X.H.; Eldridge, D.J. Is the removal of aboveground shrub biomass an effective technique to restore a shrub-encroached grassland? Restor. Ecol. 2019, 27, 1348–1356. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Predicting long-term dynamics of soil salinity and sodicity on a global scale. Proc. Natl. Acad. Sci. USA 2020, 117, 33017–33027. [Google Scholar] [CrossRef]
- Wu, Z.H.; Che, M.L.; Zhang, S.T.; Duo, L.H.; Lei, S.G.; Lu, Q.Q.; Yan, Q.W. Remote sensing monitoring and driving force analysis of salinized soil in grassland mining area. Sustainability 2022, 14, 741. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.L.; Zhang, F.; Chan, N.W.; Kung, H.T.; Liu, S.H.; Deng, L.F. Estimation of soil salt content using machine learning techniques based on remote-sensing fractional derivatives, a case study in the Ebinur Lake Wetland National Nature Reserve, Northwest China. Ecol. Indic. 2020, 119, 106869. [Google Scholar] [CrossRef]
- Yang, J. Development prospect of the research on salt-affected soils in China. Acta Pedol. Sin. 2008, 45, 837–845. [Google Scholar]
- Maestre, F.T.B.; Matthew, A.; Puche, M.D.; Belén Hinojosa, M.; Martínez, I.; García-Palacios, P.; Castillo, A.P.; Soliveres, S.; Luzuriaga, A.L.; Sánchez, A.M.; et al. Shrub encroachment can reverse desertification in semi-arid Mediterranean grasslands. Ecol. Lett. 2009, 12, 930–941. [Google Scholar] [CrossRef]
- Hering, R.; Hauptfleisch, M.; Geissler, K.; Marquart, A.; Schoenen, M.; Blaum, N. Shrub encroachment is not always land degradation: Insights from ground-dwelling beetle species niches along a shrub cover gradient in a semi-arid Namibian savanna. Land Degrad. Dev. 2019, 30, 14–24. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yu, Q.; Lu, X.T.; Trumbore, S.E.; Yang, J.J.; Han, X.G. Impacts of leguminous shrub encroachment on neighboring grasses include transfer of fixed nitrogen. Oecologia 2016, 180, 1213–1222. [Google Scholar] [CrossRef]
- Zhang, G.F.; Zhao, W.Z.; Zhou, H.; Yang, Q.Y.; Wang, X.F. Extreme drought stress shifts net facilitation to neutral interactions between shrubs and sub-canopy plants in an arid desert. Oikos 2018, 127, 381–391. [Google Scholar] [CrossRef]
- Parajuli, R.; O’Brien, M.J.; Timilsina, B.; Pugnaire, F.I.; Schob, C.; Ghimire, S.K. Facilitation by a dwarf shrub enhances plant diversity of human-valued species at high elevations in the Himalayas of Nepal. Basic Appl. Ecol. 2021, 54, 23–36. [Google Scholar] [CrossRef]
- Rong, Q.Q.; Liu, J.T.; Cai, Y.P.; Lu, Z.H.; Zhao, Z.Z.; Yue, W.C.; Xia, J.B. “Fertile island” effects of Tamarix chinensis Lour. on soil N and P stoichiometry in the coastal wetland of Laizhou Bay, China. J. Soils Sediments 2016, 16, 864–877. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Bai, J.H.; Liu, Q.; Lu, Q.Q.; Gao, Z.Q.; Wang, J.J. Spatial and seasonal variations of soil carbon and nitrogen content and stock in a tidal salt marsh with Tamarix chinensis, China. Wetlands 2016, 36, S145–S152. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Zhang, Z.M.; Dong, G.; Yuan, X.K. Spatial variability in soil fertility related to the “fertile islands” effect of Vitex negundo. Fresenius Environ. Bull. 2017, 26, 72–79. [Google Scholar]
- Ross, M.S.; Sah, J.P. Forest Resource Islands in a Sub-tropical Marsh: Soil-Site Relationships in Everglades Hardwood Hammocks. Ecosystems 2011, 14, 632–645. [Google Scholar] [CrossRef][Green Version]
- Schob, C.; Armas, C.; Pugnaire, F.I. Direct and indirect interactions co-determine species composition in nurse plant systems. Oikos 2013, 122, 1371–1379. [Google Scholar] [CrossRef]
- Iwaoka, C.; Imada, S.; Taniguchi, T.; Du, S.; Yamanaka, N.; Tateno, R. The impacts of soil fertility and salinity on soil nitrogen dynamics mediated by the soil microbial community beneath the halophytic shrub tamarisk. Microb. Ecol. 2018, 75, 985–996. [Google Scholar] [CrossRef]
- Li, X.Q.; Xia, J.B.; Zhao, X.M.; Chen, Y.P. Effects of planting Tamarix chinensis on shallow soil water and salt content under different groundwater depths in the Yellow River Delta. Geoderma 2019, 335, 104–111. [Google Scholar] [CrossRef]
- Woods, S.R.; Archer, S.R.; Schwinning, S. Seedling responses to water pulses in shrubs with contrasting histories of grassland encroachment. PLoS ONE 2014, 9, e87278. [Google Scholar] [CrossRef]
- Madrigal-Gonzalez, J.; Kelt, D.A.; Meserve, P.L.; Squeo, F.A.; Gutierrez, J.R. Shrub-ephemeral plants interactions in semiarid north-central Chile: Is the nurse plant syndrome manifested at the community level? J. Arid Environ. 2016, 126, 47–53. [Google Scholar] [CrossRef]
- Peng, H.Y.; Li, X.Y.; Li, G.Y.; Zhang, Z.H.; Zhang, S.Y.; Li, L.; Zhao, G.Q.; Jiang, Z.Y.; Ma, Y.J. Shrub encroachment with increasing anthropogenic disturbance in the semiarid Inner Mongolian grasslands of China. Catena 2013, 109, 39–48. [Google Scholar] [CrossRef]
- Daryanto, S.; Fu, B.J.; Zhao, W.W. Evaluating the use of fire to control shrub encroachment in global drylands: A synthesis based on ecosystem service perspective. Sci. Total Environ. 2019, 648, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.R.; Travieso, G.; Galindo, N.; Acevedo, M.; Puettmann, K.; Salas-Eljatib, C. Shrub influences on seedling performance when restoring the slow-growing conifer Pilgerodendron uviferum in southern bog forests. Restor. Ecol. 2020, 28, 396–407. [Google Scholar] [CrossRef]
- Anthelme, F.; Dangles, O. Plant-plant interactions in tropical alpine environments. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 363–372. [Google Scholar] [CrossRef]
- Zhou, Y.; Boutton, T.W.; Wu, X.B. Woody plant encroachment amplifies spatial heterogeneity of soil phosphorus to considerable depth. Ecology 2019, 100, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Albert, C.H.; Ibanez, S.; Saccone, P.; Zinger, L.; Choler, P.; Clement, J.C.; Lavergne, S.; Geremia, R.A. Microbes on the cliff: Alpine cushion plants structure bacterial and fungal communities. Front. Microbiol. 2013, 4, 64. [Google Scholar] [CrossRef]
- Wang, S.; Cao, W.; Wang, X.; Li, W.; Li, X.; Wang, J. Distribution of soil moisture and salt of Tamarix chinensis plantation in desert saline-alkali land of Hexi Corridor Region. Chin. J. Appl. Ecol. 2019, 30, 2531–2540. [Google Scholar]
- Wang, Z.; Huang, W.; Wang, X.; Yang, X.; Zhai, H.; Zhao, J.; Chai, S. Comparative study on production performance of 10 alfalfa species in the moderate and mild saline alkali land in Jiuquan Desert irrigation area. J. Anim. Sci. Vet. Med. 2021, 40, 14–20. [Google Scholar]
- Bao, S.; Jiang, R.; Yang, C.; Xu, G.; Han, X. Soil Chemical Analysis of Agriculture, 3rd ed.; Chinese Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–581. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; States Department of Agriculture: Washington, DC, USA, 1954.
- Zhang, J. Quantitative Ecology, 3rd ed.; Science Press: Beijing, China, 2018. [Google Scholar]
- He, Y.F.; D’Odorico, P.; De Wekker, S.F.J. The role of vegetation-microclimate feedback in promoting shrub encroachment in the northern Chihuahuan desert. Glob. Chang. Biol. 2015, 21, 2141–2154. [Google Scholar] [CrossRef]
- Soliveres, S.; Eldridge, D.J. Do changes in grazing pressure and the degree of shrub encroachment alter the effects of individual shrubs on understorey plant communities and soil function? Funct. Ecol. 2014, 28, 530–537. [Google Scholar] [CrossRef]
- Bai, Y.X.; Zhang, Y.Q.; Michalet, R.; She, W.W.; Jia, X.; Qin, S.G. Responses of different herb life-history groups to a dominant shrub species along a dune stabilization gradient. Basic Appl. Ecol. 2019, 38, 1–12. [Google Scholar] [CrossRef]
- He, Y.F.; D’Odorico, P.; De Wekker, S.F.J.; Fuentes, J.D.; Litvak, M. On the impact of shrub encroachment on microclimate conditions in the northern Chihuahuan desert. J. Geophys. Res-Atmos. 2010, 115, D21. [Google Scholar] [CrossRef]
- He, Y.F.; D’Odorico, P.; De Wekker, S.F.J. The relative importance of climate change and shrub encroachment on nocturnal warming in the southwestern United States. Int. J. Climatol. 2015, 35, 475–480. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.Y.; Huang, H.; Wu, X.C.; Yu, K.L.; Wei, J.Q.; Zhang, C.C.; Wang, P.; Hu, X.; D’Odorico, P. Does phenology play a role in the feedbacks underlying shrub encroachment? Sci. Total Environ. 2019, 657, 1064–1073. [Google Scholar] [CrossRef]
- Belay, T.A.; Moe, S.R. Assessing the Effects of Woody Plant traits on understory herbaceous cover in a semiarid rangeland. Environ. Manag. 2015, 56, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.D.; Peltzer, D.A. Per-gram competitive effects and contrasting soil resource effects in grasses and woody plants. J. Ecol. 2021, 109, 74–84. [Google Scholar] [CrossRef]
- Prieto, I.; Armas, C.; Pugnaire, F.I. Hydraulic lift promotes selective root foraging in nutrient-rich soil patches. Funct. Plant Biol. 2012, 39, 804–812. [Google Scholar] [CrossRef]
- Prieto, I.; Kikvidze, Z.; Pugnaire, F.I. Hydraulic lift: Soil processes and transpiration in the Mediterranean leguminous shrub Retama sphaerocarpa (L.) Boiss. Plant Soil 2010, 329, 447–456. [Google Scholar] [CrossRef]
- Nagy, R.C.; Fusco, E.J.; Balch, J.K.; Finn, J.T.; Mahood, A.; Allen, J.M.; Bradley, B.A. A synthesis of the effects of cheatgrass invasion on US Great Basin carbon storage. J. Appl. Ecol. 2020, 58, 327–337. [Google Scholar] [CrossRef]
- Hu, X.; Li, Z.C.; Li, X.Y.; Liu, Y. Influence of shrub encroachment on CT-measured soil macropore characteristics in the Inner Mongolia grassland of northern China. Soil Tillage Res. 2015, 150, 1–9. [Google Scholar] [CrossRef]
- Li, C.J.; Li, Y.; Ma, J.A. Spatial heterogeneity of soil chemical properties at fine scales induced by Haloxylon ammodendron (Chenopodiaceae) plants in a sandy desert. Ecol. Res. 2011, 26, 385–394. [Google Scholar] [CrossRef]
- Predick, K.I.; Archer, S.R.; Aguillon, S.M.; Keller, D.A.; Throop, H.L.; Barnes, P.W. UV-B radiation and shrub canopy effects on surface litter decomposition in a shrub-invaded dry grassland. J. Arid Environ. 2018, 157, 13–21. [Google Scholar] [CrossRef]
- Bachar, A.; Soares, M.I.M.; Gillor, O. The effect of resource islands on abundance and diversity of bacteria in arid soils. Microb. Ecol. 2012, 63, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.H.; Boulangeat, I.; Conradi, T.; Davis, M.; Svenning, J.C. The importance of ecological memory for trophic rewilding as an ecosystem restoration approach. Biol. Rev. 2019, 94, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ogle, K.; Barber, J.J.; Barron-Gafford, G.A.; Bentley, L.P.; Young, J.M.; Huxman, T.E.; Loik, M.E.; Tissue, D.T. Quantifying ecological memory in plant and ecosystem processes. Ecol. Lett. 2015, 18, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.E.; Wright, J.; Ashbaker, S. Comparison of invasive shrub Honeysuckle eradication tactics for amateurs: Stump treatment versus regrowth spraying of Lonicera maackii. Restor. Ecol. 2012, 20, 788–793. [Google Scholar] [CrossRef]
- Moreno-de las Heras, M.; Turnbull, L.; Wainwright, J. Seed-bank structure and plant-recruitment conditions regulate the dynamics of a grassland-shrubland Chihuahuan ecotone. Ecology 2016, 97, 2303–2318. [Google Scholar] [CrossRef]
- Ma, M.; Baskin, C.C.; Zhao, Y.; An, H. Light controls alpine meadow community assembly during succession by affecting species recruitment from the seed bank. Ecol. Appl. 2023, 33, e2787. [Google Scholar] [CrossRef]
- Shuyskaya, E.V.; Rakhamkulova, Z.F.; Lebedeva, M.P.; Kolesnikov, A.V.; Safarova, A.; Borisochkina, T.I.; Toderich, K.N. Different mechanisms of ion homeostasis are dominant in the recretohalophyte Tamarix ramosissima under different soil salinity. Acta Physiol. Plant. 2017, 39, 81. [Google Scholar] [CrossRef]
| Soil Properties | Outside | Beneath | Beneath/Outside | SVS (m3) |
|---|---|---|---|---|
| Soil moisture (%) | 6.42 | 10.66 | 1.66 | 80.21 |
| SOM (g/kg) | 1.11 | 1.68 | 1.52 | 23.96 |
| AN (mg/kg) | 21.21 | 28.42 | 1.34 | 23.96 |
| AP (mg/kg) | 12.55 | 21.03 | 1.68 | 23.90 |
| AK (mg/kg) | 229.29 | 504.16 | 2.20 | 23.04 |
| TN (g/kg) | 0.06 | 0.14 | 2.47 | 19.25 |
| TP (g/kg) | 0.44 | 0.58 | 1.33 | 21.56 |
| TK (g/kg) | 2.60 | 8.04 | 3.09 | 25.63 |
| Soluble salt (g/kg) | 7.56 | 3.22 | 0.43 | 23.96 |
| Ca2+ (g/kg) | 1.52 | 0.41 | 0.27 | 23.90 |
| Mg2+ (g/kg) | 0.44 | 0.13 | 0.30 | 18.56 |
| K+ (g/kg) | 0.05 | 0.02 | 0.41 | 22.59 |
| Na+ (g/kg) | 0.25 | 0.12 | 0.48 | 22.59 |
| HCO3− (g/kg) | 0.18 | 0.05 | 0.27 | 24.18 |
| Cl− (g/kg) | 0.22 | 0.03 | 0.14 | 23.96 |
| SO42− (g/kg) | 4.68 | 0.13 | 0.03 | 24.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, X.; Cao, W. Fertile Island Soils Promote the Restoration of Shrub Patches in Burned Areas in Arid Saline Land. Fire 2023, 6, 341. https://doi.org/10.3390/fire6090341
Wang S, Wang X, Cao W. Fertile Island Soils Promote the Restoration of Shrub Patches in Burned Areas in Arid Saline Land. Fire. 2023; 6(9):341. https://doi.org/10.3390/fire6090341
Chicago/Turabian StyleWang, Shilin, Xiaojun Wang, and Wenxia Cao. 2023. "Fertile Island Soils Promote the Restoration of Shrub Patches in Burned Areas in Arid Saline Land" Fire 6, no. 9: 341. https://doi.org/10.3390/fire6090341
APA StyleWang, S., Wang, X., & Cao, W. (2023). Fertile Island Soils Promote the Restoration of Shrub Patches in Burned Areas in Arid Saline Land. Fire, 6(9), 341. https://doi.org/10.3390/fire6090341






