Effects of Fire Frequency Regimes on Flammability and Leaf Economics of Non-Graminoid Vegetation
Abstract
1. Introduction
2. Materials and Methods
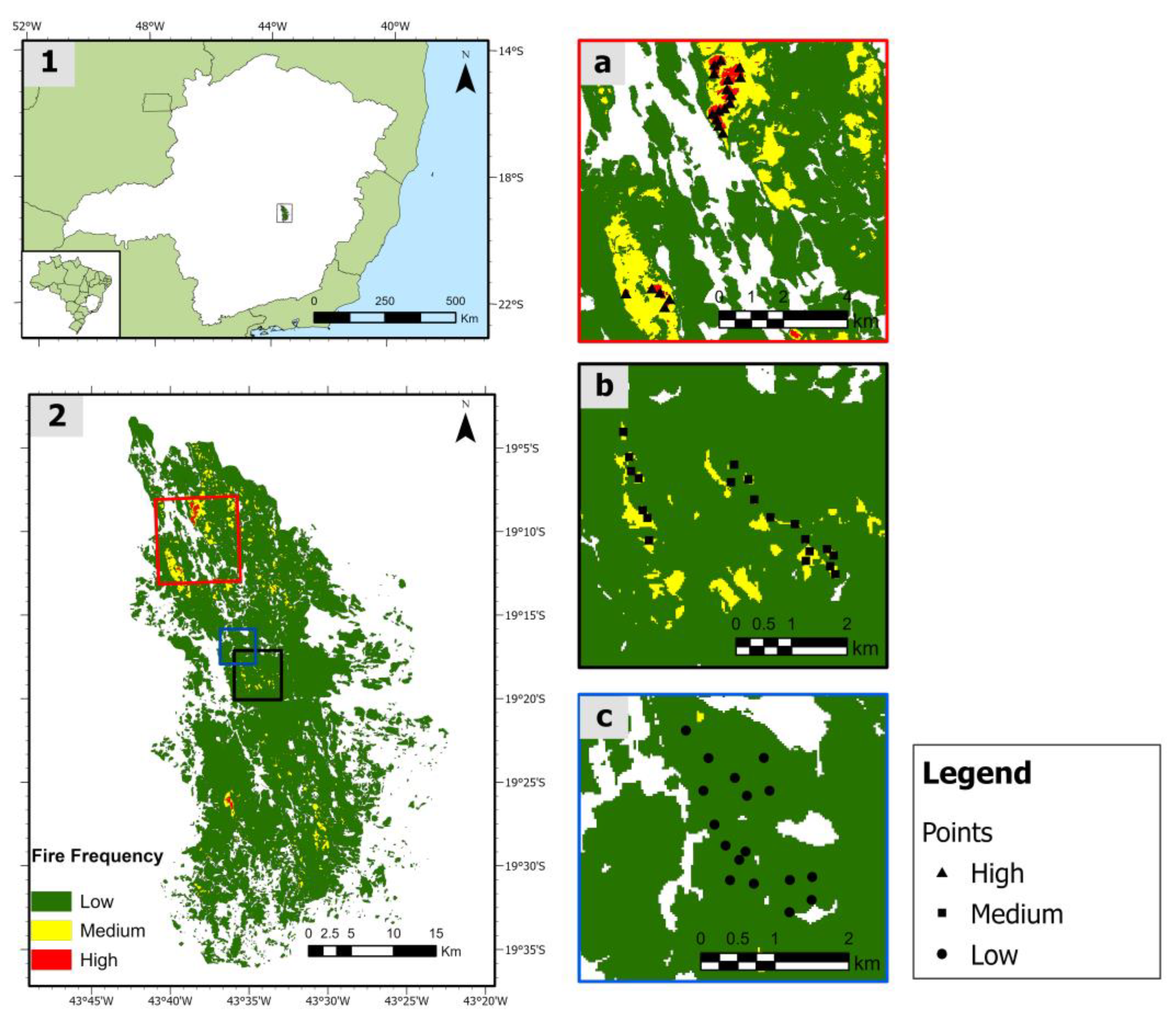
2.1. Study Site
2.2. Fire Regime Classification
2.3. Sampling
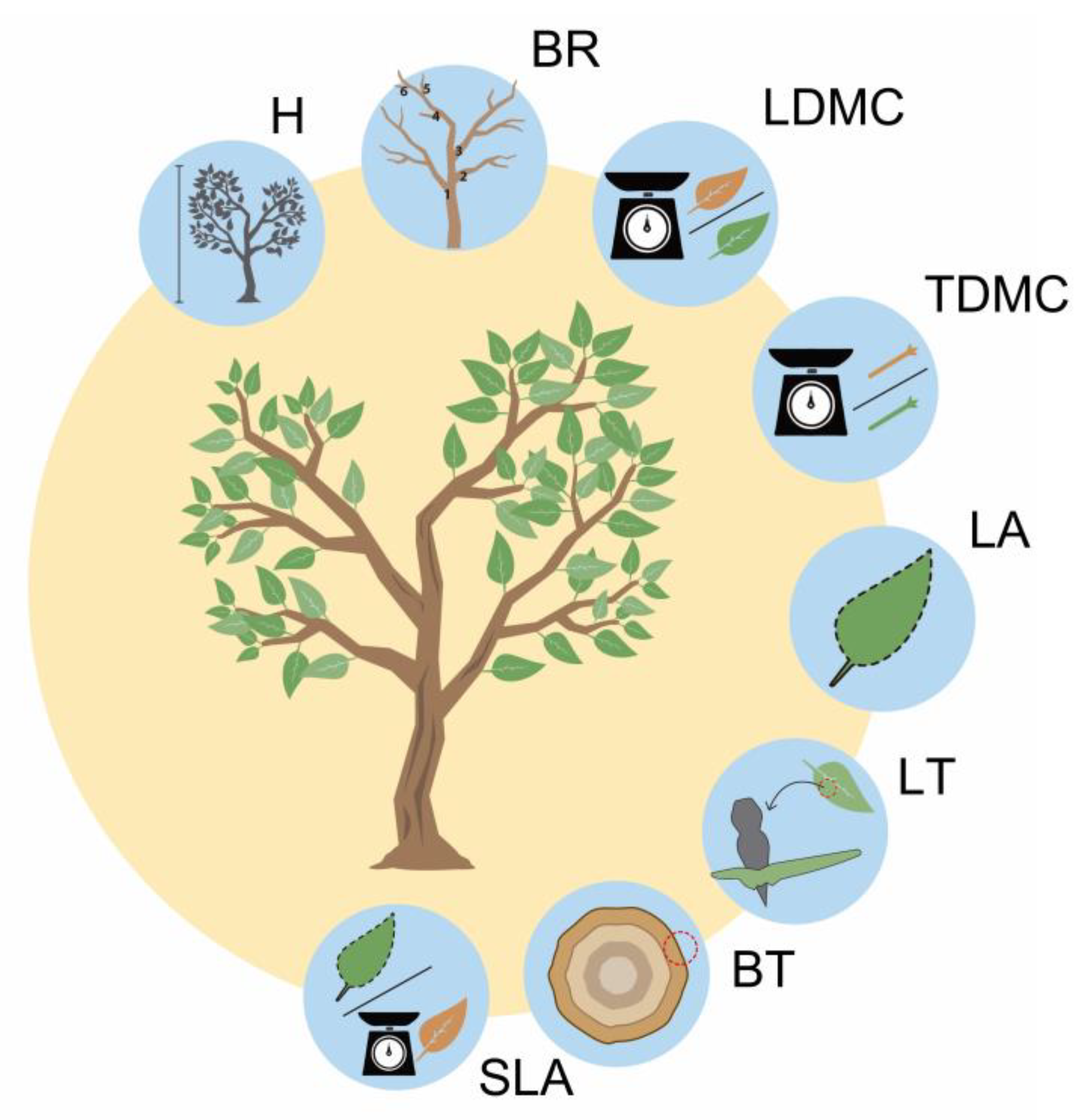
2.4. Functional Traits
3. Data Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition | Unit |
| BR | Branching architecture | # order of ramifications |
| BT | Bark Thickness | mm |
| H | Height | m |
| LA | Leaf Area | mm2 |
| LDMC | Leaf Dry Matter Content | mg g−1 |
| LT | Leaf Toughness | kgf |
| SLA | Specific Leaf Area | mm2 mg−1 |
| TDMC | Twig Dry Matter Content | mg g−1 |
References
- Bond, W.; Keeley, J. Fire as a Global ‘Herbivore’: The Ecology and Evolution of Flammable Ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, M.; Hanan, N.P.; Scholes, R.J.; Ratnam, J.; Augustine, D.J.; Cade, B.S.; Gignoux, J.; Higgins, S.I.; Le Roux, X.; Ludwig, F.; et al. Determinants of Woody Cover in African Savannas. Nature 2005, 438, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.E.R.; Anderson, T.M.; Sankaran, M.; Higgins, S.I.; Archibald, S.; Hoffmann, W.A.; Hanan, N.P.; Williams, R.J.; Fensham, R.J.; Felfili, J.; et al. Savanna Vegetation-Fire-Climate Relationships Differ among Continents. Science 2014, 343, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.F.; Grether, R.; de Queiroz, L.P.; Skema, C.; Pennington, R.T.; Hughes, C.E. Recent Assembly of the Cerrado, a Neotropical Plant Diversity Hotspot, by in Situ Evolution of Adaptations to Fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef] [PubMed]
- Dantas, V.; Batalha, M.A.; Pausas, J.G. Fire Drives Functional Thresholds on the Savanna–Forest Transition. Ecology 2013, 94, 2454–2463. [Google Scholar] [CrossRef]
- Ewing, A.L.; Engle, D.M. Effects of Late Summer Fire on Tallgrass Prairie Microclimate and Community Composition. Am. Midl. Nat. 1988, 120, 212. [Google Scholar] [CrossRef]
- Veldman, J.W.; Buisson, E.; Durigan, G.; Fernandes, G.W.; Le Stradic, S.; Mahy, G.; Negreiros, D.; Overbeck, G.E.; Veldman, R.G.; Zaloumis, N.P.; et al. Toward an Old-Growth Concept for Grasslands, Savannas, and Woodlands. Front. Ecol. Environ. 2015, 13, 154–162. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Orthen, B.; do Nascimento, P.K.V. Comparative Fire Ecology of Tropical Savanna and Forest Trees: Fire Traits of Savanna and Forest Trees. Funct. Ecol. 2003, 17, 720–726. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bradstock, R.A.; Keith, D.A.; Keeley, J.E. Plant Functional Traits in Relation to Fire in Crown-Fire Ecosystems. Ecology 2004, 85, 1085–1100. [Google Scholar] [CrossRef]
- Cowling, R.M.; Pressey, R.L. Rapid Plant Diversification: Planning for an Evolutionary Future. Proc. Natl. Acad. Sci. USA 2001, 98, 5452–5457. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an Evolutionary Pressure Shaping Plant Traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, A.; Rosalem, P.; Zanzarini, V.; Camargos, L.S.; Martins, A.R. From Ashes to Flowers: A Savanna Sedge Initiates Flowers 24 h after Fire. Ecology 2019, 100, e02648. [Google Scholar] [CrossRef] [PubMed]
- Zirondi, H.L.; Ooi, M.K.J.; Fidelis, A. Fire-triggered Flowering Is the Dominant Post-fire Strategy in a Tropical Savanna. J. Veg. Sci. 2021, 32, e12995. [Google Scholar] [CrossRef]
- Fidelis, A.; Appezzato-da-Glória, B.; Pillar, V.D.; Pfadenhauer, J. Does Disturbance Affect Bud Bank Size and Belowground Structures Diversity in Brazilian Subtropical Grasslands? Flora-Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 110–116. [Google Scholar] [CrossRef]
- Melo-de-Pinna, G.F.D.A.; Edson-Chaves, B.; Menezes-e-Vasconcelos, K.; de Lemos, R.C.; Santos-da-Cruz, B.; Devecchi, M.F.; Pirani, J.R. Underground System of Geoxylic Species of Homalolepis Turcz. (Simaroubaceae, Sapindales) from the Brazilian Cerrado. Braz. J. Bot. 2022, 45, 515–525. [Google Scholar] [CrossRef]
- Simon, M.F.; Pennington, T. Evidence for Adaptation to Fire Regimes in the Tropical Savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 173, 711–723. [Google Scholar] [CrossRef]
- Alves, R.J.V.; Silva, N.G. O fogo é sempre um vilão nos campos rupestres? Biodivers. Bras. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Kolbek, J.; Alves, R. Impacts of Cattle, Fire and Wind in Rocky Savannas, Southeastern Brazil. Acta Univ. Carol. Environ. 2008, 22, 111–130. [Google Scholar]
- Ribeiro, K.T.; Fernandes, G.W. Patterns of Abundance of a Narrow Endemic Species in a Tropical and Infertile Montane Habitat. Plant Ecol. 2000, 147, 205–217. [Google Scholar] [CrossRef]
- Fernandes, A.F.; Oki, Y.; Fernandes, G.W.; Moreira, B. The Effect of Fire on Seed Germination of Campo Rupestre Species in the South American Cerrado. Plant Ecol. 2021, 222, 45–55. [Google Scholar] [CrossRef]
- Le Stradic, S.; Silveira, F.A.O.; Buisson, E.; Cazelles, K.; Carvalho, V.; Fernandes, G.W. Diversity of Germination Strategies and Seed Dormancy in Herbaceous Species of Campo Rupestre Grasslands: Germination in Tropical Grasslands. Austral Ecol. 2015, 40, 537–546. [Google Scholar] [CrossRef]
- Moreira, B.; Castellanos, M.C.; Pausas, J.G. Genetic Component of Flammability Variation in a Mediterranean Shrub. Mol. Ecol. 2014, 23, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Conceição, A.A.; Rapini, A.; do Carmo, F.F.; Brito, J.C.; Silva, G.A.; Neves, S.P.S.; Jacobi, C.M. Rupestrian Grassland Vegetation, Diversity, and Origin. In Ecology and Conservation of Mountaintop Grasslands in Brazil; Fernandes, G.W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 105–127. ISBN 978-3-319-29807-8. [Google Scholar]
- Conceição, A.A.; Alencar, T.G.; Souza, J.M.; Moura, A.D.C.; Silva, G.A. Massive Post-Fire Flowering Events in a Tropical Mountain Region of Brazil: High Episodic Supply of Floral Resources. Acta Bot. Bras. 2013, 27, 847–850. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E.; Schwilk, D.W. Flammability as an Ecological and Evolutionary Driver. J. Ecol. 2017, 105, 289–297. [Google Scholar] [CrossRef]
- Schwilk, D.W. Dimensions of Plant Flammability. New Phytol. 2015, 206, 486–488. [Google Scholar] [CrossRef]
- Grootemaat, S.; Wright, I.J.; Bodegom, P.M.; Cornelissen, J.H.C.; Cornwell, W.K. Burn or Rot: Leaf Traits Explain Why Flammability and Decomposability Are Decoupled across Species. Funct. Ecol. 2015, 29, 1486–1497. [Google Scholar] [CrossRef]
- Murray, B.R.; Hardstaff, L.K.; Phillips, M.L. Differences in Leaf Flammability, Leaf Traits and Flammability-Trait Relationships between Native and Exotic Plant Species of Dry Sclerophyll Forest. PLoS ONE 2013, 8, e79205. [Google Scholar] [CrossRef]
- Alam, M.A.; Wyse, S.V.; Buckley, H.L.; Perry, G.L.W.; Sullivan, J.J.; Mason, N.W.H.; Buxton, R.; Richardson, S.J.; Curran, T.J. Shoot Flammability Is Decoupled from Leaf Flammability, but Controlled by Leaf Functional Traits. J. Ecol. 2020, 108, 641–653. [Google Scholar] [CrossRef]
- Krix, D.W.; Phillips, M.L.; Murray, B.R. Relationships among Leaf Flammability Attributes and Identifying Low-Leaf-Flammability Species at the Wildland–Urban Interface. Int. J. Wildland Fire 2019, 28, 295. [Google Scholar] [CrossRef]
- Chuvieco, E.; Aguado, I.; Dimitrakopoulos, A.P. Conversion of Fuel Moisture Content Values to Ignition Potential for Integrated Fire Danger Assessment. Can. J. For. Res. 2004, 34, 2284–2293. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A Handbook of Protocols for Standardised and Easy Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2003, 51, 335. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The World-Wide ‘Fast-Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation Classification by Reference to Strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A Global Method for Calculating Plant CSR Ecological Strategies Applied across Biomes World-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Franco, A.C. Comparative Growth Analysis of Tropical Forest and Savanna Woody Plants Using Phylogenetically Independent Contrasts. J. Ecol. 2003, 91, 475–484. [Google Scholar] [CrossRef]
- Simpson, K.J.; Ripley, B.S.; Christin, P.; Belcher, C.M.; Lehmann, C.E.R.; Thomas, G.H.; Osborne, C.P. Determinants of Flammability in Savanna Grass Species. J. Ecol. 2016, 104, 138–148. [Google Scholar] [CrossRef]
- Cochrane, M.A.; Laurance, W.F. Synergisms among Fire, Land Use, and Climate Change in the Amazon. AMBIO J. Hum. Environ. 2008, 37, 522–527. [Google Scholar] [CrossRef]
- Piñol, J.; Terradas, J.; Lloret, F. Climate Warming, Wildfire Hazard, and Wildfire Occurrence in Coastal Eastern Spain. Clim. Chang. 1998, 38, 345–357. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of Invasive Alien Plants on Fire Regimes. BioScience 2004, 54, 677. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting Changes in Community Composition and Ecosystem Functioning from Plant Traits: Revisiting the Holy Grail: Plant Response and Effect Groups. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Keeley, J.E. Reexamining Fire Suppression Impacts on Brushland Fire Regimes. Science 1999, 284, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- De Paula Loiola, P.; Cianciaruso, M.V.; Silva, I.A.; Batalha, M.A. Functional Diversity of Herbaceous Species under Different Fire Frequencies in Brazilian Savannas. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 674–681. [Google Scholar] [CrossRef]
- Alvarado, S.T.; Fornazari, T.; Cóstola, A.; Morellato, L.P.C.; Silva, T.S.F. Drivers of Fire Occurrence in a Mountainous Brazilian Cerrado Savanna: Tracking Long-Term Fire Regimes Using Remote Sensing. Ecol. Indic. 2017, 78, 270–281. [Google Scholar] [CrossRef]
- Figueira, J.E.C.; Ribeiro, K.T.; Ribeiro, M.C.; Jacobi, C.M.; França, H.; de Oliveira Neves, A.C.; Conceição, A.A.; Mourão, F.A.; Souza, J.M.; de Knegt Miranda, C.A. Fire in Rupestrian Grasslands: Plant Response and Management. In Ecology and Conservation of Mountaintop Grasslands in Brazil; Fernandes, G.W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 415–448. ISBN 978-3-319-29807-8. [Google Scholar]
- Alves, R.J.V.; Cardin, L.; Kropf, M.S. Angiosperm Disjunction “Campos Rupestres—Restingas”: A Re-Evaluation. Acta Bot. Bras. 2007, 21, 675–685. [Google Scholar] [CrossRef]
- Silveira, F.A.O.; Negreiros, D.; Barbosa, N.P.U.; Buisson, E.; Carmo, F.F.; Carstensen, D.W.; Conceição, A.A.; Cornelissen, T.G.; Echternacht, L.; Fernandes, G.W.; et al. Ecology and Evolution of Plant Diversity in the Endangered Campo Rupestre: A Neglected Conservation Priority. Plant Soil 2016, 403, 129–152. [Google Scholar] [CrossRef]
- Alves, R.J.V.; Kolbek, J. Can Campo Rupestre Vegetation Be Floristically Delimited Based on Vascular Plant Genera? Plant Ecol. 2010, 207, 67–79. [Google Scholar] [CrossRef]
- Fernandes, G.W. (Ed.) Ecology and Conservation of Mountaintop Grasslands in Brazil; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-29807-8. [Google Scholar]
- Rapini, A.; Ribeiro, P.L.; Lambert, S.M.; Pirani, J.R. A Flora dos Campos Rupestres da Cadeia do Espinhaço. Megadiversidade 2008, 4, 16–24. [Google Scholar]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167. [Google Scholar] [CrossRef]
- Silva, D.M.; Batalha, M.A. Defense Syndromes against Herbivory in a Cerrado Plant Community. Plant Ecol. 2011, 212, 181–193. [Google Scholar] [CrossRef]
- Spasojevic, M.J.; Aicher, R.J.; Koch, G.R.; Marquardt, E.S.; Mirotchnick, N.; Troxler, T.G.; Collins, S.L. Fire and Grazing in a Mesic Tallgrass Prairie: Impacts on Plant Species and Functional Traits. Ecology 2010, 91, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Popović, Z.; Bojović, S.; Marković, M.; Cerdà, A. Tree Species Flammability Based on Plant Traits: A Synthesis. Sci. Total Environ. 2021, 800, 149625. [Google Scholar] [CrossRef] [PubMed]
- Alessio, G.A.; Peñuelas, J.; Llusià, J.; Ogaya, R.; Estiarte, M.; De Lillis, M. Influence of Water and Terpenes on Flammability in Some Dominant Mediterranean Species. Int. J. Wildland Fire 2008, 17, 274. [Google Scholar] [CrossRef]
- De Lillis, M.; Bianco, P.M.; Loreto, F. The Influence of Leaf Water Content and Isoprenoids on Flammability of Some Mediterranean Woody Species. Int. J. Wildland Fire 2009, 18, 203. [Google Scholar] [CrossRef]
- Xanthopoulos, G.; Wakimoto, R.H. A Time to Ignition–Temperature–Moisture Relationship for Branches of Three Western Conifers. Can. J. For. Res. 1993, 23, 253–258. [Google Scholar] [CrossRef]
- Abedi, M.; Omidipour, R.; Hosseini, S.V.; Bahalkeh, K.; Gross, N. Fire Disturbance Effects on Plant Taxonomic and Functional Β-diversity Mediated by Topographic Exposure. Ecol. Evol. 2022, 12, e8552. [Google Scholar] [CrossRef] [PubMed]
- Keddy, P.A. Assembly and Response Rules: Two Goals for Predictive Community Ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Montserrat-Martí, G.; Charles, M.; Jones, G.; Wilson, P.; Shipley, B.; Sharafi, M.; Cerabolini, B.E.L.; Cornelissen, J.H.C.; Band, S.R.; et al. Is Leaf Dry Matter Content a Better Predictor of Soil Fertility than Specific Leaf Area? Ann. Bot. 2011, 108, 1337–1345. [Google Scholar] [CrossRef]
- Cianciaruso, M.V.; Silva, I.A.; Batalha, M.A.; Gaston, K.J.; Petchey, O.L. The Influence of Fire on Phylogenetic and Functional Structure of Woody Savannas: Moving from Species to Individuals. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 205–216. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary Ecology of Resprouting and Seeding in Fire-prone Ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- Batalha, M.A.; Silva, I.A.; Cianciaruso, M.V.; De Carvalho, G.H. Trait Diversity on the Phylogeny of Cerrado Woody Species. Oikos 2011, 120, 1741–1751. [Google Scholar] [CrossRef]
- Mason, C.M.; Donovan, L.A. Does Investment in Leaf Defenses Drive Changes in Leaf Economic Strategy? A Focus on Whole-Plant Ontogeny. Oecologia 2015, 177, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Schwilk, D.W. Flammability Is a Niche Construction Trait: Canopy Architecture Affects Fire Intensity. Am. Nat. 2003, 162, 725–733. [Google Scholar] [CrossRef]
- Rundel, P.W. Fire as an Ecological Factor. In Physiological Plant Ecology I; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1981; pp. 501–538. ISBN 978-3-642-68092-2. [Google Scholar]
- Souza, J.P.; Albino, A.L.S.; Prado, C.H.B.A. Evidence of the Effects of Fire on Branching and Leaf Development in Cerrado Trees. Acta Bot. Bras. 2017, 31, 677–685. [Google Scholar] [CrossRef]
- Tilman, D. Plant Strategies and the Dynamics and Structure of Plant Communities. (MPB-26), Volume 26; Princeton University Press: Princeton, NJ, USA, 1988; ISBN 978-0-691-20959-3. [Google Scholar]
- Fernandes, P.M.; Cruz, M.G. Plant Flammability Experiments Offer Limited Insight into Vegetation–Fire Dynamics Interactions. New Phytol. 2012, 194, 606–609. [Google Scholar] [CrossRef]
- Negreiros, D.; Le Stradic, S.; Fernandes, G.W.; Rennó, H.C. CSR Analysis of Plant Functional Types in Highly Diverse Tropical Grasslands of Harsh Environments. Plant Ecol. 2014, 215, 379–388. [Google Scholar] [CrossRef]
- Carbone, L.M.; Aguilar, R. Contrasting Effects of Fire Frequency on Plant Traits of Three Dominant Perennial Herbs from Chaco Serrano: Fire Modulates Plant Traits. Austral Ecol. 2016, 41, 778–790. [Google Scholar] [CrossRef]
- Santacruz-García, A.C.; Bravo, S.; del Corro, F.; Ojeda, F. A Comparative Assessment of Plant Flammability through a Functional Approach: The Case of Woody Species from Argentine Chaco Region: A Comparative Assessment of Plant Flammability. Austral Ecol. 2019, 44, 1416–1429. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Barbosa, N.P.U.; Alberton, B.; Barbieri, A.; Dirzo, R.; Goulart, F.; Guerra, T.J.; Morellato, L.P.C.; Solar, R.R.C. The Deadly Route to Collapse and the Uncertain Fate of Brazilian Rupestrian Grasslands. Biodivers. Conserv. 2018, 27, 2587–2603. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Arantes-Garcia, L.; Barbosa, M.; Barbosa, N.P.U.; Batista, E.K.L.; Beiroz, W.; Resende, F.M.; Abrahão, A.; Almada, E.D.; Alves, E.; et al. Biodiversity and Ecosystem Services in the Campo Rupestre: A Road Map for the Sustainability of the Hottest Brazilian Biodiversity Hotspot. Perspect. Ecol. Conserv. 2020, 18, 213–222. [Google Scholar] [CrossRef]
- Barbosa, N.P.U.; Fernandes, G.W. Rupestrian Grassland: Past, Present and Future Distribution. In Ecology and Conservation of Mountaintop Grasslands in Brazil; Fernandes, G.W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 531–544. ISBN 978-3-319-29807-8. [Google Scholar]
- Schaefer, C.E.G.R.; Corrêa, G.R.; Candido, H.G.; Arruda, D.M.; Nunes, J.A.; Araujo, R.W.; Rodrigues, P.M.S.; Fernandes Filho, E.I.; Pereira, A.F.S.; Brandão, P.C.; et al. The Physical Environment of Rupestrian Grasslands (Campos Rupestres) in Brazil: Geological, Geomorphological and Pedological Characteristics, and Interplays. In Ecology and Conservation of Mountaintop Grasslands in Brazil; Fernandes, G.W., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 15–53. ISBN 978-3-319-29807-8. [Google Scholar]
- De Carvalho Barbosa, B.; Siqueira Cappi, V.; Pontes Ribeiro, S.; Fernandes, W. Avaliação da capacidade de rebrotamento pós-distúrbio das plantas lenhosas típicas dos campos rupestres. Ecol. Austral 2014, 24, 350–355. [Google Scholar] [CrossRef]
- Le Stradic, S.; Hernandez, P.; Fernandes, G.W.; Buisson, E. Regeneration after Fire in Campo Rupestre: Short- and Long-Term Vegetation Dynamics. Flora 2018, 238, 191–200. [Google Scholar] [CrossRef]
| Fire Regime | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Low | Medium | High | Deviance | Resid. Df | Resid. Dev | F | R² | p-Value 1 | F-Test p-Value (LMM vs. LM) |
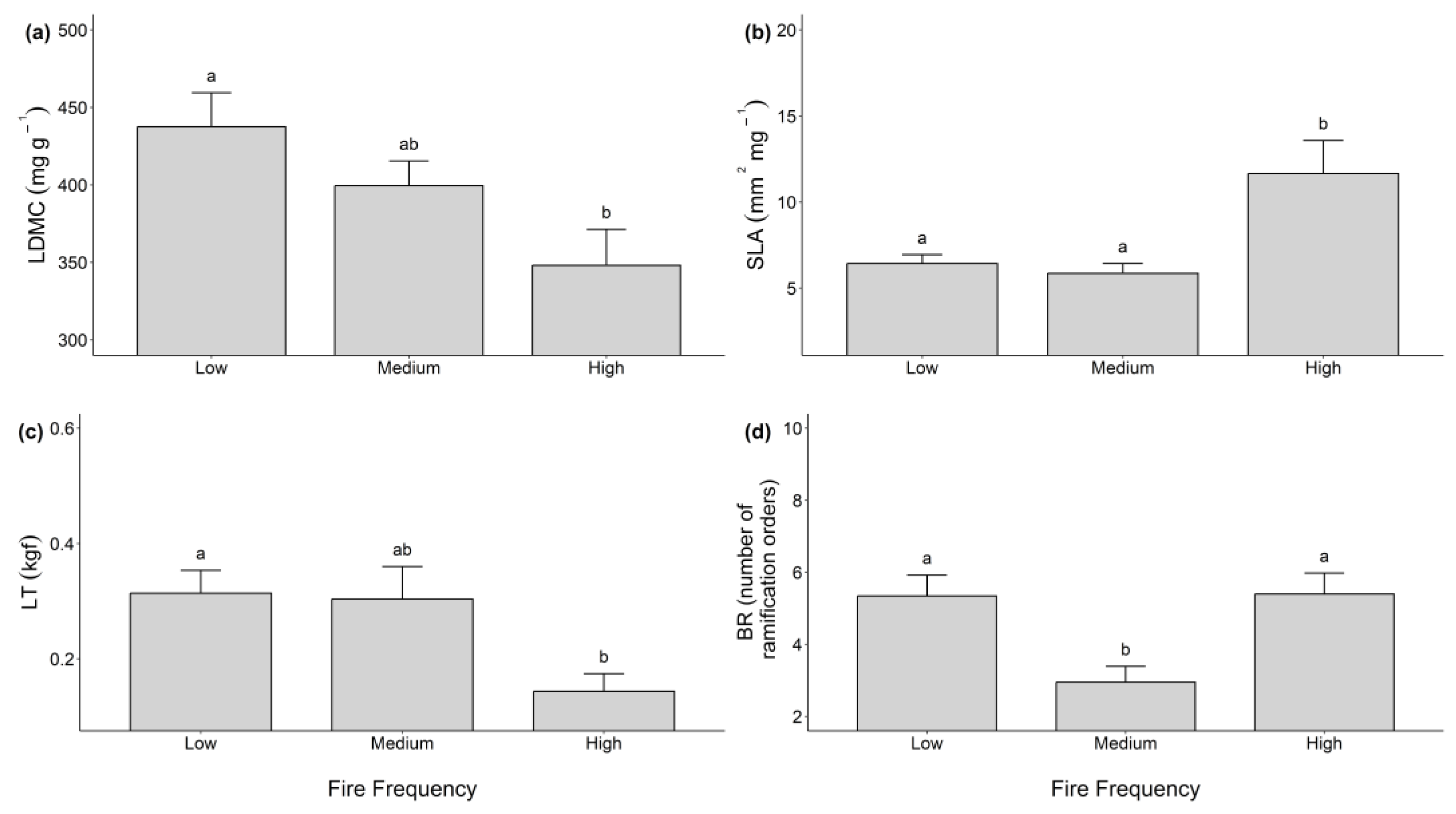
| LDMC (mg g−1) | 437.57 ± 41.98 a | 399.45 ± 52.78 ab | 348.14 ± 49.66 b | 81,323 | 68 | 541920 | 5.1022 | 0.131 | 0.0086 | 0.254 |
| SLA (mm2 mg−1) | 6.44 ± 2.44 a | 5.86 ± 3.06 b | 11.66 ± 3.29 b | 477.09 | 68 | 1832.7 | 8.8512 | 0.207 | 0.0003 | 0.341 |
| LT (kgf) | 0.313 ± 0.13 a | 0.303 ± 0.10 ab | 0.143 ± 0.14 b | 0.36524 | 58 | 2.4403 | 4.3404 | 0.13 | 0.0175 | 0.7892 |
| BR (# order of ramifications) | 5.34 ± 1.10 a | 2.95 ± 1.38 b | 5.39 ± 1.48 a | 102.28 | 68 | 373.56 | 9.3097 | 0.215 | 0.0002 | 0.4088 |
| H (m) | 1.14 ± 0.23 a | 0.79 ± 0.29 a | 0.95 ± 0.31 a | 1.43 | 68 | 16.855 | 2.8847 | 0.078 | 0.0627 | 0.138 |
| TDMC (mg g−1) | 450.44 ± 52.33 a | 469.23 ± 67.57 a | 398.15 ± 71.03 a | 55,424 | 56 | 611651 | 2.5372 | 0.08 | 0.0881 | 0.9545 |
| LA (mm²) | 1435.61 ± 2492.42 a | 1252.39 ± 3133.57 a | 3250.32 ± 3360.79 a | 408,041 | 67 | 2.00 × 10⁸ | 0.0523 | 0.029 | 0.9491 | 0.6831 |
| BT (mm) | 3.44 ± 3.36 a | 3.89 ± 4.28 a | 4.23 ± 4.58 a | 6.1287 | 65 | 3320.5 | 0.06 | 0.002 | 0.9418 | 0.9887 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamounier Moura, A.; Negreiros, D.; Fernandes, G.W. Effects of Fire Frequency Regimes on Flammability and Leaf Economics of Non-Graminoid Vegetation. Fire 2023, 6, 265. https://doi.org/10.3390/fire6070265
Lamounier Moura A, Negreiros D, Fernandes GW. Effects of Fire Frequency Regimes on Flammability and Leaf Economics of Non-Graminoid Vegetation. Fire. 2023; 6(7):265. https://doi.org/10.3390/fire6070265
Chicago/Turabian StyleLamounier Moura, Arthur, Daniel Negreiros, and Geraldo Wilson Fernandes. 2023. "Effects of Fire Frequency Regimes on Flammability and Leaf Economics of Non-Graminoid Vegetation" Fire 6, no. 7: 265. https://doi.org/10.3390/fire6070265
APA StyleLamounier Moura, A., Negreiros, D., & Fernandes, G. W. (2023). Effects of Fire Frequency Regimes on Flammability and Leaf Economics of Non-Graminoid Vegetation. Fire, 6(7), 265. https://doi.org/10.3390/fire6070265







