Abstract
Understanding how fire severity affects resprouting plants during post-fire regeneration is key to anticipating Mediterranean vegetation vulnerability in a context of increasingly large fires with high intensity and severity due to climate change. Here, we monitored the water status, leaf gas exchange, and plant growth in holm oaks (Quercus ilex) of central Spain burned with different fire severity throughout the first post-fire year. The Q. ilex burned with high severity (HB+) showed higher water potential and shoot growth than those burned with low severity (LB+) or unburned (B−), especially during spring and summer. In summer, resprouting HB+ and LB+ plants exhibited higher carbon assimilation than unburned ones. Moreover, we also found that plants with higher water availability and growth, i.e., HB+ individuals, had higher specific leaf area and lower water use efficiency. Overall, our study shows that holm oak forests exhibit high plasticity to fire and that Q. ilex burned with high severity have a faster short-term regeneration than those burned with low severity. However, this rapid regeneration is based on a less conservative water-use strategy, which could jeopardize their populations in case of extreme drought events increasingly common in the current context of climate change.
1. Introduction
Fire plays a major role as a disturbing factor in numerous terrestrial ecosystems [1], being a key ecological factor for understanding the composition and structure of many of these ecosystems [2]. In fact, the presence of fire in Mediterranean ecosystems has been a recurrent disturbance during the last thousands of years, shaping their landscape and determining the distribution and characteristics of the Mediterranean vegetation [3,4]. However, anthropogenic influence on the fire regime has become more pronounced in recent decades, reflecting the effects of climate change and land use changes, and the subsequent development of firefighting and fuel management [5]. In this sense, the increase in temperatures and drought events in Mediterranean areas due to climate change [6] have contributed to a substantial increase in fire risk [7,8], which could increase even further according to climate projections, with large fires becoming more frequent and severe in the coming decades [9,10].
Plant species have developed different mechanisms to survive or persist after a fire, which have traditionally been divided into two regeneration strategies: seeders and resprouters [11]. The two strategies are not mutually exclusive, but rather represent the extremes of a continuous gradient of responses to fire [4]. In seeder species, individuals die during fire but populations persist through recruitment of new seedlings that emerge from seed banks they had previously stored in the soil or canopy [12,13]. By contrast, resprouter species survive the fire despite losing all or much of their aboveground biomass, subsequently regenerating from various types of buds protected from fire [14,15]. Thus, resprouting carries a cost of storing resources belowground to maintain and protect the bud bank and support rapid post-disturbance resprout, whereas seeders allocate the corresponding resources to other functions such as fast growth and reproduction [16,17,18]. Therefore, the two strategies result in two distinct functional groups with different morphological and physiological traits [19,20,21].
Resprouting is a common functional strategy in many plant lineages under different environments and disturbance types, which has been extensively reviewed in the literature [22,23,24]. Moreover, resprouting is morphologically very diverse [4], with buds located in a variety of organs such as branches, roots, root crown, rhizomes, lignotubers, or bulbs, which may be related to the disturbance regime [23]. In this regard, there are major differences between fire and other disturbances since the fire not only defoliates the plant, but also can have lethal effects on meristematic tissues, depletes the litter and changes nutrient dynamics, and kills interacting species [23,25]. Several studies show that plant persistence declines with increasing fire frequency and severity due to a decrease in bud availability, their protection, and the resources to fund regrowth [26,27,28], but the magnitude of the effects could be variable among the species and timescales [29,30]. Anyway, the functional and ecophysiological response of resprouting plants to fire is not fully understood, which is of paramount importance to better understand and protect Mediterranean-type ecosystems.
Here, we studied the effect of fire on the functional response of holm oak (Quercus ilex) individuals burned with different severity. This resprouter species is dominant in sclerophyllous woods and maquis vegetation of the central–western Mediterranean basin [31], so it has been widely studied in regard to fire-related aspects as diverse as paleoecology or ecophysiology, among others [32,33,34,35]. However, to our knowledge, the effect of fire severity on the regeneration capacity of Q. ilex from a functional perspective has not yet been specifically addressed, which is of vital importance in a scenario of climate change such as the current one, in which the large fires are becoming more frequent and severe in Mediterranean environments. We hypothesize that functionality of more severely burned holm oaks could be negatively affected during short-term post-fire regeneration. This hypothesis, based on previous studies reported in other resprouter species [26,27,28,29,30], must be specifically tested for Q. ilex. To this end, we designed a field experiment in which we monitored the water status, leaf gas exchange, and plant growth of holm oaks burned with different severity during their first year of post-fire regeneration. The ultimate objective of the work was to evaluate the degree of regeneration and the functional response of Q. ilex individuals affected by different fire severity.
2. Materials and Methods
2.1. Study Site, Wildfire, and Experimental Design
The study site was located in a holm oak forest in the center of the Iberian Peninsula, with an altitude of ca. 700 m and a northwest gentle slope (39°50′ N, 4°05′ W). The climate is continental Mediterranean, with a mean annual temperature of 15.8 °C, mean annual rainfall of 342 mm, and a pronounced summer drought (Toledo meteorological station, 39°53′ N, 4°02′ W; [36]). Vegetation is dominated by Quercus ilex subsp. rotundifolia Lam., but other species such as Retama sphaerocarpa (L.) Boiss., Stipa tenacissima L., Juniperus oxycedrus L., Pistacia terebinthus L., Rhamnus lycioides L., Daphne gnidium L., and different annual herbaceous species are also present. The parent rock is dominated by pre-Hercynian and Hercynian igneous rocks and the soils are regosols with a pH of around 7.5 and soil organic matter ranging from 5 to 16% [37].
The study area was affected by a wildfire on 28 June 2019 (Figure 1a), which occurred under extreme temperature conditions and high wind intensity that favored the rapid spread of the fire and made it very difficult to extinguish, affecting a total of 1600 ha with different levels of severity. Thus, after the fire, 30 holm oak individuals of similar diameter at breast height (mean DBH of 19 cm) were randomly selected in an extension of ca. 5 ha and subsequently assigned to the three following experimental treatments (n = 10 per treatment): B−, unburned trees adjacent to a burnt area with similar environmental conditions and soil properties; LB+, trees burned with low severity, i.e., whose aboveground part was only partially scorched by the flames during a fire and exhibited epicormic shoots resprouting from the branches after the fire; HB+, trees burned with high severity, i.e., whose aboveground part was almost completely eliminated by the flames during a fire and where resprout after the fire occurred through new basal shoots emerging from the ground (Figure 1b). The post-fire regeneration and functional performance of all these individuals were monitored at the end of each season (autumn 29 November 2019, winter 28 February 2020, spring 2 June 2020, summer 2 September 2020) during the first post-fire year. The meteorological conditions during the study period were recorded by a weather station close to the study area (39°53′ N, 4°02′ W; [36]).

Figure 1.
(a) Overview of the study area during (picture modified from [38]) and after the fire. (b) Detail of an unburned Q. ilex (B−), and resprouts of Q. ilex burned with low (LB+) and high (HB+) severity.
2.2. Plant Functional Monitoring
2.2.1. Water Status
The topsoil moisture (SM) was monitored seasonally at three points on the south face of all plants under study using a volumetric soil water content meter (Model HH2; Delta-T Devices Ltd., Cambridge, UK), which was gravimetrically calibrated beforehand with the natural soil from the study area at different stages of a dryness cycle (R2 = 0.95, p < 0.001).
Predawn shoot water potential (Ψpd) was also recorded seasonally in three south-facing shoots per plant using a Scholander-type pressure chamber. Ψpd was used as an indicator of plant water stress, assuming the absence of night-time transpiration and the possible effect of solute concentration [39,40].
2.2.2. Leaf Gas Exchange
Net carbon assimilation (A) and stomatal conductance (g) were monitored seasonally in three south-facing leaves per plant using a portable IRGA (Model LI-6400; Li-Cor Biosciences Inc., Lincoln, NE, USA). The measures were taken at ca. 8:00 solar time, with chamber parameters set to match the external weather conditions in each season (reference [CO2] at 400 ppm, photosynthetically active radiation (PAR) at 1000–1500 µmol m−2 s−1, leaf temperature at 20–25 °C, and vapor pressure deficit (VPD) at 1.5 kPa approximately).
The intrinsic water use efficiency (WUEi) was inferred as:
where A and g were the net carbon assimilation and stomatal conductance measured previously in the field, respectively.
2.2.3. Plant Growth
For recording shoot growth (SG), three south-facing shoots per plant were tagged at the beginning of the experiment and their maximum lengths were measured seasonally using a flexometer.
For monitoring leaf growth and structure, specific leaf area (SLA) was measured seasonally in three south-facing leaves per plant. To this end, fresh leaves were collected in the field and subsequently scanned using the software ImageJ v.1.53c (National Institutes of Health, Bethesda, MD, USA) to obtain their leaf area (LA). Finally, they were dried (70 °C, 48 h). The SLA was calculated as:
where LA and DW were the leaf area and dry weight, respectively.
3. Statistical Analysis
The generalized estimating equations (GEE) procedure extends the generalized linear model with maximum likelihood (GzLM) to allow for analysis of repeated measurements or other correlated observations, such as the observations recorded in our study individuals during their functional monitoring throughout the first post-fire year. Thus, a GEE test with post hoc least significant difference (LSD) pairwise comparisons was used to assess the effect of fire severity, time, and their interaction on SM, Ψpd, A, WUEi, SG, and SLA variables during the entire study period (n = 40 per treatment). Moreover, since the GEE only indicates whether or not there is a general effect of the different factors during the entire study period, a GzLM test with post hoc LSD pairwise comparisons was also conducted to assess the effect of fire severity in each season (n = 10 per treatment), i.e., autumn, winter, spring, and summer. Following Akaike’s information criterion corrected for finite samples (AICc) after testing various error distributions and link functions, a normal distribution with an identity link was assumed for SM, Ψpd, A, WUEi, and SG variables, and a gamma distribution with a logarithmic link for SLA.
Statistical analyses were implemented in SPSS 28 (IBM Corp., Armonk, NY, USA) and graphing was performed in OriginPro 2019 (OriginLab Corp., Northampton, MA, USA).
4. Results
4.1. Meteorology
The mean temperature recorded during the study period was 17.2 °C, with mean minimum and maximum values of 4.2 °C and 34.6 °C during the winter and summer, respectively. Total accumulated rainfall during the 19–20 meteorological year was 313.5 mm, with spring being the wettest period with a recorded precipitation of 45.8% of the total (Figure 2).
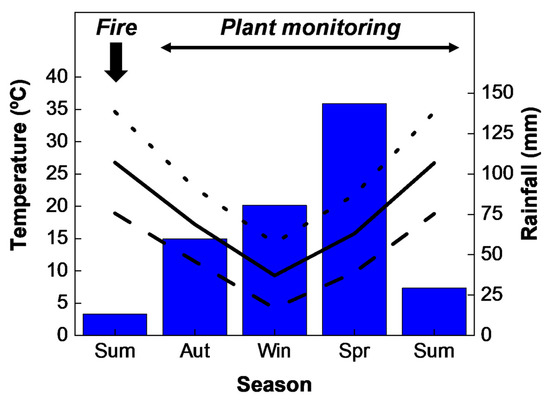
Figure 2.
Mean maximum (dotted line), medium (solid line), and minimum (dashed line) temperature and accumulated rainfall (bars) seasonally recorded during the study period.
4.2. Water Status
No statistically significant differences among treatments (i.e., B−, LB+, and HB+) were observed in soil moisture (SM) during the entire study period (Table 1 and Table 2). In autumn, values of SM around 15% were recorded in all treatments, which gradually decreased until reaching values below 3% in summer (Figure 3a).

Table 1.
Results of the GEE test (χ2 and p values) to evaluate the effect of fire severity, time, and their interaction on SM, Ψpd, A, WUEi, SG, and SLA variables during the entire study period. Statistically significant differences (p ≤ 0.05) are shown in boldface.

Table 2.
Results of the GzLM test (χ2 and p values) to evaluate the effect of fire severity on SM, Ψpd, A, WUEi, SG, and SLA variables in each season during the study period. Statistically significant differences (p ≤ 0.05) are shown in boldface. Lowercase letters denote statistically homogeneous subsets from post hoc LSD pairwise comparisons within each season.
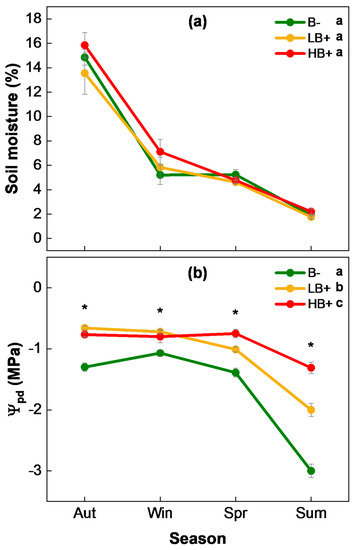
Figure 3.
(a) Soil moisture (SM) and (b) predawn shoot water potential (Ψpd) seasonally recorded (mean ± s.e.) for Q. ilex unburned (B−), and burned with low (LB+) and high (HB+) severity. The asterisks (*) show statistically significant differences among treatments within each season (p ≤ 0.05). Lowercase letters denote statistically homogeneous subsets from post hoc pairwise comparisons for the entire study period.
Regarding predawn shoot water potential (Ψpd), the plants burned with high severity (HB+) showed the highest Ψpd during the entire study period, with low-severity-burned plants (LB+) exhibiting intermediate values and unburned ones (B−) with more negative Ψpd (Table 1 and Table 2). Immediately after the fire, during autumn and winter, HB+ and LB+ plants had a significantly higher Ψpd than B−. During spring and summer, HB+ plants exhibited a very stable Ψpd of around −1.0 MPa, whereas LB+ and B− plants dropped to values of −2.0 and −3.0 MPa, respectively (Figure 3b).
4.3. Leaf Gas Exchange
No statistically significant differences among treatments were observed in net carbon assimilation (A) during the entire study period (Table 1 and Table 2). During autumn, winter, and spring, all plants showed stable values of A between 8 and 13 µmol CO2 m−2 s−1. In summer, A dropped drastically and significant differences between HB+ and LB+ plants (ca. 4 µmol CO2 m−2 s−1) with respect to B− ones (1.4 µmol CO2 m−2 s−1) were found (Figure 4a).
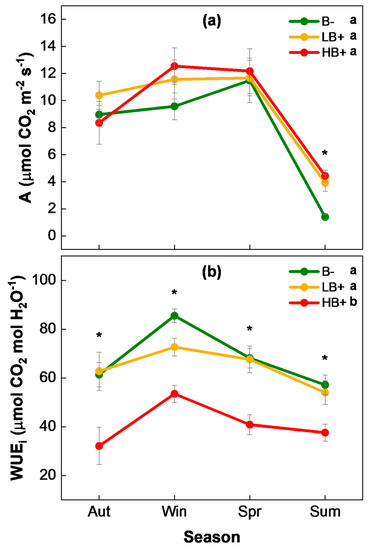
Figure 4.
(a) Net carbon assimilation (A) and (b) intrinsic water use efficiency (WUEi) seasonally recorded (mean ± s.e.) for Q. ilex unburned (B−), and burned with low (LB+) and high (HB+) severity. The asterisks (*) show statistically significant differences among treatments within each season (p ≤ 0.05). Lowercase letters denote statistically homogeneous subsets from post hoc pairwise comparisons for the entire study period.
Regarding intrinsic water use efficiency (WUEi), the HB+ plants showed a significantly lower WUEi than LB+ and B− ones during the entire study period (Table 1 and Table 2). The WUEi was never higher than 55 µmol CO2 mol H2O−1 for the HB+ individuals, whereas the LB+ and B− ones remained with values between 60 and 80 µmol CO2 mol H2O−1. In all plants under study, the maximum values of WUEi were recorded in winter (Figure 4b).
4.4. Plant Growth
Statistically significant differences among the three experimental treatments (HB+, LB+, and B−) in shoot growth (SG) were observed during the entire study period (Table 1 and Table 2). In autumn, the LB+ plants showed slightly higher SG than HB+ and B− ones, all of them with values below 1.0 mm day−1. Vegetative growth stopped in winter, to be reactivated again in spring, where the HB+ plants experienced much higher SG (4.2 mm day−1) than LB+ and B− ones (no more than ca. 1.0 mm day−1). In summer, the HB+ individuals still maintained some growth despite high temperatures and low rainfall, whereas for LB+ and B− treatments the plants practically stopped their growth (Figure 5a). The mortality of the studied individuals was practically null and only one HB+ individual had not resprouted at the end of the study period.

Figure 5.
(a) Shoot growth (SG) and (b) specific leaf area (SLA) seasonally recorded (mean ± s.e.) for Q. ilex unburned (B−), and burned with low (LB+) and high (HB+) severity. The asterisks (*) show statistically significant differences among treatments within each season (p ≤ 0.05). Lowercase letters denote statistically homogeneous subsets from post hoc pairwise comparisons for the entire study period.
Regarding specific leaf area (SLA), the HB+ plants showed the highest SLA during the entire study period, with LB+ plants exhibiting intermediate values and B− ones with lower SLA (Table 1 and Table 2). The HB+ and LB+ plants had values of ca. 60–80 and 50–60 cm2 g−1, respectively, with maximum recordings in autumn and summer. Nevertheless, B− plants showed more stable values during the entire study period, around 40–45 cm2 g−1 (Figure 5b).
5. Discussion
All resprouters are able to allocate resources to where they are needed for plant recovery from disturbance, but resprouting from different types of organs could in turn reflect different types of disturbance [23]. It is often assumed that the higher the severity of the disturbance, the lower the height at which buds resprout new shoots [15,22]. In fact, this is what occurred in our study, where we found that Q. ilex burned with low severity had epicormic shoots resprouting from the branches, whereas Q. ilex burned with high severity had basal shoots resprouting from the ground. However, most emphasis has been placed on whether a species resprouts or not and not much on intraspecific differences or among distinct types of resprouting [16,41], which could have important implications for the speed of regeneration and ecosystem functioning.
From the point of view of water availability, Q. ilex burned with high severity showed a better water status (Ψpd) than Q. ilex burned with low severity and unburned ones, which was especially clear during summer drought months. In this sense, several studies have shown that resprouts of Mediterranean species generally exhibit a better water status during the first few years after fire than unburned plants [42,43,44], which has also been previously observed in Q. ilex [34]. This reinforces the idea that fire has a negative effect at the population level, as many individuals may be killed during the fire, but it could even be positive at the functional level for the surviving individuals [45,46]. This would be possible due to the fact that most of the aboveground leaf biomass of plants is generally consumed during a fire, with a consequent reduction in leaf area index and transpiration rates [47]. However, the root system of resprouters remains practically intact after a fire, which ensures a water supply similar to that before the fire and allows an increase in water availability until the plant recovers within the next post-fire years [48,49]. This fact also explains the better water status of the high-severity-burned holm oaks compared to the low-severity ones that still maintain part of their leaf canopy, which could have important implications for their subsequent post-fire regeneration.
Regarding leaf gas exchange, previous studies in Q. ilex show that resprouts from burned plants had photosynthetic activity (A) similar to that of unburned plants during milder weather periods, but higher photosynthetic rates during summer drought [34], which is in line with our observations and the general pattern found in other Mediterranean species [42,43,44]. However, we found that resprouts from plants burned with high severity showed a lower intrinsic water use efficiency (WUEi) than those burned with low severity or unburned ones. This agrees with a previous observation in other Mediterranean species after fire from δ13C data [45], a proxy of WUEi [50], and it could be supported by the higher water availability recorded in plants burned with high severity. However, our results contrast with the observations of Fleck et al. [51,52], who found higher WUEi in burned Q. ilex individuals compared to unburned ones, although this was observed on 15-month-old resprouts in a population very close to the sea. This could indicate that Q. ilex have high plasticity to adapt their leaf gas exchange to water availability, but also that factors such as the time since fire (age of resprout) or intraspecific differences between populations of the same species or different subspecies may also play a role. To determine the importance of these factors, more studies in different populations and time scales are needed.
The Q. ilex burned with high severity also showed a higher shoot growth (SG) than Q. ilex burned with low severity and unburned ones, especially during the spring and summer of the first post-fire year. This higher growth recorded in severely burned plants is supported by a higher water availability and a less conservative water-use strategy, as mentioned above, which could in turn jeopardize the plant in case of extreme drought. In this regard, resprouting plants rely on the mobilization of stored carbohydrates to fund respiration and regrowth until the plant has recovered photosynthetic capacity to support these costs [16,23,53]. However, root function must also be sustained, so if CO2 uptake is limited by stomatal closure due to drought, then carbohydrates could be depleted leading to death [54,55]. Likewise, it has been documented that resprouts have a higher risk of cavitation than undisturbed plants [55,56], so they could be endangered in case of severe and prolonged drought [24]. On the other hand, we also found that shoots with higher growth, i.e., shoots of Q. ilex burned with high severity, also had leaves with higher specific leaf area (SLA). In this sense, there is extensive evidence of the positive relationship between SLA and leaf lifespan with photosynthetic and respiration rates [57]. Moreover, it shows the high plasticity of Q. ilex leaves to adapt to the environment and stress conditions, as previously reported [35], developing, in this case, more sclerophyllous leaves (i.e., low SLA) in those plants that have a lower water availability (unburned ones), which allows it to better adapt to drought even though this entails higher construction costs [58,59,60].
6. Conclusions
Overall, our study shows that holm oak forests exhibit high plasticity to fire and that Q. ilex burned with high severity have a faster regeneration than those burned with low severity, at least during the first year after a fire. However, this rapid regeneration is based on poor water use efficiency, so that plant recovery could be compromised in case of extreme post-fire drought events, which are becoming increasingly common in Mediterranean ecosystems due to climate change. Therefore, it is essential to carry out further long-term studies, and on different populations and environments in order to clarify the general response of this species to different fire severity and thus be able to anticipate its response and that of the ecosystems it inhabits to present and future threats.
Author Contributions
Conceptualization, M.B.H. and A.P.; methodology, A.P. and M.B.H.; investigation, A.P. and M.B.H.; formal analysis, A.P.; writing—original draft preparation, A.P.; writing—review and editing, A.P. and M.B.H.; visualization, A.P. and M.B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Castilla-La Mancha, through the 2023-GRIN-34447 project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank O. Rioja, A. Landaeta, and A. Carrión for their help during the data processing in the laboratory. We also thank A. Blanco for her priceless help and support at all stages of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowman, D.M.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P. Fire in the Earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Naveh, Z. The evolutionary significance of fire in the Mediterranean region. Vegetatio 1975, 29, 199–208. [Google Scholar] [CrossRef]
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterranean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: New York, NY, USA, 2012. [Google Scholar]
- Bowman, D.M.; Kolden, C.A.; Abatzoglou, J.T.; Johnston, F.H.; van der Werf, G.R.; Flannigan, M. Vegetation fires in the Anthropocene. Nat. Rev. Earth Environ. 2020, 1, 500–515. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef]
- Jolly, W.M.; Cochrane, M.A.; Freeborn, P.H.; Holden, Z.A.; Brown, T.J.; Williamson, G.J.; Bowman, D.M. Climate-induced variations in global wildfire danger from 1979 to 2013. Nat. Commun. 2015, 6, 7537. [Google Scholar] [CrossRef]
- Ruffault, J.; Curt, T.; Moron, V.; Trigo, R.M.; Mouillot, F.; Koutsias, N.; Pimont, F.; Martin-StPaul, N.; Barbero, R.; Dupuy, J.-L. Increased likelihood of heat-induced large wildfires in the Mediterranean Basin. Sci. Rep. 2020, 10, 13790. [Google Scholar] [CrossRef]
- Turco, M.; Rosa-Cánovas, J.J.; Bedia, J.; Jerez, S.; Montávez, J.P.; Llasat, M.C.; Provenzale, A. Exacerbated fires in Mediterranean Europe due to anthropogenic warming projected with non-stationary climate-fire models. Nat. Commun. 2018, 9, 3821. [Google Scholar] [CrossRef]
- Bond, W.J.; van Wilgen, B.W. Fire and Plants; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Keeley, J.E. Role of fire in seed germination of woody taxa in California chaparral. Ecology 1987, 68, 434–443. [Google Scholar] [CrossRef]
- Trabaud, L. Postfire plant community dynamics in the Mediterranean Basin. In The Role of Fire in Mediterranean-Type Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Springer: New York, NY, USA, 1994; pp. 1–15. [Google Scholar]
- Bond, W.J.; Midgley, J.J. Ecology of sprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Klimesova, J.; Klimes, L. Bud banks and their role in vegetative regeneration—A literature review and proposal for simple classification and assessment. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 115–129. [Google Scholar] [CrossRef]
- Moreira, B.; Tormo, J.; Pausas, J.G. To resprout or not to resprout: Factors driving intraspecific variability in resprouting. Oikos 2012, 121, 1577–1584. [Google Scholar] [CrossRef]
- Pate, J.S.; Froend, R.H.; Bowen, B.J.; Hansen, A.; Kuo, J. Seedling growth and storage characteristics of seeder and resprouter species of Mediterranean-type ecosystems of SW Australia. Ann. Bot. 1990, 65, 585–601. [Google Scholar] [CrossRef]
- Schwilk, D.W.; Ackerly, D.D. Is there a cost to resprouting? Seedling growth rate and drought tolerance in sprouting and nonsprouting Ceanothus (Rhamnaceae). Am. J. Bot. 2005, 92, 404–410. [Google Scholar] [CrossRef]
- Ackerly, D. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 2004, 74, 25–44. [Google Scholar] [CrossRef]
- Bell, D.T. Ecological response syndromes in the flora of southwestern Western Australia: Fire resprouters versus reseeders. Bot. Rev. 2001, 67, 417–440. [Google Scholar] [CrossRef]
- Vilagrosa, A.; Hernandez, E.I.; Luis, V.C.; Cochard, H.; Pausas, J.G. Physiological differences explain the co-existence of different regeneration strategies in Mediterranean ecosystems. New Phytol. 2014, 201, 1277–1288. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.; Midgley, J.J.; Lamont, B.; Ojeda, F.; Burrows, G.; Enright, N.; Knox, K. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards understanding resprouting at the global scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Albert-Belda, E.; Gómez-Muñoz, B.; Moreno, J.M. High fire frequency reduces soil fertility underneath woody plant canopies of Mediterranean ecosystems. Sci. Total Environ. 2021, 752, 141877. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Pérez, B.; Moreno, J.M. Resprouting of the Mediterranean-type shrub Erica australis with modified lignotuber carbohydrate content. J. Ecol. 2003, 91, 348–356. [Google Scholar] [CrossRef]
- Enright, N.; Fontaine, J.; Westcott, V.; Lade, J.; Miller, B. Fire interval effects on persistence of resprouter species in Mediterranean-type shrublands. Plant Ecol. 2011, 212, 2071–2083. [Google Scholar] [CrossRef]
- Paula, S.; Ojeda, F. Belowground starch consumption after recurrent severe disturbance in three resprouter species of the genus Erica. Botany 2009, 87, 253–259. [Google Scholar] [CrossRef]
- Fernández, C.; Vega, J.A.; Fonturbel, T. Does fire severity influence shrub resprouting after spring prescribed burning? Acta Oecol. 2013, 48, 30–36. [Google Scholar] [CrossRef]
- Wright, B.R.; Clarke, P.J. Resprouting responses of Acacia shrubs in the Western Desert of Australia—Fire severity, interval and season influence survival. Int. J. Wildland Fire 2007, 16, 317–323. [Google Scholar] [CrossRef]
- de Rigo, D.; Caudullo, G. Quercus ilex in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 152–153. [Google Scholar]
- Carcaillet, C.; Barakat, H.N.; Panaïotis, C.; Loisel, R. Fire and late-Holocene expansion of Quercus ilex and Pinus pinaster on Corsica. J. Veg. Sci. 1997, 8, 85–94. [Google Scholar] [CrossRef]
- de Román, M.; de Miguel, A.M. Post-fire, seasonal and annual dynamics of the ectomycorrhizal community in a Quercus ilex L. forest over a 3-year period. Mycorrhiza 2005, 15, 471–482. [Google Scholar] [CrossRef]
- Fleck, I.; Hogan, K.; Llorens, L.; Abadía, A.; Aranda, X. Photosynthesis and photoprotection in Quercus ilex resprouts after fire. Tree Physiol. 1998, 18, 607–614. [Google Scholar] [CrossRef]
- Limousin, J.; Rambal, S.; Ourcival, J.; Rocheteau, A.; Joffre, R.; Rodriguez-Cortina, R. Long-term transpiration change with rainfall decline in a Mediterranean Quercus ilex forest. Glob. Chang. Biol. 2009, 15, 2163–2175. [Google Scholar] [CrossRef]
- AEMET. Agencia Estatal de Meteorología—Datos abiertos—AEMET OpenData. Available online: https://opendata.aemet.es/centrodedescargas/inicio,15/01/2023 (accessed on 12 June 2023).
- GEODE. Mapas IGME—Portal de Cartografía del IGME: GEODE—Cartografía Geológica Digital Continua a Escala 1:50,000. Available online: https://info.igme.es/cartografiadigital/geologica/Geode.aspx (accessed on 15 January 2023).
- Monroy, J. Un Año del Incendio Histórico Que Amenazó las Urbanizaciones; La Tribuna de Toledo: Toledo, Spain, 2020. [Google Scholar]
- Donovan, L.A.; Richards, J.H.; Linton, M.J. Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 2003, 84, 463–470. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Pausas, J.G.; Keeley, J.E. Epicormic resprouting in fire-prone ecosystems. Trends Plant Sci. 2017, 22, 1008–1015. [Google Scholar] [CrossRef]
- Clemente, A.S.; Rego, F.C.; Correia, O.A. Growth, water relations and photosynthesis of seedlings and resprouts after fire. Acta Oecol. 2005, 27, 233–243. [Google Scholar] [CrossRef]
- DeSouza, J.; Silka, P.; Davis, S. Comparative physiology of burned and unburned Rhus laurina after chaparral wildfire. Oecologia 1986, 71, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Fleck, I.; Diaz, C.; Pascual, M.; Iniguez, F. Ecophysiological differences between first-year resprouts after wildfire and unburned vegetation of Arbutus unedo and Coriaria myrtifolia. Acta Oecologica 1995, 16, 55–69. [Google Scholar]
- Parra, A.; Moreno, J.M. Post-fire environments are favourable for plant functioning of seeder and resprouter Mediterranean shrubs, even under drought. New Phytol. 2017, 214, 1118–1131. [Google Scholar] [CrossRef]
- Parra, A.; Moreno, J.M. Drought differentially affects the post-fire dynamics of seeders and resprouters in a Mediterranean shrubland. Sci. Total Environ. 2018, 626, 1219–1229. [Google Scholar] [CrossRef]
- McMichael, C.; Hope, A.; Roberts, D.; Anaya, M. Post-fire recovery of leaf area index in California chaparral: A remote sensing-chronosequence approach. Int. J. Remote Sens. 2004, 25, 4743–4760. [Google Scholar] [CrossRef]
- Rambal, S. Fire and water yield: A survey and predictions for global change. In The Role of Fire in Mediterranean-Type Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Springer: New York, NY, USA, 1994; pp. 96–116. [Google Scholar]
- Silva, J.S.; Rego, F.C.; Mazzoleni, S. Soil water dynamics after fire in a Portuguese shrubland. Int. J. Wildland Fire 2006, 15, 99–111. [Google Scholar] [CrossRef]
- Werner, C.; Schnyder, H.; Cuntz, M.; Keitel, C.; Zeeman, M.J.; Dawson, T.; Badeck, F.-W.; Brugnoli, E.; Ghashghaie, J.; Grams, T.E. Progress and challenges in using stable isotopes to trace plant carbon and water relations across scales. Biogeosciences 2012, 9, 3083–3111. [Google Scholar] [CrossRef]
- Fleck, I.; Grau, D.; Sanjosé, M.; Vidal, D. Carbon isotope discrimination in Quercus ilex resprouts after fire and tree-fell. Oecologia 1996, 105, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Fleck, I.; Grau, D.; Sanjosé, M.; Vidal, D. Influence of fire and tree-fell on physiological parameters in Quercus ilex resprouts. Ann. Sci. For. 1996, 53, 337–348. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Schulze, E.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Pratt, R.B.; Jacobsen, A.L.; Ramirez, A.R.; Helms, A.M.; Traugh, C.A.; Tobin, M.F.; Heffner, M.S.; Davis, S.D. Mortality of resprouting chaparral shrubs after a fire and during a record drought: Physiological mechanisms and demographic consequences. Glob. Chang. Biol. 2014, 20, 893–907. [Google Scholar] [CrossRef]
- Ramirez, A.; Pratt, R.; Jacobsen, A.; Davis, S. Exotic deer diminish post-fire resilience of native shrub communities on Santa Catalina Island, southern California. Plant Ecol. 2012, 213, 1037–1047. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef]
- Villar, R.; Merino, J. Comparison of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytol. 2001, 151, 213–226. [Google Scholar] [CrossRef]
- Salleo, S.; Nardini, A.; Lo Gullo, M. Is sclerophylly of Mediterranean evergreens an adaptation to drought? New Phytol. 1997, 135, 603–612. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).