Prescribed Burning Effect on the Richness, Diversity and Forest Structure of an Endemic Reforested Pinus canariensis Stand (Canary Islands)
Abstract
1. Introduction
2. Materials and Methods
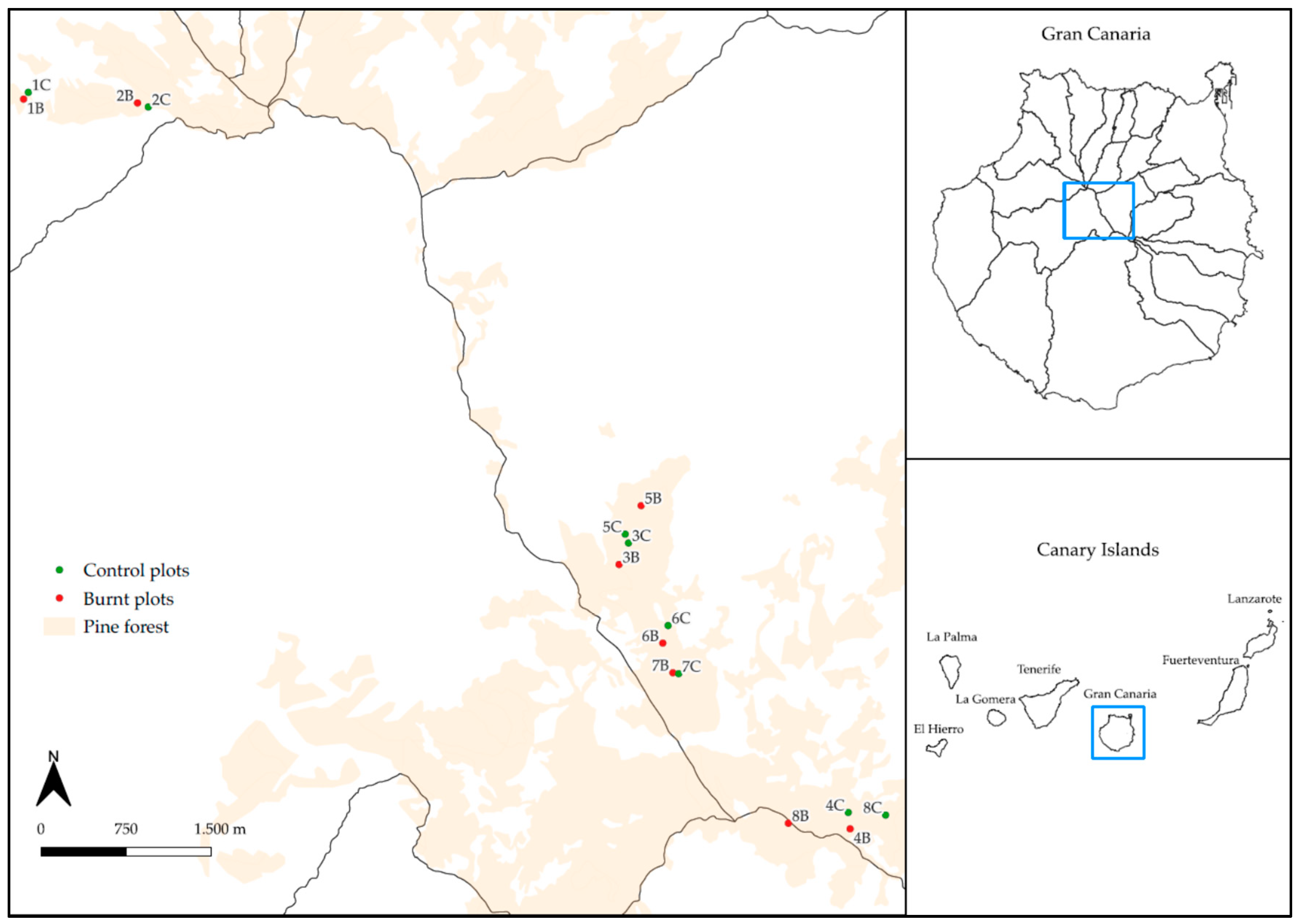
2.1. Study Site
2.2. Design of the Experiment
2.3. Statistical Analyses
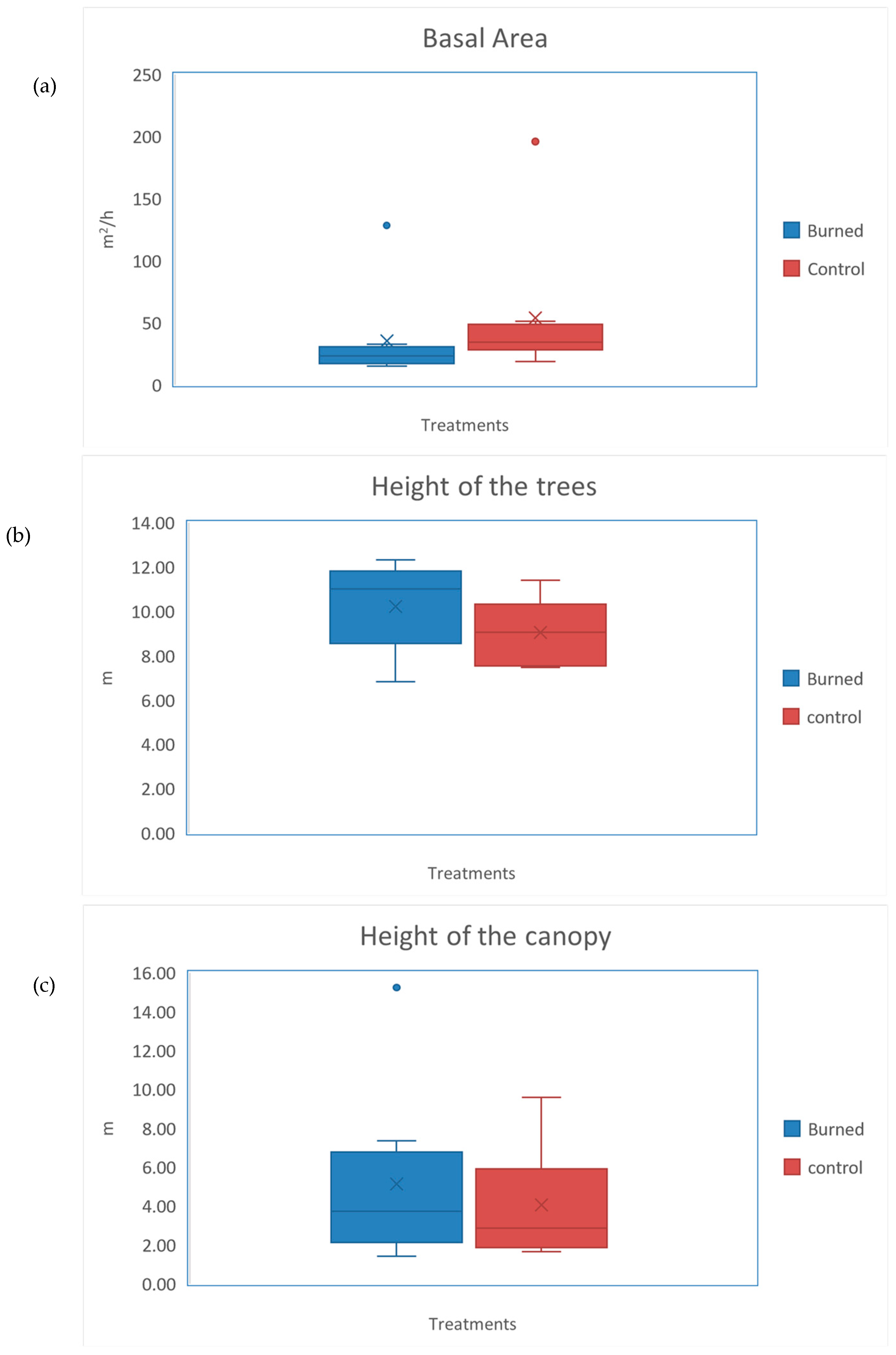
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Family [41] | Life form [42] | Origin * [42] |
|---|---|---|---|
| Adenocarpus foliolosus (Aiton) DC. | Fabaceae | Phanerophyte | NS* |
| Allium sp. | Amaryllidaceae | Bulb geophyte | |
| Argyranthemum adauctum (Link) Humphries | Asteraceae | Chamaephyte | NS* |
| Asphodelus ramosus L. | Xanthorrhoeaceae | Bulb geophyte | NP |
| Avena sp. | Poaceae | Therophyte | |
| Carduus tenuiflorus Curtis | Asteraceae | Therophyte | NP |
| Convulvulus canariensis L. | Convolvulaceae | Epiphyte | NS |
| Erysimum virescens (Webb ex Christ) Wettst. | Brassicaceae | Nanophanerophyte | NS* |
| Ferula linkii Webb | Apiaceae | Bulb geophyte | NS* |
| Galium aparine L. | Rubiaceae | Therophyte | NP |
| Geranium dissectum L. | Geraniaceae | Therophyte | NP |
| Geranium molle L. | Geraniaceae | Therophyte | NP |
| Hirschfeldia incana (L.) Lagr.-Foss. | Brassicaceae | Hemicryptophyte | NP |
| Hordeum murinum L. | Poaceae | Therophyte | NP |
| Hypericum grandifolium Choisy | Clusiaceae | Phanerophyte | NS |
| Juncus acutus L. | Juncaceae | Chamaephyte | NP |
| Lavandula canariensis Mill. subsp. canariae Upson & S. Andrews | Lamiaceae | Nanophanerophyte | NS* |
| Marrubium vulgare L. | Lamiaceae | Nanophanerophyte | ISN |
| Micromeria canariensis (P. Pérez) Puppo | Lamiaceae | Chamaephyte | NS* |
| Pteridium aquilinum (L.) Kuhn in Von der Decken | Dennstaedtiaceae | Rhizome geophyte | NP |
| Ranunculus cortusifolius Willd. | Ranunculaceae | Hemicryptophyte | NS |
| Sideritis dasygnaphala (Webb & Berthel.) Clos emend. Svent. | Lamiaceae | Nanophanerophyte | NS* |
| Silene vulgaris (Moench) Garcke | Lamiaceae | Therophyte | NP |
| Sonchus acaulis Dum. Cours. | Asteraceae | Hemicryptophyte | NS* |
| Trifolium sp. | Fabaceae | Therophyte | |
| Vicia lutea L. | Fabaceae | Therophyte | NP |
References
- Kornas, K. Succession regressive de la vegetation des garrigues sur les calcarires compacts dans la Montagne de la Gardiole, pres de Montpellier. Acta Soc. Bot. Pol. 1958, 27, 563–596. [Google Scholar]
- Molinier, R. La dynamique de la vegetation provençale. Collect. Bot. Appl. 1968, 60, 119–208. [Google Scholar]
- White, P.S. Pattern, process, and natural disturbance in vegetation. Bot. Rev. 1979, 45, 229–299. [Google Scholar] [CrossRef]
- Leopold, A.J.; Cain, S.A.; Cottam, C.M.; Gabrielson, I.N.; Kimball, T.L. Wildfire management in the national parks. Am. For. 1963, 69, 32–35,61–63. [Google Scholar]
- Wright, H.A.; Bailey, A.W. Fire Ecology; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Perry, D.A. Forest Ecosystems; The John Hopkins University Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Naveh, Z. The evolutionary significance of fire in the Mediterranean region. Vegetatio 1975, 29, 199–208. [Google Scholar] [CrossRef]
- Trabaud, L. Postfire plant community dynamics in the Mediterranean basin. In The Role of Fire in Mediterranean Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Springer: New York, NY, USA, 1994; pp. 1–15. [Google Scholar]
- Del Arco, M.J.; Pérez, P.L.; Rodríguez, O.; Salas, M.; Wildpret, W. Atlas Cartográfico de los Pinares Canarios II: Tenerife; Viceconsejería de Medio Ambiente y Conservación de la Naturaleza (Consejería de Política Territorial, Gobierno de Canarias): Santa Cruz de Tenerife, Spain, 1992. [Google Scholar]
- Höllermann, P. The impact of fire in Canarian ecosystems 1983–1998. Erdkunde 2000, 54, 70–75. [Google Scholar] [CrossRef]
- Silva, J.S.; Rego, F.; Fernandes, P.; Rigolot, E. Towards Integrated Fire Management: Outcomes of the European Project Fire Paradox; European Forest Institute: Joensuu, Finland, 2010; ISBN 9709525453485. [Google Scholar]
- Fernandes, P.M.; Rego, F.C.; Rigolot, E. The FIRE PARADOX project: Towards science-based fire management in Europe. For. Ecol. Manag. 2011, 261, 2177–2178. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Naranjo-Cigala, A. Wildfire impact and the “fire paradox” in a natural and endemic pine forest stand and shrubland. Fire 2018, 1, 44. [Google Scholar] [CrossRef]
- Pérez, B.; Moreno, J.M. Fire-type and forest management effects on the early vegetation dynamics of a Pinus pinaster woodland. Plant Ecol. 1998, 134, 27–41. [Google Scholar] [CrossRef]
- Gobierno de Canarias. Ley 12/1994, de 19 de diciembre, de Espacios Naturales de Canarias. Boletín Oficial de Canarias. Nº157, de 24 de Diciembre de 1994. Available online: https://www.boe.es/eli/es-cn/l/1994/12/19/12 (accessed on 8 January 2022).
- Grillo Delgado, F.; Díaz Fababú, D.; Camaño Azcárate, J. III Curso Europeo Avanzado de Manejo de Fuego; Las Palmas de Gran Canaria: Municipality of Las Palmas, Spain, 2009. [Google Scholar]
- Smith, B.; Wilson, J.B. A consumer’s guide to evenness indices. Oikos 1996, 76, 70–82. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28. [Google Scholar]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package; R Package (Version 2.5–7). 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 8 January 2022).
- Arévalo, J.R.; Fernández-Palacios, J.M. Natural regeneration of Pinus canariensis Chr. Sm. Ex DC in Buch in forest plantations after thinning. TOFSCIJ 2008, 1, 54–60. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Fernández-Palacios, J.M. From pine plantations to natural stands. Ecological restoration of a Pinus canariensis Sweet, ex Spreng forest. Plant Ecol. 2005, 181, 217–226. [Google Scholar] [CrossRef]
- Knapp, E.E.; Schwilk, D.W.; Kane, J.M.; Keeley, J.E. Role of burning season on initial understory vegetation response to prescribed fire in a mixed conifer forest. Can. J. For. Res. 2007, 37, 11–22. [Google Scholar] [CrossRef]
- Rego, F.C.; Bunting, S.C.; Silva, J.M. Changes in understory vegetation following prescribed fire in maritime pine forest. For. Ecol. Manag. 1991, 41, 21–31. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Fernández-Lugo, S.; García-Domínguez, C.; Naranjo-Cigala, A.; Grillo, F.; Calvo, L. Restoration through prescribed burning and cutting of understory vegetation in a Pinus canariensis stand. Sci. World J. 2014, 2014, 215418. [Google Scholar] [CrossRef]
- Messier, C.; Puettmann, K.J.; Coates, K.D. Managing Forests as Complex Adaptive Systems: Building Resilience to the Challenge of Global Change; Earthscan from Routledge: Oxon, NS, Canada, 2013; ISBN 9781138779693. [Google Scholar]
- Lake, J.C.; Leishman, M.R. Invasion success of exotic plants in natural ecosystems: The role of disturbance, plant attributes and freedom from herbivores. Biol. Conserv. 2004, 117, 215–226. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Fernández-Lugo, S.; Naranjo-Cigala, A.; Salas, M.; Ruíz, R.; Ramos, R.; Moreno, M. Post-fire recovery of an endemic Canarian pine forest. Int. J. Wildland Fire 2014, 23, 403–409. [Google Scholar] [CrossRef]
- Piper, F.I.; Gundale, M.J.; Fajardo, A. Extreme defoliation reduces tree growth but not C and N storage in a winter-deciduous species. Ann. Bot. 2015, 115, 1093–1103. [Google Scholar] [CrossRef]
- Palacio, S.; Hernández, R.; Maestro-Martínez, M.; Camarero, J.J. Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the re-growth ability of trees. Trees 2012, 26, 1627–1640. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Garcia-Del-Rey, E.; Otto, R.; Fernández-Palacios, J.M.; Gil-Muñoz, P.; Gil, L. Effects of wildfire on endemic breeding birds in a Pinus canariensis forest of Tenerife, Canary Islands. Écoscience 2010, 17, 298–311. [Google Scholar] [CrossRef]
- Quintana, G.; Salas, M.; Fernández, E. Contribución al estudio de las comunidades rupícolas de la vertiente norte de Gran Canaria (Islas Canarias). Lazaroa 2006, 27, 89–102. [Google Scholar]
- Verloove, F.; Déniz, E.A.; Salas, M. New records of non-native vascular plants in Gran Canaria (Spain, Canary Islands). Flora Mediterr. 2020, 30, 121–136. [Google Scholar]
- Brown, R.T.; Agee, J.K.; Franklin, J.R. Forest restoration and fire: Principles in the context of place. Conserv. Biol. 2004, 18, 903–912. [Google Scholar] [CrossRef]
- Xanthopoulos, G.; Caballero, D.; Galante, M.; Alexandrian, D.; Rigolot, E.; Marzano, R. Forest fuels management in Europe. In Proceedings of the RMRS-P-41: Fuels Management-How to Measure Success Conference Proceedings, Portland, OR, USA, 28–30 March 2006; Andrews, P.L., Butler, B.W., Eds.; Rocky Mountain Research Station, US Department of Agriculture, Forest Service: Fort Collins, CO, USA, 2006; Volume 41, pp. 29–46. [Google Scholar]
- Fernandes, P.M.; Botelho, H.S. A review of prescribed burning effectiveness in fire hazard reduction. Int. J. Wildland Fire 2003, 12, 117–128. [Google Scholar] [CrossRef]
- Hesseln, H.; Loomis, J.B.; González-Cabán, A. Comparing the economic effects of fire on hiking demand in Montana and Colorado. J. For. Econ. 2004, 10, 21–35. [Google Scholar] [CrossRef]
- Brown, R.W. Bracken in the North York Moors: Its ecological and amenity implications in national parks. In Bracken, Ecology, Land Use and Control Technology; Smith, R.T., Taylor, J.A., Eds.; Parthenon Publishing Group: Carnforth, UK, 1986; pp. 77–86. [Google Scholar]
- Mólina-Terrén, D.M.; Fry, D.L.; Grillo, F.F.; Cardil, A.; Stephens, S.L. Fire History and Management of Pinus canariensis Forests on the Western Canary Islands Archipelago, Spain. For. Ecol. Manag. 2016, 382, 184–192. [Google Scholar] [CrossRef]
- Gobierno de Canarias. Banco de Datos de Biodiversidad de Canarias. Available online: https://www.biodiversidadcanarias.es/biota/ (accessed on 12 January 2023).
- Raunkiaer, C. Types biologiques pour la géographie botanique. Oversigt over Det Kongelige Danske Videnskabernes Selskabs Fohrandlinger. Bull. Soc. Acad. Sci. Dan. 1905, 5, 347–438. [Google Scholar]
| Altitude | Slope | Aspect | Rock | Litter | Herbs | Litter Depth | ||
|---|---|---|---|---|---|---|---|---|
| Plot | (m) | (°Sex.) | (% Cover) | (cm) | Years | |||
| 1B | 1449 | 5 | N | 5 | 16.8 | 53.8 | 1.0 | 9 |
| 1C | 1453 | 5 | N | 5 | 25.3 | 56.0 | 5.4 | |
| 2B | 1480 | 30 | S | 4 | 86.5 | 1.0 | 3.4 | 9 |
| 2C | 1480 | 30 | S | 0 | 74.7 | 9.3 | 6.6 | |
| 3B | 1580 | 10 | N | 10 | 91.9 | 1.9 | 4.2 | 9 |
| 3C | 1712 | 12 | N | 10 | 86.6 | 2.4 | 5.3 | |
| 4B | 1750 | 12 | N | 30 | 79.6 | 0.6 | 1.7 | 4 |
| 4C | 1800 | 14 | N | 8 | 74.6 | 8.5 | 4.1 | |
| 5B | 1615 | 13 | N | 0 | 32.0 | 61.0 | 2.3 | 3 |
| 5C | 1686 | 20 | N | 0 | 86.7 | 7.0 | 7.7 | |
| 6B | 1750 | 5 | N | 25 | 86.4 | 2.6 | 2.6 | 5 |
| 6C | 1739 | 5 | N | 0 | 93.2 | 0.5 | 5.9 | |
| 7B | 1793 | 10 | S | 1 | 69.8 | 1.3 | 1.1 | 2 |
| 7C | 1770 | 9 | S | 2 | 66.8 | 1.0 | 5.4 | |
| 8B | 1888 | 8 | N | 10 | 22.8 | 62.3 | 2.1 | 6 |
| 8C | 1934 | 8 | N | 18 | 61.8 | 10.5 | 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo, J.R.; Bernardos, M.; González-Montelongo, C.; Grillo, F. Prescribed Burning Effect on the Richness, Diversity and Forest Structure of an Endemic Reforested Pinus canariensis Stand (Canary Islands). Fire 2023, 6, 150. https://doi.org/10.3390/fire6040150
Arévalo JR, Bernardos M, González-Montelongo C, Grillo F. Prescribed Burning Effect on the Richness, Diversity and Forest Structure of an Endemic Reforested Pinus canariensis Stand (Canary Islands). Fire. 2023; 6(4):150. https://doi.org/10.3390/fire6040150
Chicago/Turabian StyleArévalo, José Ramón, María Bernardos, Cristina González-Montelongo, and Federico Grillo. 2023. "Prescribed Burning Effect on the Richness, Diversity and Forest Structure of an Endemic Reforested Pinus canariensis Stand (Canary Islands)" Fire 6, no. 4: 150. https://doi.org/10.3390/fire6040150
APA StyleArévalo, J. R., Bernardos, M., González-Montelongo, C., & Grillo, F. (2023). Prescribed Burning Effect on the Richness, Diversity and Forest Structure of an Endemic Reforested Pinus canariensis Stand (Canary Islands). Fire, 6(4), 150. https://doi.org/10.3390/fire6040150








