Experimental Study on the Microstructural Characterization of Retardation Capacity of Microbial Inhibitors to Spontaneous Lignite Combustion
Abstract
:1. Introduction
2. Experimental Material
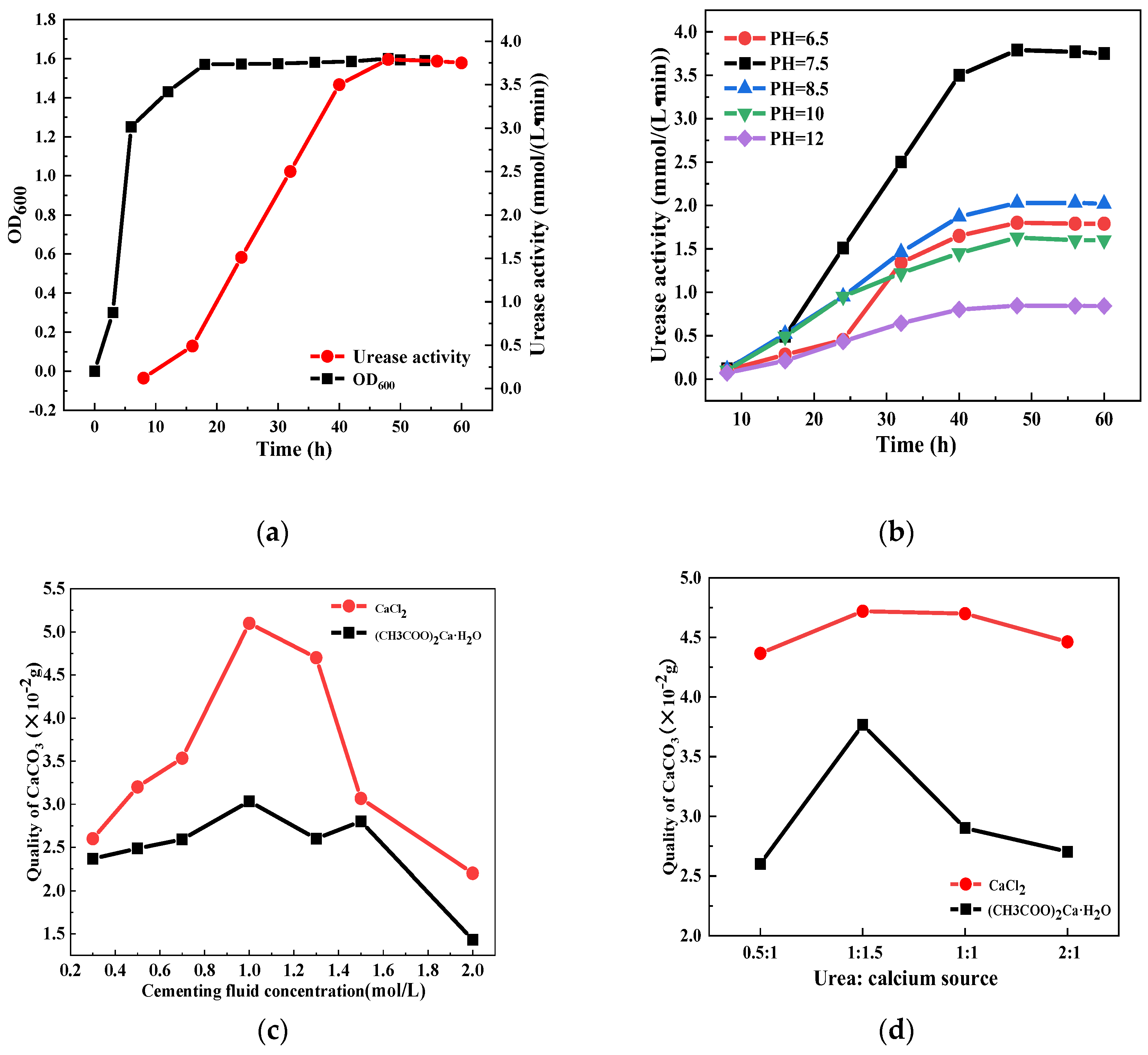
2.1. Preparation of Biological Inhibitors
2.2. Preparation of the Experimental Coal Sample
3. Experimental Methods
3.1. Electron Microscope Experiment of Retarded Lignite
3.2. Low-Temperature Nitrogen Adsorption Experiment
3.2.1. Calculation of the Specific Surface Area
3.2.2. Calculation of the Aperture Distribution
3.3. NMR and FTIR Tests
3.3.1. Nuclear Magnetic Resonance Carbon Spectrum Test
3.3.2. Fourier Infrared Spectroscopy Experiment
4. Results
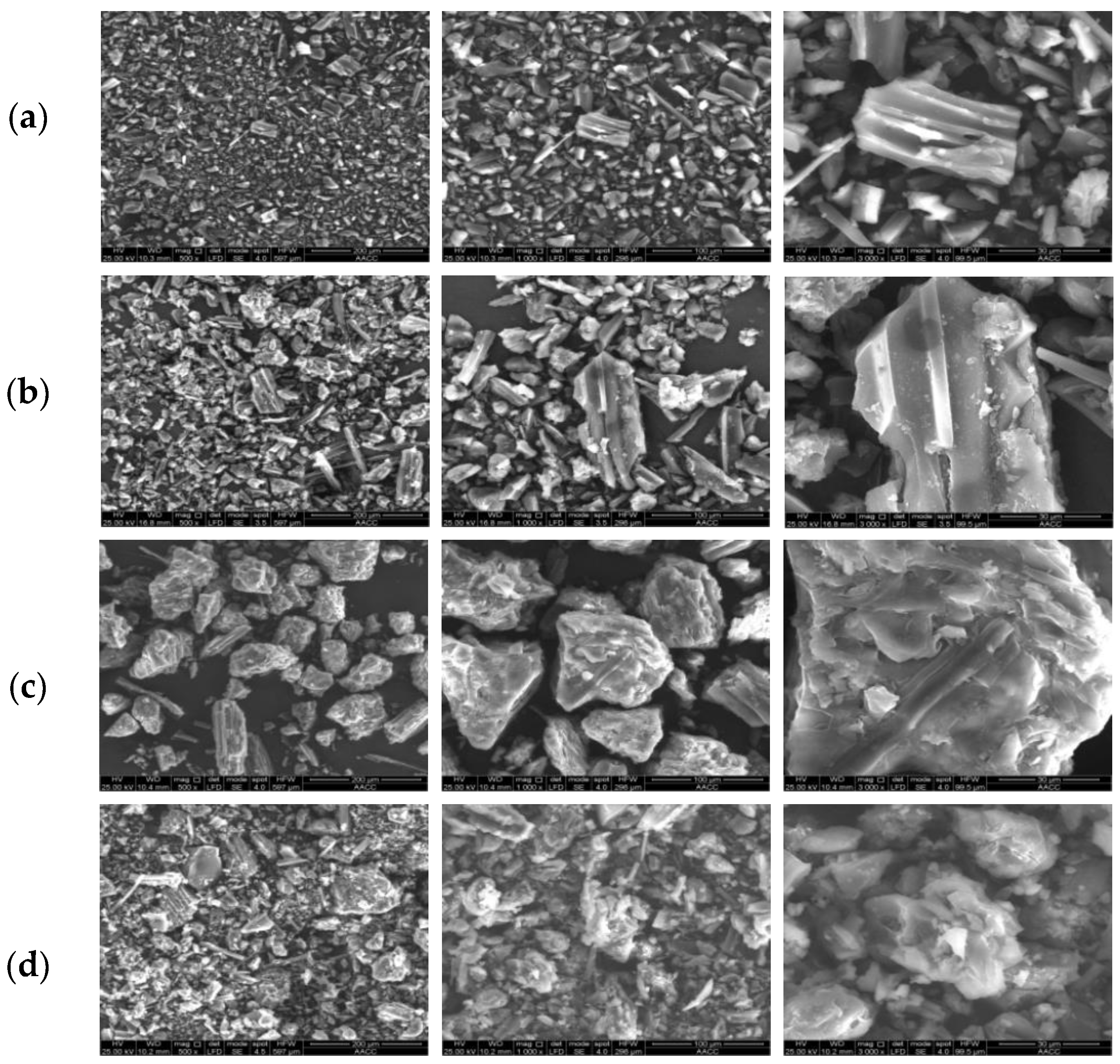
4.1. Scanning Electron Microscope Analysis of Lignite’s Surface Structure
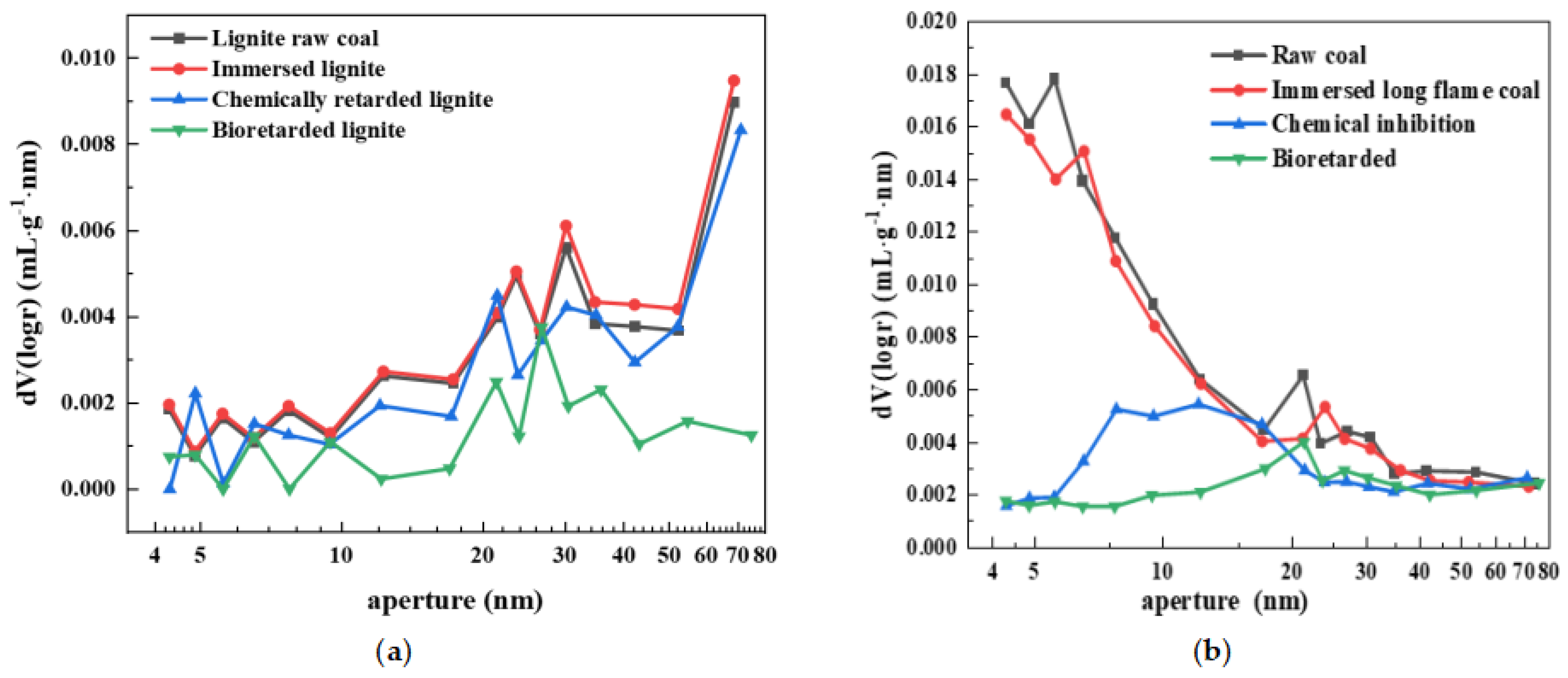
4.2. Experimental Analysis of the Inhibited Lignite’s Pore Size
4.2.1. Aperture Distribution Characteristics
4.2.2. Pore Structure Characteristic Parameter Analysis
4.3. Nuclear Magnetic Resonance Carbon Spectrum Characteristics and Infrared Spectrum Experimental Analysis
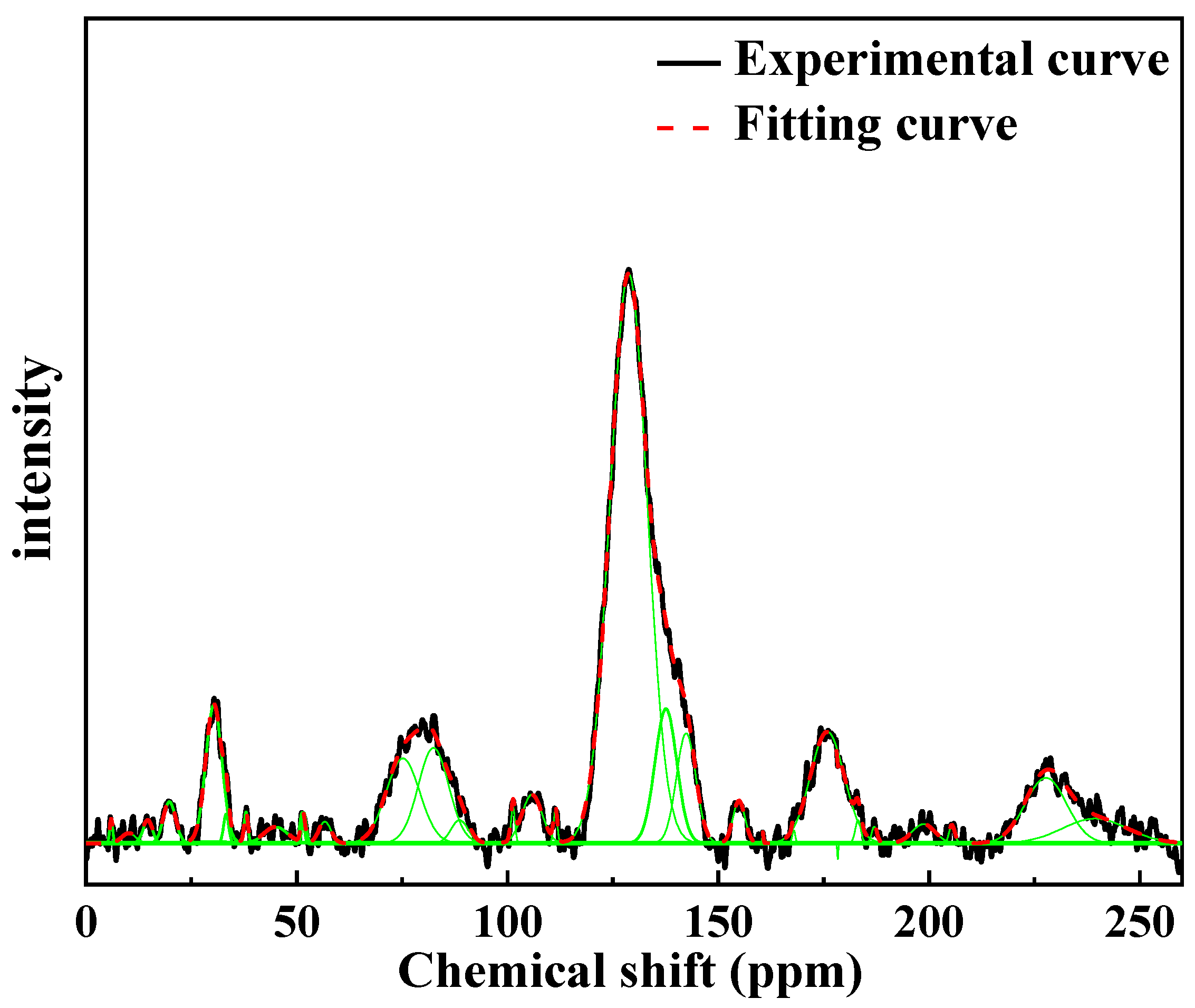
4.3.1. 13C-NMR Characteristic Analysis
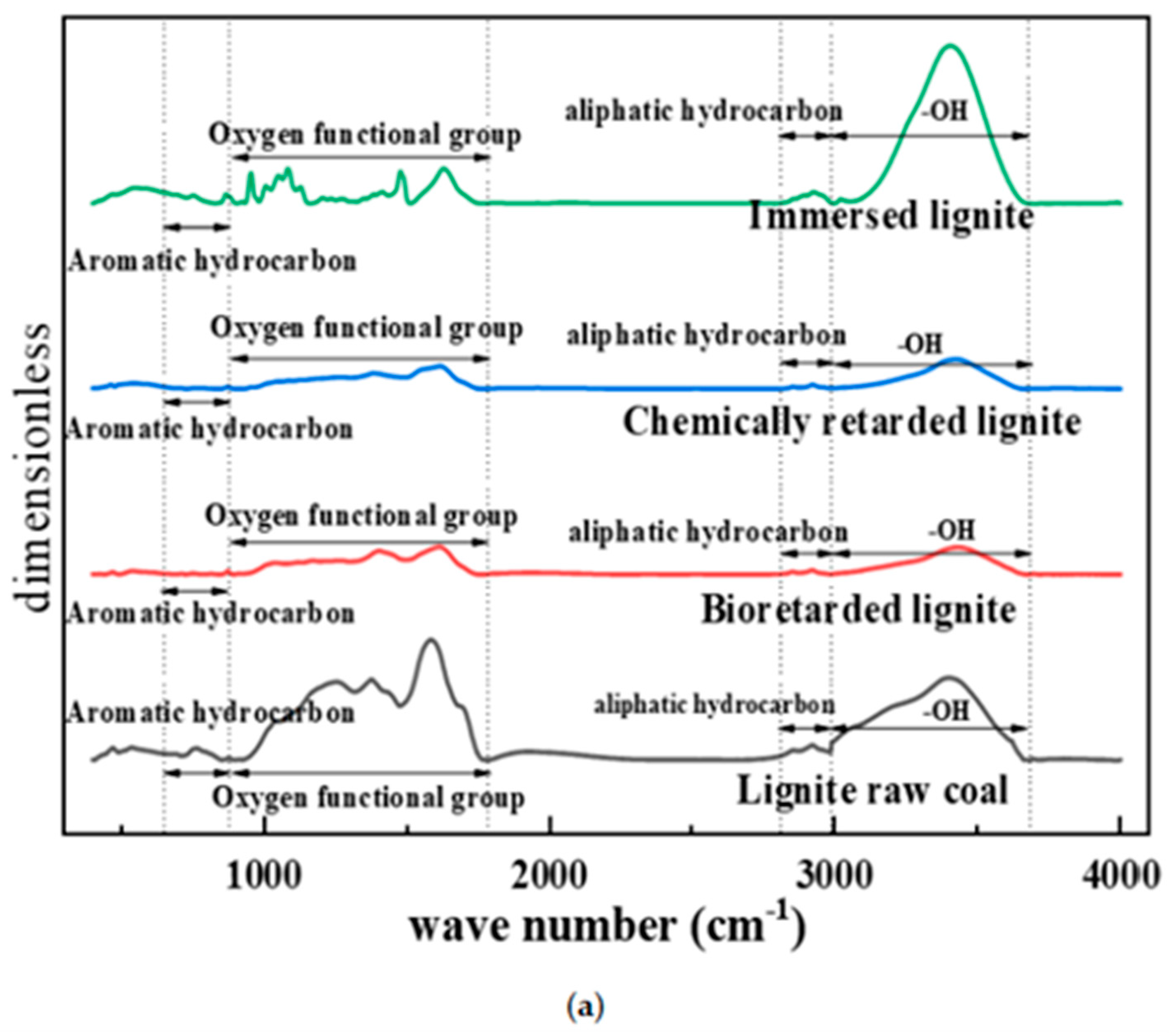
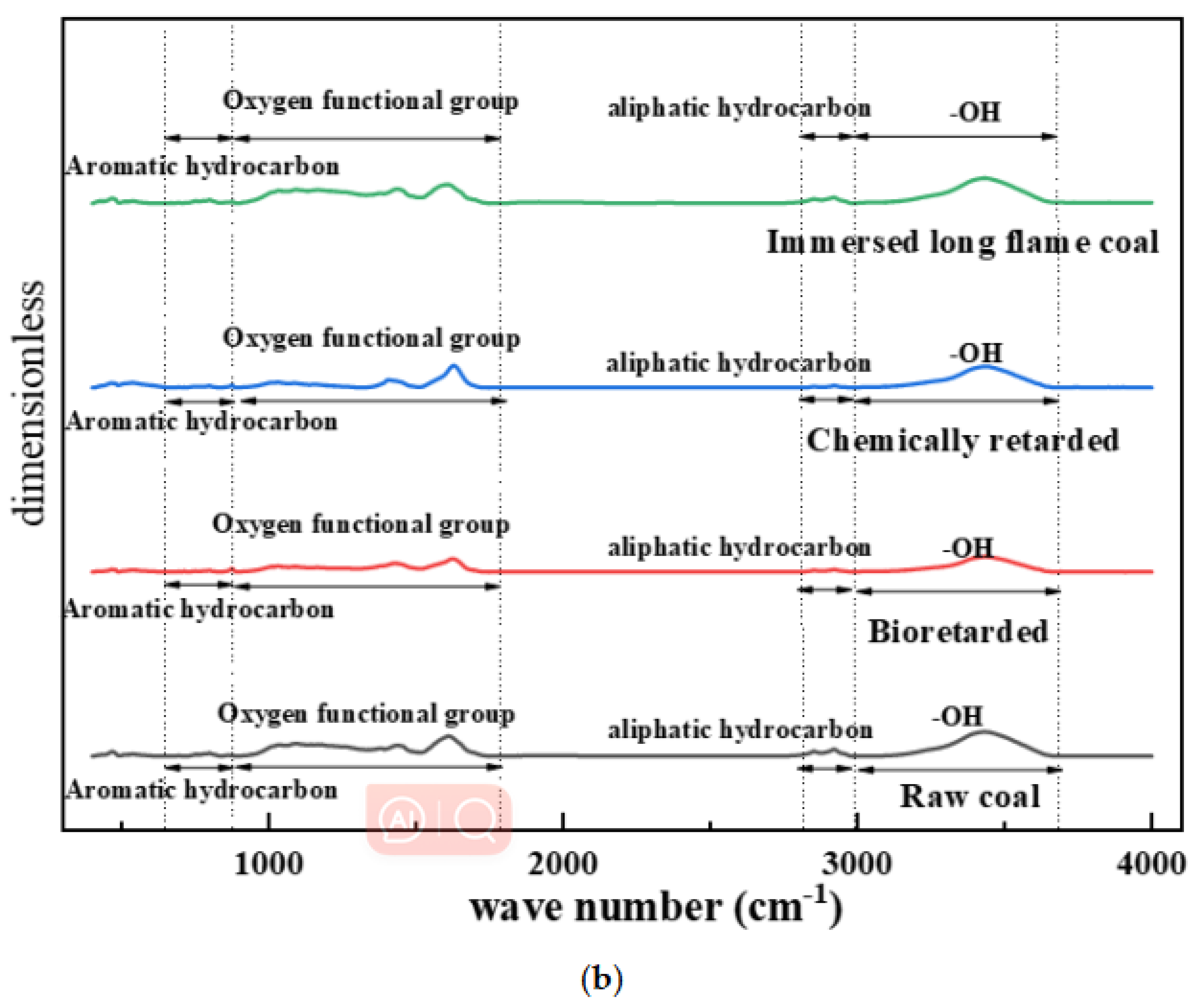
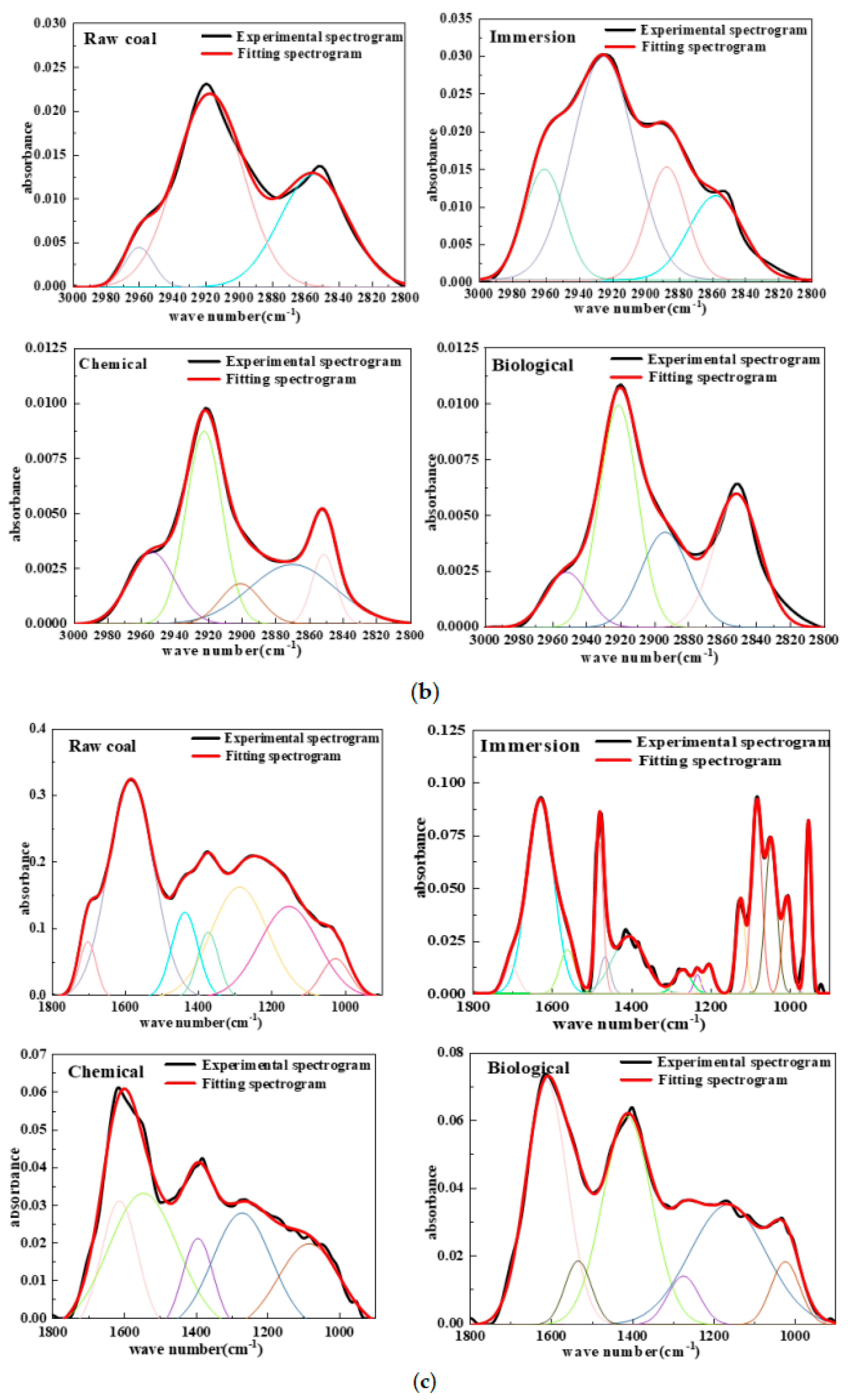
4.3.2. Infrared Spectrum Analysis of Coal Samples before and after Inhibition
- (1)
- Aromatic hydrocarbons
- (2)
- Fatty hydrocarbons
- (3)
- Hydroxyl group
- (4)
- Functional groups that contain oxygen
4.3.3. Analysis of the Spectral Peak Characteristics of Functional Groups
5. Conclusions
- (1)
- Based on SEM images, the changing characteristics of the surface micro-morphology of treated coal samples were analyzed. The surface of lignite is mainly composed of dissolution caves and pores. Compared with raw coal, a large number of deposited calcium carbonate particles are obviously attached to the surface of lignite after resistance treatment, which plays a role in physical oxygen insulation.
- (2)
- The pore size distribution, total pore volume, and specific surface area of lignite after different treatments were studied. The holes of lignite mainly exist in the form of parallel slate-like slits and open-wedge holes on all sides. Based on the pore distribution map, it was observed that the primary pores of lignite can be effectively blocked by microbial inhibitors compared with raw coal.
- (3)
- Based on the fitting results of the infrared spectra of coal samples, three main active groups—hydroxyl group, carboxyl group, and methyl/methylene group—were selected for analysis. The results show that the active group contents of lignite molecules, including the hydroxyl group, carboxyl group, and methyl/methylene group after microbial inhibition is lower than that of raw coal. In particular, the content of methyl/methylene involved in the initial oxidation reaction is reduced by 96.5% compared with the baseline content of raw coal, indicating that the biological inhibitor can simultaneously block the oxidation of the three active groups.
- (4)
- The performance difference between biological and chemical inhibition with respect to coal spontaneous combustion was further compared, and it was observed that calcium carbonate produced via biological inhibition showed a denser spherical distribution than that produced via chemical inhibition, indicating that the adhesion of calcium carbonate produced via biological inhibition was better. Based on the pore size distribution map, it is observed that the total pore volume and specific surface area of the coal sample after biological resistance treatment are more reduced compared to chemical resistance treatments, and the number of pores is significantly reduced. The results show that biological inhibition is more effective than chemical inhibition in preventing the spontaneous combustion of lignite at the physical level. According to the results of NMR experiments, the first three components of the coal sample are aromatic carbon, fatty carbon, and carbonyl carbon. The results of FTIR analyses showed that the consumption effect of biological inhibitors on methyl/methylene was better than that of chemical inhibitors, and the spontaneous combustion effect was improved.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, Y.; Huang, Z.; Zhang, Y.; Ding, H.; Hu, X.; Gao, Y.; Sun, C. Study on the Effect of Oxygen on Free Radical Generation in Coal. Combust. Sci. Technol. 2022, 6, 1–8. [Google Scholar] [CrossRef]
- Xu, L.; Li, Q.; Myers, M.; Chen, Q.; Li, X. Application of Nuclear Magnetic Resonance Technology to Carbon Capture, Utilization and Storage: A Review. J. Rock. Mech. Geotech. Eng. 2019, 11, 892–908. [Google Scholar] [CrossRef]
- Niu, H.; Tao, M.; Bu, Y.; Li, S.; Yang, Y.; Sun, Q.; Mao, Z. Spontaneous combustion characteristics and mechanism of water-immersed and air-dried brown coal. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 7413–7431. [Google Scholar] [CrossRef]
- Yu, S.-L.; Liu, X. Study on Multi-Indicator Quantitative Risk Evaluation Methods for Different Periods of Coal Spontaneous Combustion in Coal Mines. Combust. Sci. Technol. 2022, 9, 1–4. [Google Scholar] [CrossRef]
- Bosikov, I.I.; Martyushev, N.V.; Klyuev, R.V.; Savchenko, I.A.; Kukartsev, V.V.; Kukartsev, V.A.; Tynchenko, Y.A. Modeling and Complex Analysis of the Topology Parameters of Ventilation Networks When Ensuring Fire Safety While Developing Coal and Gas Deposits. Fire 2023, 6, 95. [Google Scholar] [CrossRef]
- Xu, Y.; Dehghanpour, H.; Ezulike, O.; Virues, C. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wei, J.; Zhao, Y.; Shen, L. Fire prevention and control using gel-stabilization foam to inhibit spontaneous combustion of coal: Characteristics and engineering applications. Fuel 2020, 264, 116903. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, S.; Hu, X.; Cai, J.; Tang, Z.; Xu, Q. Whole process inhibition of a composite superabsorbent polymer-based antioxidant on coal spontaneous combustion. Arab. J. Sci. Eng. 2018, 43, 5999–6009. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Yu, X.; Wu, M.; Zhao, Y.; Lu, Y.; Pan, R.; Niu, H.; Hu, S. Development of a novel composite inhibitor modified with proanthocyanidins and mixed with ammonium polyphosphate. Energy 2020, 213, 118901. [Google Scholar] [CrossRef]
- Zhai, X.W.; Pan, W.J.; Xiao, Y.; Wang, S.R.; Ouyang, L.M. Inhibition of coal spontaneous combustion by an environment-friendly, water-based fire extinguishing agent. J. Therm. Anal. Calorim. 2021, 144, 325–334. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Wang, D. Experimental study on the inhibition of primary and secondary oxidation of coal by chlorine salt inhibitors. Min. Saf. Environ. Prot. 2015, 42, 1–4. [Google Scholar] [CrossRef]
- Chi, K.; Wang, J.; Ma, L.; Wang, J.; Zhou, C. Synergistic Inhibitory Effect of Free Radical Scavenger/Inorganic Salt Compound Inhibitor on Spontaneous Combustion of Coal. Combust. Sci. Technol. 2022, 194, 2146–2162. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, G.; Chu, R.; Wu, G.; Gao, M.; Dai, J.; Bai, L. Evolution mechanism of active groups and thermal effectsof Chinese lignite in low-temperature oxidation. Chem. Eng. Commun. 2019, 207, 861–870. [Google Scholar] [CrossRef]
- Zheng, L. Test and analysis on salty retardants performance to restrain coal oxidized spontaneous combustion. Coal Sci. Technol. 2010, 38, 70–72. [Google Scholar]
- Lv, H.; Li, B.; Deng, J.; Ye, L.; Gao, W.; Shu, C.M.; Bi, M. A novel methodology for evaluating the inhibitory effect of chloride salts on the ignition risk of coal spontaneous combustion. Energy 2021, 231, 121093. [Google Scholar] [CrossRef]
- Tang, Y. Experimental investigation of applying MgCl2 and phosphates to synergistically inhibit the spontaneous combustion of coal. J. Energy Inst. 2018, 91, 639–645. [Google Scholar] [CrossRef]
- He, S.; Qi, J.; Zhou, C.; Tang, Y.; Liu, H. Quantum chemistry of metal ions (Na+, Ba2+, Ca2+) inhibiting the activity of oxygen-containing functional groups in coal. Coal Mine Saf. 2023, 54, 149–155. [Google Scholar]
- Soto, F.; Alves, M.; Valdés, J.C.; Armas, O.; Crnkovic, P.; Rodrigues, G.; Lacerda, A.; Melo, L. Experimental study on thermo-responsive inhibitors inhibiting coal spontaneous combustion. Fuel Process Technol. 2018, 175, 113–122. [Google Scholar] [CrossRef]
- Lanfang, Z. Performance analysis of salt retarders for inhibiting spontaneous combustion of coal oxidation. Coal Sci. Technol. 2010, 5, 70–72. [Google Scholar]
- Zhai, X.; Zhou, Y.; Song, B.; Pan, W.; Wang, J. Comparative study on the inhibiting effect of dissolvable tiny-foam extinguishing agent and chlorine salts on coal spontaneous combustion. Environ. Sci. Pollut. Res. 2023, 30, 80591–80601. [Google Scholar] [CrossRef]
- Deng, B.; Qiao, L.; Wang, Y.; Mu, X.; Deng, C.; Jin, Z. Study on the effect of inorganic and organic sodium on coal spontaneous combustion. Fuel 2023, 353, 129256. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Zhang, Y.; Wang, J.; Zhou, C. Experimental study on synergistic inhibition of coal spontaneous combustion by magnesium chloride and carbon/oxygen free radical catcher. Min. Saf. Environ. Prot. 2021, 3, 6–11+16. [Google Scholar] [CrossRef]
- Choi, K.H.; Seo, D.J.; Kim, Y.J.; Cho, S.S.; Han, Y.J.; Yang, I.; Kim, C.W.; Oh, K.; An, J.C.; Park, J.I. Molecular Characteristics of Catalytic Nitrogen Removal from Coal Tar Pitch over γ-Alumina-Supported NiMo and CoMo Catalysts. Int. J. Mol. Sci. 2023, 24, 11793. [Google Scholar] [CrossRef] [PubMed]
- Hongying, X.; Mengmeng, M.; Peng, C. Experimental study on inhibition of spontaneous combustion of coal by composite inhibitor by infrared spectrum. Saf. Environ. Prot. Min. Ind. 2019, 46, 40–44. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.; Shu, P.; Zhai, F.; Chen, S.; Wang, K.; Deng, J.; Kang, F.; Li, L. Preparation and properties of hydrotalcite microcapsules for coal spontaneous combustion prevention. Process Saf. Environ. Prot. 2021, 152, 536–548. [Google Scholar] [CrossRef]
- Pandey, J.; Mohalik, N.K.; Mishra, R.K.; Khalkho, A.; Kumar, D.; Singh, V.K. Investigation of the Role of Fire Retardants in Preventing Spontaneous Heating of Coal and Controlling Coal Mine Fires. Fire Technol. 2015, 51, 227–245. [Google Scholar] [CrossRef]
- Gao, K.; Bick, P.; Suleiman, M.T.; Li, X.; Helm, J.; Brown, D.G.; Zouari, N. Wind Erosion Mitigation Using Microbial-Induced Carbonate Precipitation. J. Geotech. Geoenviron. Eng. 2023, 149, 04023053. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Jin, Y.; Li, Q.; Xu, J. Application of microbially induced calcium carbonate precipitation (MICP) in sand embankments for scouring/erosion control. Mar. Georesour. Geotechnol. 2021, 39, 1459–1471. [Google Scholar] [CrossRef]
- Dai, X.; Li, W.; Chen, S.; Zeng, J.; Tong, C.; Zhou, J.; Xiang, T.; Zhang, J.; Li, C.; Ye, Y.; et al. Experimental Study on Coastal Sediment Reinforcement by Induced Carbonate Precipitation by Different Enzyme Sources. Water 2023, 15, 1484. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Bai, Y.; Long, H.; Cai, Y.; Zhang, J. Joint Characterization and Fractal Laws of Pore Structure in Low-Rank Coal. Sustainability 2023, 15, 9599. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, G.; Halenda, P.P. The determination of pore volume and area distribution in porous substances. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Wu, G.; Ye, Z.; Zhang, L.; Tang, J. Bituminous Coal Sorption Characteristics and Its Modeling of the Main Coal Seam Gas Component in the Huaibei Coalfield, China. Sustainability 2023, 15, 9822. [Google Scholar] [CrossRef]
- Drozd-Rzoska, A.; Rzoska, S.J.; Kalabinski, J. Energy Calculation and Simulation of Methane Adsorbed by Coal with Different Metamorphic Grades. ACS Omega 2020, 5, 14976–14989. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Liang, Z.; Jiang, Z.; Wu, W. Method Selection for Analyzing the Mesopore Structure of Shale—Using a Combination of Multifractal Theory and Low-Pressure Gas Adsorption. Energies 2023, 16, 2464. [Google Scholar] [CrossRef]
- Dong, X.-W.; Wang, A.; Yaning, M. The microscopic pore structure of coal spontaneous combustion propensity to its effects. Coal Sci. Technol. 2014, 42, 41–45+49. [Google Scholar]
- Kan, H.; Wang, Y.; Mo, W.L.; Wei, X.Y.; Mi, H.Y.; Ma, K.J.; Zhu, M.X.; Guo, W.C.; Guo, J.; Niu, J.M. Effect of solvent swelling with different enhancement methods on the microstructure and pyrolysis performance of Hefeng subbituminous coal. Fuel 2023, 332 Pt 1, 2023.P1. [Google Scholar] [CrossRef]
- Nakashima, Y.; Sawatsubashi, T.; Fujii, S. Nondestructive quantification of moisture in powdered low-rank coal by a unilateral nuclear magnetic resonance scanner. Int. J. Coal Prep. Util. 2022, 42, 1421–1434. [Google Scholar] [CrossRef]
- Cui, B.; Wu, B.; Wang, M.; Jin, X.; Shen, Y.; Chang, L. Structural characteristics of coking coal quality on the quality of the coke research. J. Coal Chem. Ind. 2023, 03, 106–111. [Google Scholar]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. Int. J. Mol. Sci. 2015, 16, 6227. [Google Scholar] [CrossRef]


| Sample | Mad (%) | Aad (%) | Vad (%) | FCad (%) | C (%) | H (%) | O (%) | N (%) | S (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lignite | 0.86 | 6.81 | 23.15 | 69.18 | 65.00 | 4.54 | 29.19 | 0.93 | 0.34 |
| Coal Sample | PV (mL/g) | Dpv | Ps (m2/g) | Dps | |
|---|---|---|---|---|---|
| Lignite | Raw coal | 0.00476 | - | 1.340 | - |
| Bioretarded | 0.00150 | −68.49% | 0.348 | −74.03% | |
| Chemically inhibited | 0.00258 | −45.79% | 1.004 | −25.07% | |
| Immersed | 0.00479 | 0.63% | 1.335 | −0.37% | |
| Long-flame coal | Raw coal | 0.009637 | - | 3.051 | - |
| Bioretarded | 0.0033540 | −65.19 | 0.952 | −68.79 | |
| Chemically inhibited | 0.0047644 | −50.56 | 1.185 | −61.16 | |
| Immersed | 0.008547 | −11.31 | 2.032 | −33.39 | |
| Peak Type | Peak Number | Peak Position | Functional Group | Peak Attribution |
|---|---|---|---|---|
| Oxygen-containing functional groups | 1 | 3697~3590 | –OH | Free hydroxyl group |
| 2 | 3500~3200 | An intermolecular hydrogen bond between a phenol hydroxyl group, an alcohol hydroxyl group, and an amino group | ||
| 3 | 1790~1715 | C=O | Stretching vibration of the carbonyl group | |
| 4 | 1715~1690 | –COOH | Carboxyl group | |
| 5 | 1275~1010 | C–O–C | Ether bond | |
| Aliphatic hydrocarbon | 6 | 2975~2950 | –CH3 | Methyl group with asymmetric stretching vibration |
| 7 | 1470~1430 | Methyl with shear vibration | ||
| 8 | 1380~1370 | |||
| 9 | 2940~2915 | –CH2 | Methylene with asymmetric stretching vibration | |
| 10 | 2870~2845 | Methylene with symmetric stretching vibration | ||
| Aromatic hydrocarbon | 11 | 3090~3030 | –CH | Aromatic CH with stretching vibration |
| 12 | 1620~1490 | C=C | C=C with stretching vibration in the aromatic ring | |
| 13 | 900~700 | —— | Out-of-plane bending vibration of polyhedron-substituted aromatics |
| Coal Sample | D–CH3/–CH2 | D–OH | D–COOH | |
|---|---|---|---|---|
| Lignite | Raw coal | - | - | - |
| Bioretarded | −96.6% | −33.5% | −70.7% | |
| Chemically inhibited | −94.4% | −33.8% | −77.9% | |
| Immersed | −67.7% | 268.2% | −73.3% | |
| Long-flame coal | Raw coal | - | - | - |
| Bioretarded | −90.7% | −39.6% | −43.7% | |
| Chemically inhibited | −92.2% | −21.5% | −42.5% | |
| Immersed | −73.1% | −4.68 | 1.20% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, R.; Chen, X.; Zou, X.; Li, D.; Wang, S. Experimental Study on the Microstructural Characterization of Retardation Capacity of Microbial Inhibitors to Spontaneous Lignite Combustion. Fire 2023, 6, 452. https://doi.org/10.3390/fire6120452
Wang Y, Liu R, Chen X, Zou X, Li D, Wang S. Experimental Study on the Microstructural Characterization of Retardation Capacity of Microbial Inhibitors to Spontaneous Lignite Combustion. Fire. 2023; 6(12):452. https://doi.org/10.3390/fire6120452
Chicago/Turabian StyleWang, Yanming, Ruijie Liu, Xiaoyu Chen, Xiangyu Zou, Dingrui Li, and Shasha Wang. 2023. "Experimental Study on the Microstructural Characterization of Retardation Capacity of Microbial Inhibitors to Spontaneous Lignite Combustion" Fire 6, no. 12: 452. https://doi.org/10.3390/fire6120452
APA StyleWang, Y., Liu, R., Chen, X., Zou, X., Li, D., & Wang, S. (2023). Experimental Study on the Microstructural Characterization of Retardation Capacity of Microbial Inhibitors to Spontaneous Lignite Combustion. Fire, 6(12), 452. https://doi.org/10.3390/fire6120452








